The Medtronic MiniMed™ 780G (MM780G) system uses an algorithm that includes autocorrection bolus (AB) delivery. This study evaluates the impact of omitted meal boluses and the system settings, glucose target and active insulin time (AIT), on the AB.
MethodRetrospective observational study on data uploaded by all MiniMed 780G users in our healthcare area, obtained through the remote monitoring platform Care Connect, from April to August 2023. Downloads with a sensor usage time <95% were excluded.
Results235 downloads belonging to 235 users were analysed. AB delivery was significantly higher at 2 h AIT (36.08 ± 13.17%) compared to the rest of settings (2.25–4 h) (26.43 ± 13.2%) (p < 0.001). AB differences based on the glucose target were not found.
Patients with <3 meal boluses per day had higher AB delivery (46.91 ± 19.00% vs 27.53 ± 11.54%) (p < 0.001) and had more unfavourable glucometric parameters (GMI 7.12 ± 0.45%, TIR 67.46 ± 12.89% vs GMI 6.78 ± 0.3%, TIR 76.51 ± 8.37%) (p < 0.001). However, the 2-h AIT group presented similar TAR, TIR and GMI regardless of the number of meal boluses.
ConclusionThe fewer user-initiated boluses, the greater the autocorrection received. The active insulin time of 2 h entails a more active autocorrection pattern that makes it possible to more effectively compensate for the omission of meal boluses without increasing hypoglycaemias.
El sistema Medtronic MiniMed™ 780G (MM780G) utiliza un algoritmo que incluye la administración automática de bolus de autocorrección (BA). Este estudio evalua la influencia de la omisión de bolus prandiales y de los parámetros modificables del sistema, objetivo glucémico y duración de insulina activa (DIA), sobre el BA.
MétodoEstudio observacional retrospectivo sobre las descargas de todos los usuarios del dispositivo MM780G de nuestro área de salud, obtenidas a través de la plataforma de seguimiento remoto Care Connect, de abril a agosto de 2023. Se excluyeron descargas con un tiempo de uso del sensor < 95%.
ResultadosSe analizaron 235 descargas pertenecientes a 235 usuarios. El BA fue significativamente mayor con DIA de 2 horas (36,08% ± 13,17%) frente al resto de duraciones (2,25 - 4 horas) (26,43% ± 13,2%) (p < 0,001). No observamos diferencias del BA en función del objetivo glucémico.
Los pacientes con < 3 bolus prandiales diarios recibían mayor BA (46,91 ± 19,00 % vs 27,53 ± 11,54%) (p < 0,001) y tenían parámetros glucométricos más desfavorables (GMI 7,12 ± 0.45%, TIR 67,46 ± 12,89% vs GMI 6,78 ± 0,3%, TIR 76,51 ± 8,37) (p < 0,001). Sin embargo, el grupo de DIA de 2 horas, presentó similares TAR, TIR y GMI independientemente del número de bolus prandiales.
ConclusiónLa autocorrección es mayor cuanto menos bolus prandiales se administran. La DIA de 2 horas conlleva un patrón de autocorrección más activo que permite compensar más eficazmente la omisión de bolus prandiales sin incremento de hipoglucemias.
Managing type 1 diabetes is challenging for both professionals and patients. In recent years, significant technological advances have emerged, such as continuous glucose monitoring (CGM), which has enabled the development of advanced hybrid closed-loop (AHCL) systems. These systems modulate basal insulin administration automatically and require user intervention to manually input prandial boluses and notify physical exercise.
