Obese patients often find it difficult to adhere to long-term low-calorie diets. One of the reasons for dietary failure is the permanent feeling of hunger. Ghrelin is an orexigenic hormone, secreted by enterochromaffin cells in the gastric fundus.
The aim of this study was to analyze changes in plasma ghrelin levels after PENS of dermatome T6 associated to a low-calorie diet, as well as changes in appetite and weight loss, as compared to a control group on a low-calorie alone.
Material and methodsA prospective, non-randomized study was conducted including 20 patients who underwent PENS of dermatome T6 associated to a low-calorie diet before undergoing bariatric surgery to lose weight (Group 1), and 20 patients who were only prescribed a low-calorie diet before surgery (Group 2). In Group 1, plasma ghrelin levels were measured at 5 timepoints: before the first PENS session (Sample 1a); after the first PENS session (Sample 1b); before the last PENS session (Sample 2a); after the last PENS session (Sample 2b); and one month after treatment completion (Sample 3). In Group 2, only two samples were collected: before the start of the diet (Sample 1) and after 12 weeks of diet (Sample 2).
ResultsAfter 12 weeks of treatment, BMI decreases of 8.42±2.6% and 1.32±0.98% were seen in Group 1 and Group 2 respectively (p=0.007). A significant decrease was seen in ghrelin levels between samples 1a and 2a, and between samples 1a and 3. In Group 2, a non-significant increase was seen in ghrelin levels.
ConclusionPENS of dermatome T6 was associated to decreased plasma ghrelin levels. This therapy, associated to a low-calorie diet, achieves a BMI reduction greater than 8% after 12 weeks of treatment.
Los pacientes con obesidad, con frecuencia, tienen dificultad para adherirse a una dieta baja en calorías durante largos períodos de tiempo. Una de las causas del fracaso dietético es la sensación continua de hambre. La grelina es un péptido orexígeno, secretado por células enterocromafines del fundus gástrico.
El objetivo de este estudio fue analizar las variaciones de los valores plasmáticos de grelina tras PENS del dermatoma T6 asociado a dieta hipocalórica, así como la modificación del apetito y la pérdida de peso, comparándolo con un grupo control en el que solo se pautó una dieta hipocalórica.
Material y métodosRealizamos un estudio prospectivo no aleatorizado, incluyendo 20 pacientes sometidos a PENS del dermatoma T6, asociado a dieta hipocalórica, como tratamiento previo a ser sometidos a una técnica de cirugía bariátrica y con el fin de reducir peso (grupo 1), y 20 pacientes a los que se les pautó exclusivamente dieta hipocalórica previa a la intervención quirúrgica (grupo 2). En el grupo 1 se analizaron los niveles de grelina plasmática en 5 momentos diferentes del procedimiento: antes de realizar la primera sesión de PENS (muestra 1a), al finalizar la primera sesión de PENS (muestra 1b), antes de realizar la última sesión de PENS (muestra 2a), al finalizar la última sesión de PENS (muestra 2b) y un mes después de haber finalizado el tratamiento (muestra 3). En el grupo 2 se obtuvieron solo 2 muestras, antes de comenzar la dieta (muestra 1) y tras 12 semanas de dieta (muestra 2).
ResultadosTras 12 semanas de tratamiento se observó una pérdida de IMC del 8,42±2,6% en el grupo 1 y del 1,32±0,98% en el grupo 2 (p=0,007). En el grupo 1 se apreció un descenso significativo de los valores de grelina entre las muestras 1a y 2a, y entre las muestras 1a y 3. En el grupo 2 se observó un aumento no significativo de los niveles de grelina entre las muestras 1 y 2.
ConclusiónEl PENS del dermatoma T6 se asoció con una disminución en los valores de grelina plasmática. Esta terapia, asociada a una dieta hipocalórica, consigue una pérdida de IMC superior al 8% en 12 semanas de tratamiento.
