Type 1 diabetes mellitus (DM1) is characterized by complex therapeutic recommendations that can interfere with and affect patient health-related quality of life (HRQoL). Being aware of the impact of the disease and its treatment is very important in clinical practice, as it allows us to detect needs, establish changes in treatment, identify barriers that complicate self-care, and offer support in decision making.1,2 In order to evaluate this impact we need specific tools that are more sensitive to the fluctuations of the disease and which afford more detailed information than the generic HRQoL assessment tools.1
Until recently there were only two specific questionnaires for measuring HRQoL in diabetes: the Diabetes Quality of Life Measure (DQoL) and the Audit of Diabetes-Dependent Quality of Life (ADDQoL) tool.
The DQoL3 is the oldest and most widely used in this regard. It was designed for the Diabetes Control and Complications Trial, and there is also a validated and adapted Spanish version (EsDQoL).4 Although data warranting the validity of its contents and internal consistency have been produced by some studies, a number of limitations have also been found: items with low α coefficients, scant applicability of the social/vocational concern subscale, and items explained in outdated vocabulary.5 No sensitivity to changes in the intensification of insulin therapy has been demonstrated.6 Neither has sensitivity been demonstrated among patients subjected to continuous subcutaneous insulin infusion.7
A Spanish language version of the ADDQol8 has been adapted and validated for Argentina, and the data referring to its psychometric properties supports its adequate internal consistency. However, the structure of this questionnaire is complex, since it measures importance and impact separately.9 Its items are formulated on the basis of a hypothetical situation which the patient may or may not be able to imagine (how life would be without diabetes),5,9 and which may represent a complex cognitive task. In fact, this type of formulation is advised against by the United States Food and Drug Administration.
We have recently published a validation of a new questionnaire, the Life with Type 1 Diabetes (Vida con Diabetes tipo 1 [ViDa1]) questionnaire,10 designed to meet the need for a useful and validated tool capable of measuring HRQoL in individuals with DM1 and which contemplates the most relevant implications of living with DM1 nowadays. We have sought to offer a tool that is easy to administer and useful in both clinical practice and in research.
The DQoL and the ADDQoL are both used in patients with DM1 and DM2, despite the fact that these are two very different diseases. Indeed, DM1 has a greater impact upon the life of the patient from the time of diagnosis. The questionnaires specifically fail to address a series of aspects that are important for the HRQoL of patients with DM1, and which refer to disease care-related activities such as diet carbohydrate count, the self-measurement of blood glucose and concern regarding hypoglycemia. They are also beginning to show their age, while the concerns and needs of people with DM1 have evolved in recent years.