The MiniMed™ 780G (MM780G) advanced hybrid closed-loop system by Medtronic includes a prandial bolus feature called bolus wizard (BW), which requires user interaction to enter the amounts of carbohydrate. In MM780G, basal insulin administration is determined by a PID (proportional-integral-derivative) algorithm and has 2 components: microboluses and autocorrection boluses (AB), both administered every 5 min.1 In situations in which basal insulin needs increase, leading to glucose levels > 120 mg/dL despite maximum basal doses, the algorithm administers AB, calculated based on algorithm control parameters rather than programmed sensitivity factors.2 Over time, the algorithm adjusts the sensitivity factor based on previous data; this learning process uses up 6 six days of total daily insulin doses and estimates of fasting glucose and plasma insulin concentration.3
The MM780G system allows programming 3 parameters that influence the insulin administration algorithm: the glycemic target, the duration of insulin action (DIA), and the carbohydrate/insulin ratio. The latter is used to estimate the bolus administered with meals. The glycemic target can be set at 100 mg/dL, 110 mg/dL, or 120 mg/dL, with a temporary target of 150 mg/dL for situations with increased risk of hypoglycemia. The DIA indicates the time insulin remains active after bolus administration, ranging from 2 to 8 h; it helps determine if previous bolus insulin persists, thus avoiding hypoglycemia due to overcorrection.1 Although the DIA in the MiniMed™ 780G system is considered in calculating the bolus, the algorithm modifies it to administer the most effective and safe final amount, based on the hypoglycemia risk in the upcoming hours.4
The MM780G system has consistently shown increased time in range across various settings and populations, including adults and children.5–8 Automatic AB administration improves glycemic control, enhancing adaptation to daily glucose variability without user intervention.3 These ABs mitigate glycemic changes with increased insulin demand and help address delays, omissions, or inaccuracies in prandial bolus estimations seen in the routine clinical practice. However, the capacity of ABs to resolve non-adherence issues remains unknown.
Our study aims to analyze the influence of omitting prandial boluses and the modifiable parameters of the MiniMed™ 780G system (glycemic target and DIA) on the percentage of insulin delivered as AB and glycemic parameters.
MethodsWe conducted a retrospective, observational real-life study that analyzed 14-day data downloads from the MiniMed™ 780G system in patients with type 1 diabetes from the Cáceres Health Area, Extremadura, Spain, who were users of this device from April 1st, 2023 through August 31st, 2023.
Information was downloaded using the Carelink System™ software and provided by Care Connect, a remote monitoring program based on digital technologies for patients using Medtronic devices, through which routine follow-up is conducted in our health area. Patients participating in this program periodically download their device information for 14-day periods. Data are provided to health care professionals via a digital platform for analysis and subsequent recommendation to the patient. Informed consent for data access and processing was obtained from the patients prior to inclusion in such remote follow-up program. Available downloads during the specified period, with, at least, 95% sensor usage time and <1% temporary target, were selected to ensure adequate Smartguard™ system performance. If multiple downloads per patient were available, the latest download within the analyzed period was chosen.
The primary endpoint of the study was to analyze the percentage of insulin delivered as AB based on the number of prandial boluses administered by the user. Secondary endpoints evaluated the relationship between the percentage of insulin delivered as AB and glycemic parameters, total daily insulin dose distribution, and adjustable MM780G system parameters (glycemic target and DIA). The evaluated glycemic parameters include time in range (TIR), time above range (TAR), time below range (TBR), glucose management indicator (GMI), and coefficient of variation (CV) based on the international consensus on CGM.9 Total daily insulin dose distribution was analyzed based on parameters provided by the MM780G system in the 14-day downloads: percentage of basal insulin, percentage of insulin administered as bolus, and percentage of insulin administered as AB.
Data analysis was performed using IBM SPSS version 20.0. Results are expressed as percentages for categorical variables and as mean and standard deviation (SD) for continuous variables. The Student t-test was used for continuous variables, and Pearson's correlation coefficient was used for correlation analysis.
This study was approved by the Cáceres Health Area clinical research ethics committee (code 013-2024).
ResultsDuring the specified period, 335 patients with type 1 diabetes were treated with the MM780G system in the Cáceres Health Area, 331 of whom were included in the Care Connect platform. We selected 235 downloads from 235 users that met the inclusion criteria. The mean age was 33.55 ± 17.83 years, with 50.2% being women.
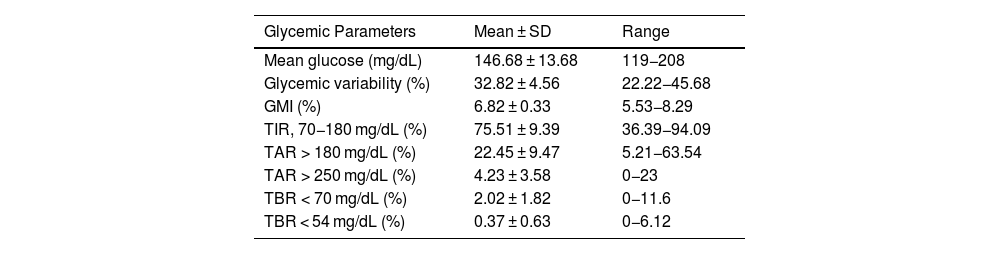
Among the glycemic parameters obtained from the downloads, notable values included a GMI of 6.82 ± 0.33% and a TIR of 75.51 ± 9.39%. Regarding the insulin dose administered by the MM780G system, 40.12% of the dose was delivered as basal insulin and 17.27% as AB, while 42.81% was administered by the patient as prandial boluses (Table 1).