About one-third of the population in developed countries is obese to some extent, and more than half is at least overweight. Obesity in itself is a health risk factor that influences the development and progression of different disorders such as dyslipidemia, ischemic heart disease, arterial hypertension, type 2 diabetes and sleep apnea–hypopnea syndrome (SAHS), thereby worsening the quality of life of patients, limiting their activities and causing psychosocial problems. There is a direct relationship between the body mass index (BMI) and morbidity and mortality risk in obese patients, derived from the associated disease conditions, which defines obesity as a disease in itself.1–3
Dietary treatment associated with physical exercise is the first therapeutic step in dealing with obesity. However, in order for such measures to be effective, patient motivation is essential. Patients with obesity often have difficulties adhering to a low-calorie diet for long periods of time. In this regard, one of the causes of dietary failure is continuous hunger sensation.4
Ghrelin is a peptide mainly produced by the enterochromaffin cells of the gastric fundus, and its release is regulated by growth hormone (GH).5 Recent studies have confirmed that ghrelin exerts an orexigenic effect; it stimulates appetite by activating neurons in the arcuate nucleus of the hypothalamus, where the appetite regulating center is located. Ghrelin also produces a positive energy balance, reducing basal metabolic expenditure and thus favoring lipid accumulation.6
Percutaneous electrical nerve stimulation (PENS) was originally developed for the treatment of urinary and fecal incontinence, stimulating the posterior tibial nerve. The mechanism of action of PENS involves the generation of a somato-somatic reflex (posterior tibial nerve, afferent pathway) that conveys the electrical impulse to the S3 root, the efferent pathway of which is the pudendal nerve, responsible for innervation of the anal sphincter.7–9
Based on the generation of a somato-autonomic reflex, stimulation of the sensory nerve endings located in dermatome T6 may trigger a reflex action, where the efferent pathways terminate in the vagus nerve branches that stimulate the gastric wall in a way similar to a gastric pacemaker. A previous study by our group10 concluded that PENS of dermatome T6 is associated with decreased appetite, and that when used in combination with adequate diet measures, the resulting weight loss is greater than in patients that only follow a diet.
The present study analyzes variations in plasma ghrelin levels after PENS targeted to dermatome T6, combined with a low-calorie diet, as well as changes in appetite and weight loss as compared to a control group receiving only a low-calorie diet.
Material and methodsA prospective, non-randomised study was conducted, involving 40 patients. Patients eligible for bariatric surgery and on the waiting list for such surgery were selected. Since most patients undergoing bariatric surgery are women, we decided to include only females in order to ensure a degree of homogeneity in the sample. The first 20 consecutive patients underwent PENS of dermatome T6, combined with a low-calorie diet (1200kcal/day) (Group 1), while the subsequent 20 patients only received a low-calorie diet (Group 2). There was no pairing between groups. Both measures were prescribed as treatment prior to bariatric surgery for the purpose of losing weight and thus reducing the surgical risk. The inclusion criteria were patients with BMI>40kg/m2 or BMI>35kg/m2, with comorbidities associated with obesity. The exclusion criteria were endocrine diseases causing obesity and/or severe psychiatric disorders.
Evaluation of candidatesPatients eligible for inclusion in the study were subject to the same screening criteria as patients scheduled for bariatric surgery, since they were programmed to undergo surgery after the mentioned treatment measures. Potential candidates were evaluated by a multidisciplinary team of surgeons, endocrinologists, psychiatrists, psychologists, anesthetists, endoscopists, radiologists and specialized nurses. It was explained to the patients that they were going to undergo a bariatric procedure after completing treatment, and that weight loss was essential in order to minimize the surgical risk.
Percutaneous electrostimulation methodPercutaneous electrical nerve stimulation of dermatome T6 was performed by the surgeons of Hospital General Universitario de Elche (Spain). Stimulation was carried out using the Urgent PC 200 Neuromodulation System® (Uroplasty, Minnetonka, MN, USA), which was originally developed for the treatment of fecal and urinary incontinence. The participants underwent a 30-min session every week for 12 consecutive weeks. The patients were placed in the supine position without anesthesia, and PENS was applied using an electrode and needle inserted into the upper left abdominal quadrant along the midclavicular line, 2cm below the costal margin at an angle of 90° to the abdominal wall and at a depth of approximately 0.5–1cm. Successful placement was confirmed by perceived movement sensation or electrical sensation at least 5cm beyond the site of needle insertion. Percutaneous electrical nerve stimulation was performed at a frequency of 20Hz and at the highest current–voltage setting (0–20mA) tolerated by the patient without causing pain.