Glycemic parameters, distribution of daily insulin dose, and adjustable parameters of the MiniMed™ 780G system configuration.
| Glycemic Parameters | Mean ± SD | Range |
|---|---|---|
| Mean glucose (mg/dL) | 146.68 ± 13.68 | 119−208 |
| Glycemic variability (%) | 32.82 ± 4.56 | 22.22−45.68 |
| GMI (%) | 6.82 ± 0.33 | 5.53−8.29 |
| TIR, 70−180 mg/dL (%) | 75.51 ± 9.39 | 36.39−94.09 |
| TAR > 180 mg/dL (%) | 22.45 ± 9.47 | 5.21−63.54 |
| TAR > 250 mg/dL (%) | 4.23 ± 3.58 | 0−23 |
| TBR < 70 mg/dL (%) | 2.02 ± 1.82 | 0−11.6 |
| TBR < 54 mg/dL (%) | 0.37 ± 0.63 | 0−6.12 |
| Distribution of daily insulin dose | ||
|---|---|---|
| Total daily insulin dose (IU) | 45.06 ± 21.44 | 8.10−129.4 |
| Basal insulin (%) | 40.12 ± 8.9 | 25−78 |
| Prandial bolus insulin (%) | 42.81 ± 11.95 | 3.74−68.4 |
| Auto-correction bolus insulin (%) | 17.27 ± 7.08 | 3.36−41.26 |
| No. of auto-correction boluses/day | 26.25 ± 10.22 | 6.6−60.1 |
| No. of prandial boluses/day | 4.77 ± 1.84 | 1.6−15.3 |
| Adjustable parameters of MiniMed™ 780G configuration | |
|---|---|
| Duration of insulin action (hours) | n (%) |
| 2.00 | 79 (33.6) |
| 2.25 | 20 (8.5) |
| 2.50 | 42 (17.9) |
| 2.75 | 15 (6.4) |
| 3.00 | 41 (17.4) |
| 3.25 | 9 (3.8) |
| 3.50 | 13 (5.5) |
| 4.00 | 16 (6.8) |
| Glucose target (mg/dL) n (%) | |
| 100 | 108 (46.0) |
| 110 | 74 (31.5) |
| 120 | 53 (22.5) |
SD, standard deviation.
Regarding the configuration of the MM780G system, the most frequently used DIA was 2 h (33.6%), and 66.4% of the devices were set with a DIA of <3 h. Additionally, 46.0% of patients had a glycemic target of 100 mg/dL (Table 1).
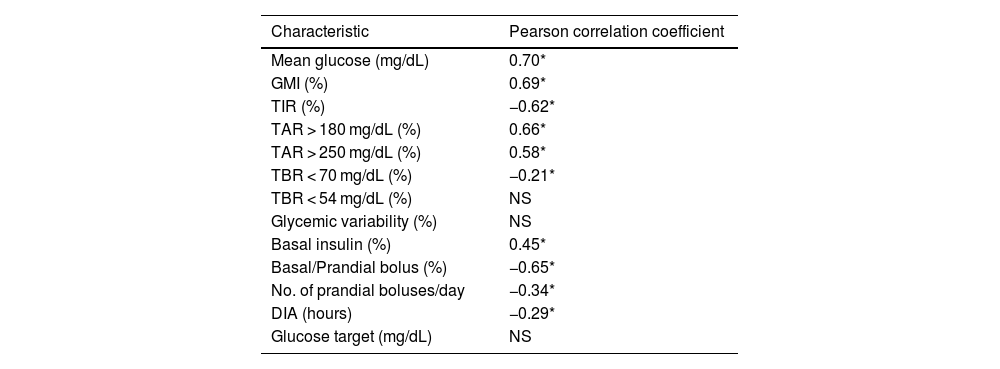
When evaluating the relationship between the percentage of insulin administered as AB and glycemic data, we found correlations with all analyzed parameters except TBR < 54 mg/dL and CV. The correlation was strongest for TIR, TAR, and GMI (r of −0.62, 0.66, and 0.69, respectively).
Regarding the modifiable parameters of the MM780G system, we found that ABs negatively correlated with DIA (r = −0.29) but not with the glycemic target (Table 2). Categorizing patients based on programmed DIA, the percentage of ABs was higher when the DIA was 2 h (36.08 ± 13.17%), a statistically significant difference vs other durations used, both individually and grouped in the range of 2 h and 15 min up to 4 h (26.43 ± 13.20%). We found no differences between them (Fig. 1) comparing other DIAs >2 h.
Correlation between the percentage of autocorrection bolus insulin and glycemic parameters and MiniMed™ 780G system parameters.
| Characteristic | Pearson correlation coefficient |
|---|---|
| Mean glucose (mg/dL) | 0.70* |
| GMI (%) | 0.69* |
| TIR (%) | −0.62* |
| TAR > 180 mg/dL (%) | 0.66* |
| TAR > 250 mg/dL (%) | 0.58* |
| TBR < 70 mg/dL (%) | −0.21* |
| TBR < 54 mg/dL (%) | NS |
| Glycemic variability (%) | NS |
| Basal insulin (%) | 0.45* |
| Basal/Prandial bolus (%) | −0.65* |
| No. of prandial boluses/day | −0.34* |
| DIA (hours) | −0.29* |
| Glucose target (mg/dL) | NS |
DIA, duration of insulin action; GMI, glucose management indicator; NS, not significant; TAR, time above range; TBR, time below range; TIR, time in range.
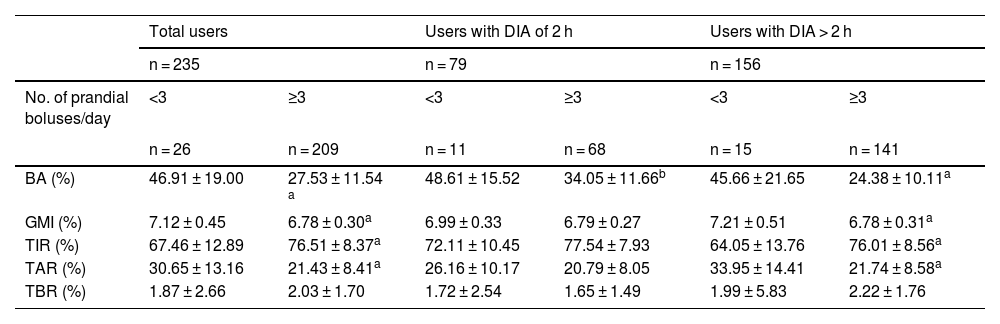
In the analysis of AB behavior based on the number of daily prandial boluses (Table 3), our data show that if the patient administers < 3 BW, the MM780G system delivers a higher percentage of ABs (46.91 ± 19.00% vs 27.53 ± 11.54%). This difference is statistically significant when considering the entire sample and remains so when grouped by DIA of 2 (48.61 ± 15.52% vs. 34.05 ± 11.66%) and > 2 h (45.66 ± 21.65% vs 24.38 ± 10.11%).
Percentage of insulin administered in autocorrection boluses based on the No. of prandial boluses per day.
| Total users | Users with DIA of 2 h | Users with DIA > 2 h | ||||
|---|---|---|---|---|---|---|
| n = 235 | n = 79 | n = 156 | ||||
| No. of prandial boluses/day | <3 | ≥3 | <3 | ≥3 | <3 | ≥3 |
| n = 26 | n = 209 | n = 11 | n = 68 | n = 15 | n = 141 | |
| BA (%) | 46.91 ± 19.00 | 27.53 ± 11.54 a | 48.61 ± 15.52 | 34.05 ± 11.66b | 45.66 ± 21.65 | 24.38 ± 10.11a |
| GMI (%) | 7.12 ± 0.45 | 6.78 ± 0.30a | 6.99 ± 0.33 | 6.79 ± 0.27 | 7.21 ± 0.51 | 6.78 ± 0.31a |
| TIR (%) | 67.46 ± 12.89 | 76.51 ± 8.37a | 72.11 ± 10.45 | 77.54 ± 7.93 | 64.05 ± 13.76 | 76.01 ± 8.56a |