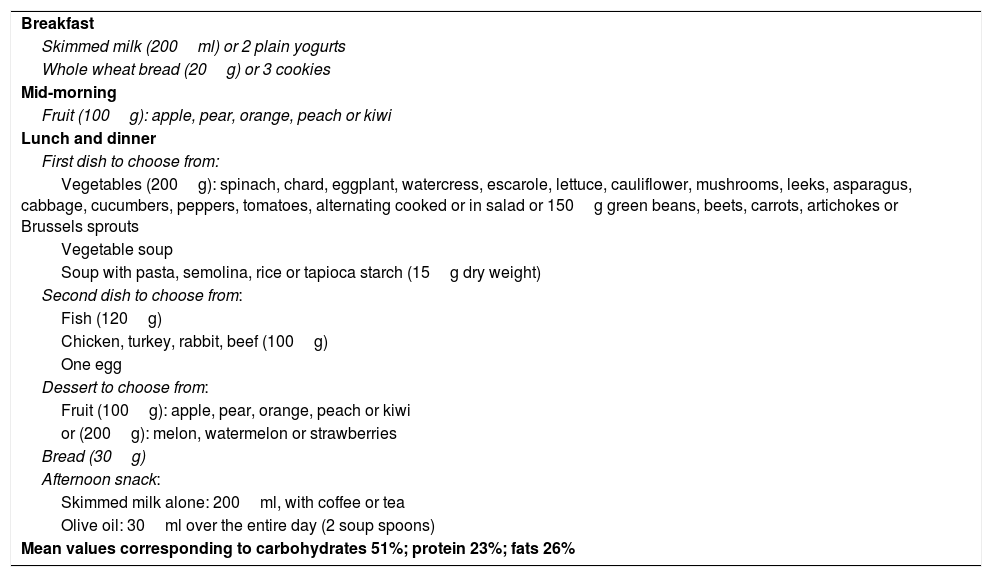
DietThe prescribed low-calorie diet provided 1200kcal/day. The diet was balanced and complied with Mediterranean diet recommendations, with a high intake of fruit and vegetables and a low intake of meat. Olive oil was the main lipid source (Table 1). This diet represents the standard protocol prescribed for patients eligible for bariatric surgery three months before the operation. Once started, the diet was to be followed until the day prior to surgery in both groups. Dietary adherence and potential problems with compliance were assessed by the nutritionist of the center through monthly visits to her office.
Diet (1200kcal) followed by all the patients enrolled in the study.
| Breakfast |
| Skimmed milk (200ml) or 2 plain yogurts |
| Whole wheat bread (20g) or 3 cookies |
| Mid-morning |
| Fruit (100g): apple, pear, orange, peach or kiwi |
| Lunch and dinner |
| First dish to choose from: |
| Vegetables (200g): spinach, chard, eggplant, watercress, escarole, lettuce, cauliflower, mushrooms, leeks, asparagus, cabbage, cucumbers, peppers, tomatoes, alternating cooked or in salad or 150g green beans, beets, carrots, artichokes or Brussels sprouts |
| Vegetable soup |
| Soup with pasta, semolina, rice or tapioca starch (15g dry weight) |
| Second dish to choose from: |
| Fish (120g) |
| Chicken, turkey, rabbit, beef (100g) |
| One egg |
| Dessert to choose from: |
| Fruit (100g): apple, pear, orange, peach or kiwi |
| or (200g): melon, watermelon or strawberries |
| Bread (30g) |
| Afternoon snack: |
| Skimmed milk alone: 200ml, with coffee or tea |
| Olive oil: 30ml over the entire day (2 soup spoons) |
| Mean values corresponding to carbohydrates 51%; protein 23%; fats 26% |
In Group 1, blood samples were collected at 5 different timepoints of the procedure:
- -
Immediately before the first PENS session (Sample 1a).
- -
At the end of the first PENS session (“peak effect”) (Sample 1b).
- -
Immediately before the last PENS session (“trough effect”) (Sample 2a).
- -
At the end of the last PENS session (Sample 2b).