| TAR (%) | 30.65 ± 13.16 | 21.43 ± 8.41a | 26.16 ± 10.17 | 20.79 ± 8.05 | 33.95 ± 14.41 | 21.74 ± 8.58a |
| TBR (%) | 1.87 ± 2.66 | 2.03 ± 1.70 | 1.72 ± 2.54 | 1.65 ± 1.49 | 1.99 ± 5.83 | 2.22 ± 1.76 |
| No. of prandial boluses/day | 3–3.99 | ≥4 | 3–3.99 | ≥4 | 3–3.99 | ≥4 |
|---|---|---|---|---|---|---|
| n = 59 | n = 150 | n = 21 | n = 47 | n = 38 | n = 103 | |
| BA (%) | 31.60 ± 11.95 | 25.92 ± 11.00a | 35.17 ± 14.96 | 33.55 ± 9.99 | 29.63 ± 9.58 | 22.44 ± 9.63a |
| GMI (%) | 6.83 ± 0.28 | 6.77 ± 0.30 | 6.75 ± 0.30 | 6.81 ± 0.26 | 6.87 ± 0.26 | 6.75 ± 0.32b |
| TIR (%) | 75.37 ± 8.86 | 76.95 ± 8.16 | 78.29 ± 9.29 | 77.21 ± 7.33 | 73.76 ± 8.30 | 76.84 ± 8.55 |
| TAR (%) | 22.54 ± 8.59 | 21.00 ± 8.32 | 20.09 ± 8.98 | 21.11 ± 7.69 | 23.89 ± 8.18 | 20.95 ± 8.63 |
| TBR (%) | 2.08 ± 1.88 | 2.02 ± 1.63 | 1.61 ± 1.65 | 1.67 ± 1.44 | 2.33 ± 1.97 | 2.18 ± 1.69 |
| No. of prandial boluses/day | 4–4.99 | ≥5 | 4–4.99 | ≥5 | 4–4.99 | ≥5 |
|---|---|---|---|---|---|---|
| n = 62 | n = 88 | n = 10 | n = 37 | n = 52 | n = 51 | |
| BA (%) | 26.56 ± 9.59 | 26.56 ± 9.59 | 34.06 ± 7.53 | 33.42 ± 10.64 | 25.11 ± 6.32 | 19.71 ± 9.26b |
| GMI (%) | 6.78 ± 0.31 | 6.76 ± 0.30 | 6.87 ± 0.26 | 6.79 ± 0.26 | 6.76 ± 0.32 | 6.74 ± 0.32 |
| TIR (%) | 76.40 ± 7.76 | 77.35 ± 8.45 | 75.14 ± 7.86 | 77.77 ± 7.18 | 76.64 ± 7.80 | 77.04 ± 9.32 |
| TAR (%) | 21.55 ± 7.76 | 20.61 ± 8.71 | 23.32 ± 8.07 | 20.51 ± 7.58 | 21.21 ± 7.74 | 20.69 ± 9.52 |
| TBR (%) | 2.01 ± 1.56 | 2.03 ± 1.68 | 1.52 ± 0.78 | 1.71 ± 1.57 | 2.10 ± 1.66 | 2.25 ± 1.73 |
AB, autocorrection bolus; DIA, duration of insulin action; GMI, glucose management indicator; NS, not significant; TAR, time above range; TBR, time below range; TIR, time in range.
We also observed significant differences in glycemic parameters based on the number of prandial boluses, with less favorable data for patients administering < 3 prandial boluses/day (GMI, 7.12 ± 0.45%; TIR, 67.46 ± 12.89%; TAR, 30.65 ± 13.16%) vs those administering, at least, 3 BW (GMI, 6.78 ± 0.3%; TIR, 76.51 ± 8.37%; TAR, 21.43 ± 8.41%). However, this difference in glycemia does not hold if the downloads are separated by DIA, as the 2 -h DIA group, receiving more ABs, shows similar TAR, TIR, and GMI regardless of the number of prandial boluses (GMI, 6.99 ± 0.33%; TIR, 72.11 ± 10.45%; TAR, 26.16 ± 10.17% for downloads with < 3 BW/day; GMI, 6.79 ± 0.27%; TIR, 77.54 ± 0.27%; TAR, 20.79 ± 8.05% in cases of ≥3 daily BW).