- -
One month after completing the 12 PENS sessions (“residual effect”) (Sample 3).
In Group 2, only two blood samples were collected:
- -
Before starting the diet (Sample 1).
- -
After 12 weeks of dietary treatment (Sample 2).
Since dietary treatment was not discontinued at any time before surgery, it made no sense to collect a third sample at 16 weeks.
The samples in all cases were collected by the same nurse, who had extensive experience in blood sampling in morbidly obese patients, under aseptic and antiseptic conditions. The samples were drawn into EDTA tubes with protease inhibitor and centrifuged at 2500rpm for 20min. The plasma fraction was extracted and frozen at −80°C until subsequent analysis, in line with the protocols of the Department of Clinical Analysis of the center.
Study variablesA specific protocol was developed for data collection in this study. The following were recorded:
- -
Sociodemographic variables: age, gender, and comorbidities.
- -
Anthropometric variables: body weight, the BMI, the percentage BMI reduction. These variables were recorded before and after the treatment.
- -
Pain perception after PENS was assessed at the end of the first stimulation session using a visual analog scale (VAS) (0=no pain, 10=unbearable pain).
- -
Complications secondary to PENS were recorded (pain, infection, etc.).
- -
Appetite was assessed using a VAS ranging from 0 (no perception) to 10 (maximum perception). Appetite was investigated before the start of treatment and after the completion of the 12 sessions.
- -
Plasma ghrelin levels were measured in 5 blood samples in Group 1 and in two samples in Group 2, collected at different timepoints of the process.
Data analysis was performed using the SPSS version 19.0 statistical package for MS Windows. Quantitative variables with a normal distribution were reported as the mean and standard deviation (SD). The median and range were calculated for variables with a non-normal distribution. Qualitative variables were reported as frequencies and percentages.
Within-group comparisons of variables were made using analysis of variance (ANOVA) for repeated samples with post hoc analysis and adjustment for multiple comparisons. Comparisons between groups were made using the Student t-test for independent data and the Mann–Whitney U-test.
Qualitative variables were compared using the chi-squared test; in cases with under 5 observations in the cell, the Fisher exact test was used. Statistical significance was considered for p<0.05.
The study was approved by the local Clinical Research Ethics Committee (Ref. CEIC-HGUE 2013/7), and all patients signed the corresponding informed consent form.
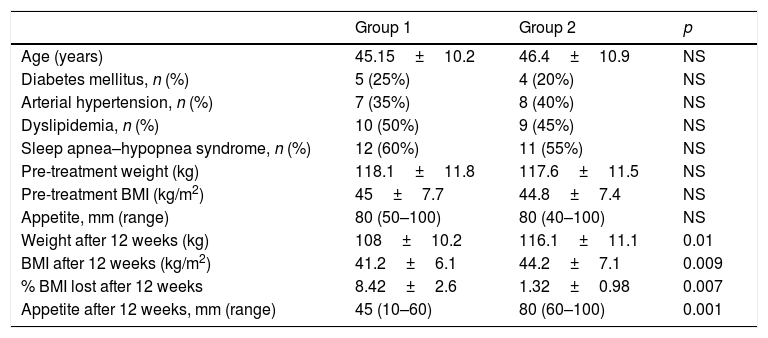
ResultsTwenty women were included in each group. There were no losses to follow-up, and all 40 patients were analyzed. Table 2 reports the anthropometric variables and appetite in each group before and after treatment.
Between-group comparison of anthropometric values and appetite before and after treatment.