DiscussionAn enhanced PID algorithm with a fuzzy logic component is a key component of the MM780G system. PID controllers are reactive and adjust insulin delivery by evaluating glucose excursions from 3 different perspectives: the proportional component estimates the deviation of the measured glucose level from the target glucose; the integral component calculates the area under the curve between the measured and target glucose levels; and the derivative component considers the rate of change of the measured glucose, all of which together dictate the amount of insulin administered.3 It has been argued that insulin secretion by pancreatic islets is controlled by proportional, integral, and derivative factors: the first phase of prandial secretion corresponds to the derivative component, the slower second phase corresponds to the integral component, while basal secretion is controlled by the proportional factor.10
Available evidence demonstrates that the MM780G system improves glycemic control safely while also providing benefits in sleep quality and treatment satisfaction.11 However, there are still limitations in the use of AHCL systems, such as their capacity to handle stress, intercurrent illnesses, exercise, and omitted prandial boluses. Omission of insulin doses is a common obstacle in the management of diabetes,12,13 also affecting patients on subcutaneous insulin infusions.14,15
In the present study, we retrospectively analyzed data from 235 downloads belonging to 235 users in real-life conditions to understand how the MM780G system works, specifically its autocorrection feature and its behavior according to the number of prandial boluses administered by the patient. We saw that the algorithm administers more AB in poorly controlled patients and in those who administer fewer prandial boluses. There was an inverse correlation between AB and the number of daily BW (R = −0.34). These data are consistent with real-life evidence from over 4000 MM780G users, showing 14.1% of AB in patients who administer a mean 4.8 BW daily.7 Other authors have also documented that AB is inversely associated with TIR, as well as the relationship between the number of prandial boluses and the degree of glycemic control. Castañeda et al. categorized users into quartiles based on the degree of glycemic control, describing 18.6 % AB and 4.2 prandial boluses per day in the worst-controlled subgroup (TIR ≤ 70.1%) vs 8.9% AB and a mean 4.9 prandial boluses per day in the best-controlled quartile (TIR > 82.2%).4
Our data show that a DIA of 2 h leads the algorithm to administer more autocorrection. This difference is due to a greater number of AB and a higher percentage of AB relative to the total insulin dose, regardless of the number of prandial boluses administered by the patient. However, no correlation was ever found between the glycemic target and autocorrection, possibly because the system's algorithm initiates AB administration when sensor glucose is >120 mg/dL.
Comparing downloads with < 3 BW daily with those with ≥ 3 BW, there is a significant increase of 70.39% in AB (p < 0.001), and poorer glycemic control when fewer boluses are self-administered. We could deduce that increased autocorrection does not compensate for prandial bolus omission. The increase in AB is smaller—21.91% (p = 0.001)—in patients with 3 BW daily vs ≥ 4 BW/daily, and now there are no differences in GMI, TAR, and TIR, probably because omissions correspond to smaller intakes rather than main meals. This corrective capacity of AB was also documented by Coutant et al.,16 who reported improved glycemic control with an AHCL system in patients who omitted boluses. We found no available evidence on the specific analysis of AB and the number of prandial boluses.
Analyzing AB provides an approach to improving our understanding of how the MM780G system works, primarily based on its PID algorithm, which determines, in our series, 57% of insulin patients receive while modifying the remaining 43% administered as prandial boluses. Therefore, it is an important part of the data we analyze. Considering this high level of system automation, in routine clinical practice, we face 2 important facts: on the one hand, the patient is responsible for the adequate administration of prandial boluses, which has become the main challenge in optimizing treatment, hence its evaluation in this study. On the other hand, we need to understand the system automated function, including AB, to optimize programming and convey necessary clarifications and recommendations to patients about their daily experiences.
The main limitation of this study is assuming the omission of prandial boluses in patients who administer <3 boluses daily, based on profile analysis through which this is relatively easy to recognize. This limitation is inherent to the methodology used (analysis of information exclusively from MM780G devices) and is partially mitigated by providing glycemic data consistent with this assumption, such as the fact that patients administering <3 BW daily have poorer glycemic parameters and require more autocorrection. Other limiting factors include the absence of clinical data and the cross-sectional nature, which does not allow for inferring causal relationships between observed facts. We also need to consider that results apply to patients using the Smartguard™ mode, at least, 95% of the time and the temporary target < 1%, which in our population corresponds to 70% of MM780G system users.
Although to maximize the benefits of AHCL therapy, pre-meal bolus administration and an accurate carbohydrate count remain important aspects they increase the treatment burden. MM780G system users who administer <3 prandial boluses daily have poorer glycemic parameters and receive a higher percentage of insulin as AB. When DIA is set to 2 h, the system administers 36% more insulin as autocorrection than with longer durations, equalizing glycemic control between those administering <3 prandial boluses daily and the rest.
FundingNone declared.
Conflicts of interestNone declared.