| Group 1 | Group 2 | p | |
|---|---|---|---|
| Age (years) | 45.15±10.2 | 46.4±10.9 | NS |
| Diabetes mellitus, n (%) | 5 (25%) | 4 (20%) | NS |
| Arterial hypertension, n (%) | 7 (35%) | 8 (40%) | NS |
| Dyslipidemia, n (%) | 10 (50%) | 9 (45%) | NS |
| Sleep apnea–hypopnea syndrome, n (%) | 12 (60%) | 11 (55%) | NS |
| Pre-treatment weight (kg) | 118.1±11.8 | 117.6±11.5 | NS |
| Pre-treatment BMI (kg/m2) | 45±7.7 | 44.8±7.4 | NS |
| Appetite, mm (range) | 80 (50–100) | 80 (40–100) | NS |
| Weight after 12 weeks (kg) | 108±10.2 | 116.1±11.1 | 0.01 |
| BMI after 12 weeks (kg/m2) | 41.2±6.1 | 44.2±7.1 | 0.009 |
| % BMI lost after 12 weeks | 8.42±2.6 | 1.32±0.98 | 0.007 |
| Appetite after 12 weeks, mm (range) | 45 (10–60) | 80 (60–100) | 0.001 |
| Group 1 | Group 2 | p | |
|---|---|---|---|
| Diabetes mellitus N (%) | 5 (25%) | 4 (20%) | NS |
| Arterial hypertension N (%) | 7 (35%) | 8 (40%) | NS |
| Dyslipidemia N (%) | 10 (50%) | 9 (45%) | NS |
| Sleep apnea–hypopnea syndrome N (%) | 12 (60%) | 11 (55%) | NS |
NS: nonsignificant.
The median pain perception score after PENS of dermatome T6 was 1 (range 0–2). There were no complications in any of the patients.
After the completion of treatment, significant weight changes were observed in Group 1 (p=0.029), but not in Group 2 (controls). Similarly, a significant decrease in appetite was seen only in Group 1 (p<0.001).
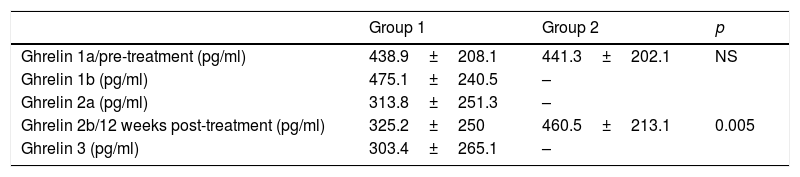
Table 3 shows the ghrelin values corresponding to the different measurements in both groups. In Group 1, a non-significant increase was observed between samples 1a and 1b. A mean decrease in ghrelin levels of 125.1pg/ml was observed between samples 1a and 2a (95% CI: 67.448–182.885; p<0.001). Likewise, on comparing samples 1a and 3, i.e., one month after the end of treatment, a mean decrease of 135.5pg/ml was observed (95% CI: 48.370–214.163; p=0.004). There were no significant differences between samples 2a and 2b.
Ghrelin values in the different samples of both groups.
| Group 1 | Group 2 | p | |
|---|---|---|---|
| Ghrelin 1a/pre-treatment (pg/ml) | 438.9±208.1 | 441.3±202.1 | NS |
| Ghrelin 1b (pg/ml) | 475.1±240.5 | – | |
| Ghrelin 2a (pg/ml) | 313.8±251.3 | – | |
| Ghrelin 2b/12 weeks post-treatment (pg/ml) | 325.2±250 | 460.5±213.1 | 0.005 |
| Ghrelin 3 (pg/ml) | 303.4±265.1 | – |
NS: non-significant.
In Group 2, only a nonsignificant increase in ghrelin values was observed between samples 1 and 2.
Neither group showed a statistically significant correlation between ghrelin levels and the quantification of appetite, or between the decrease in ghrelin levels and weight loss.
DiscussionIn the present study, PENS of dermatome T6 combined with a low-calorie diet reduced ghrelin levels, which remained lowered until at least one month after the end of treatment. Furthermore, PENS of dermatome T6 resulted in significant weight loss that was not achieved with dietary treatment alone. No statistically significant correlations were observed between ghrelin and appetite or between the changes after treatment and weight loss. However, the sample size was small.
The effect of PENS has been widely demonstrated through posterior tibial nerve stimulation in the treatment of urinary and fecal incontinence, generating a somato-somatic reflex.7–9 Our group was the first to demonstrate the application of PENS in dermatome T6 to reduce appetite and achieve weight loss.10
Pereira and Foster11 recorded a weight loss of over 20% associated with decreased appetite in two morbidly obese patients in which spinal cord stimulation was targeted to T6 and T7 level for controlling intractable lumbar pain and lumbosacral radiculitis secondary to lumbar disk herniation. These patients did not increase their physical activity or follow a diet, though they both experienced a significant reduction in appetite. The authors were the first to postulate that stimulation of the spinal cord could affect the stomach. Other investigators have reported that transcutaneous electrical gastric stimulation can alter gastric motility, delay gastric emptying and give rise to postprandial satiety.12–14 They considered that electrical stimulation was transmitted to the stomach through the abdominal wall on placing the electrode in the left upper quadrant of the abdomen. However, we believe that the effect is more likely to be a result of the generation of a somato-autonomic reflex, rather than of the transcutaneous transmission of electrical stimuli, in a way similar to transcutaneous electrical stimulation of the posterior tibial nerve in the management of incontinence.15
Moreover, it is difficult to believe that electrical impulses are able to exert an effect when having to penetrate an abdominal wall of the thickness found in morbidly obese patients, particularly taking into account that the abundant adipose tissue in these individuals is not a good electrical conductor. The same authors also suggested that the effect of gastric stimulation, which is associated with delayed gastric emptying, could also reduce ghrelin secretion in the gastric fundus and inhibit appetite through the central nervous system.12–14 Chen16 reported that gastric electrical stimulation with a gastric pacemaker can affect the central nervous system through the secretion of hormones in the stomach and the regulation of satiety and/or appetite, with the particular involvement of ghrelin in this mechanism. Based on these postulates, we carried out our study to corroborate the implication of this hormone in the stimulation-satiety-weight loss mechanism following PENS of dermatome T6.
In our study sample, appetite was seen to be reduced after treatment with PENS, and this in turn was logically associated with weight loss. We did not analyze the isolated effect of PENS without diet, but such therapy in itself does not justify relevant weight loss. As seen in a previous study by our group,10 the main effect of such stimulation is decreased appetite; all patients showed reduced or even absent hunger sensations after PENS of dermatome T6.
In the present study, we found appetite to decrease after PENS of dermatome T6, and a BMI loss of 8.42% was achieved. These data are similar to those of our initial study. Likewise, we observed a significant quantitative reduction of appetite, which decreased from 80 to 45mm as scored by the VAS. This also confirmed the previous data. As in the previous case, the technique was not perceived by the patients as being painful, and there were no associated complications. In these individuals we were unable to assess the middle- or long-term evolution of weight loss beyond one month, because the patients were subjected to bariatric surgery, taking advantage of the weight loss achieved with PENS. However, a one-year follow-up study of PENS targeted to dermatome T6 in mild to moderately obese or overweight subjects showed that the lost body weight had not been recovered 12 months after the end of treatment.17
With regard to plasma ghrelin levels, a significant decrease was observed in all the patients subjected to PENS of dermatome T6 between baseline and the end of treatment (Sample 2a). Furthermore, lowered levels persisted one month after stimulation was completed (Sample 3). Although the small sample size of our study did not allow for the correlation of the ghrelin levels with the appetite score, the lowering of hunger sensation associated with the decrease in values after stimulation suggests that this may be a mechanism of action of this therapy. A recently published study has shown that isolated PENS targeted to dermatome T6 achieves a decrease in ghrelin levels and a slight weight loss. However, when PENS of dermatome T6 is combined with a low-calorie diet, a significant weight loss occurs.18
The main limitations of our study are its small sample size, the lack of a control group receiving a diet without PENS of dermatome T6 or PENS of dermatome T6 without diet, and the absence of mid- to long-term follow-up. Further studies involving larger patient samples are therefore needed to confirm the results obtained.
ConclusionsPercutaneous electrical nerve stimulation of dermatome T6 was associated with lowered plasma ghrelin levels and decreased appetite. This therapy combined with an adequate diet achieved a BMI loss of over 8% over 12 weeks of treatment.
Financial supportFunding was received for this study from the Navarro Tripodi Foundation (research grant 2018-12).
Conflicts of interestNone declared.
Please cite this article as: Giner-Bernal L, Ruiz-Tovar J, Violeta J, Mercader M, Miralles J, Calpena R, et al. Evaluación de la grelina plasmática tras aplicación de neuroestimulación percutánea del dermatoma T6 en el tratamiento de la obesidad. Endocrinol Diabetes Nutr. 2020;67:179–185.