A complex multistep process was involved in the development of the ViDa1: (1) a literature review to gain in-depth knowledge of the existing questionnaires for measuring HRQoL in diabetes, their advantages and limitations; (2) assessment of the opinions of DM1 patients in order for us to become aware of their concerns and needs and of how they view life with diabetes; and (3) evaluation of the opinions of clinical experts such as endocrinologists, diabetes educators and psychologists.
The patient psychometric characteristics were analyzed in a multicenter study involving 578 subjects with DM1 in different hospitals in Spain. The results of the study provided evidence regarding the structure, reliability, convergent and discriminant validity, time stability and sensitivity to change of the tool.10
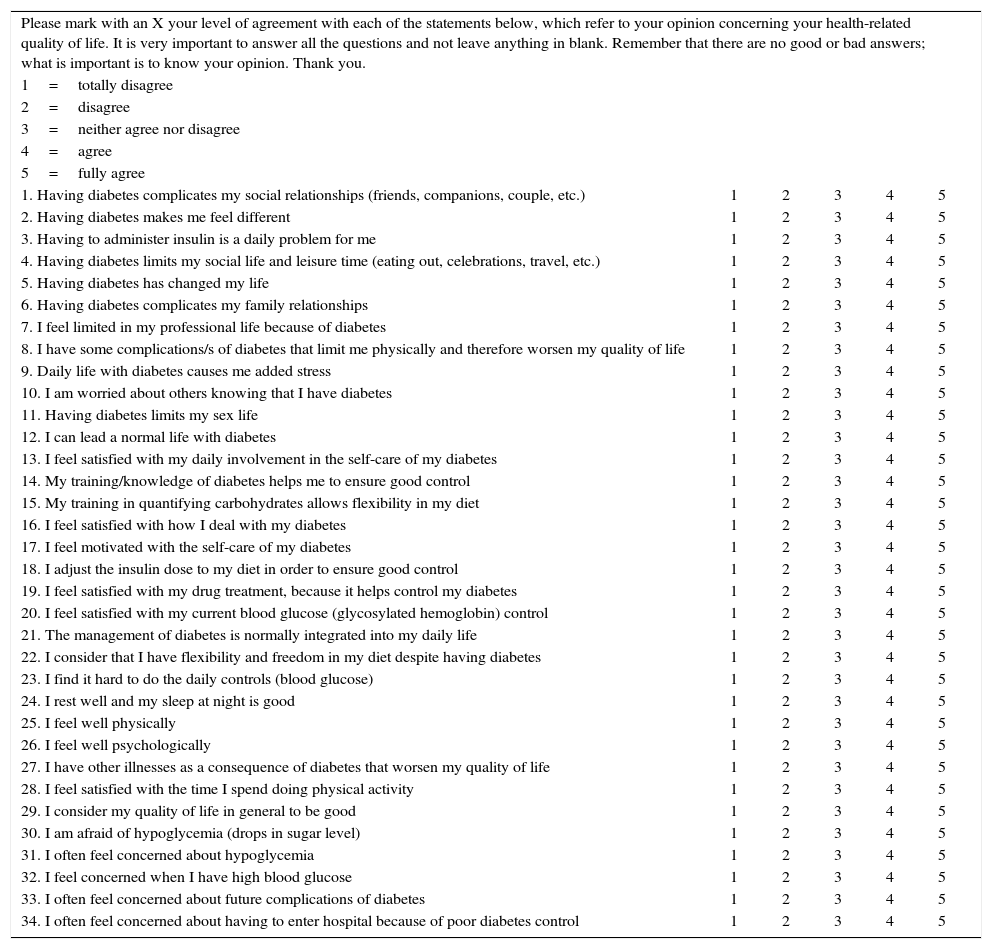
The ViDa1 comprises 34 items grouped into four different dimensions that together shape patient HRQoL: interference with life, self-care, wellbeing and concern about the disease. The questionnaire can be self-administered, with answers based on a Likert-type scale yielding a total score per subscale.
The present letter presents a translation of the original version of the ViDa1 (Table 1) with the purpose of making it available to those who wish to use it, whether in clinical practice or in research.
The ViDa1 questionnaire.
| Please mark with an X your level of agreement with each of the statements below, which refer to your opinion concerning your health-related quality of life. It is very important to answer all the questions and not leave anything in blank. Remember that there are no good or bad answers; what is important is to know your opinion. Thank you. | |||||
| 1=totally disagree | |||||
| 2=disagree | |||||
| 3=neither agree nor disagree | |||||
| 4=agree | |||||
| 5=fully agree | |||||
| 1. Having diabetes complicates my social relationships (friends, companions, couple, etc.) | 1 | 2 | 3 | 4 | 5 |
| 2. Having diabetes makes me feel different | 1 | 2 | 3 | 4 | 5 |
| 3. Having to administer insulin is a daily problem for me | 1 | 2 | 3 | 4 | 5 |
| 4. Having diabetes limits my social life and leisure time (eating out, celebrations, travel, etc.) | 1 | 2 | 3 | 4 | 5 |
| 5. Having diabetes has changed my life | 1 | 2 | 3 | 4 | 5 |
| 6. Having diabetes complicates my family relationships | 1 | 2 | 3 | 4 | 5 |
| 7. I feel limited in my professional life because of diabetes | 1 | 2 | 3 | 4 | 5 |
| 8. I have some complications/s of diabetes that limit me physically and therefore worsen my quality of life | 1 | 2 | 3 | 4 | 5 |
| 9. Daily life with diabetes causes me added stress | 1 | 2 | 3 | 4 | 5 |
| 10. I am worried about others knowing that I have diabetes | 1 | 2 | 3 | 4 | 5 |
| 11. Having diabetes limits my sex life | 1 | 2 | 3 | 4 | 5 |
| 12. I can lead a normal life with diabetes | 1 | 2 | 3 | 4 | 5 |
| 13. I feel satisfied with my daily involvement in the self-care of my diabetes | 1 | 2 | 3 | 4 | 5 |
| 14. My training/knowledge of diabetes helps me to ensure good control | 1 | 2 | 3 | 4 | 5 |
| 15. My training in quantifying carbohydrates allows flexibility in my diet | 1 | 2 | 3 | 4 | 5 |
| 16. I feel satisfied with how I deal with my diabetes | 1 | 2 | 3 | 4 | 5 |
| 17. I feel motivated with the self-care of my diabetes | 1 | 2 | 3 | 4 | 5 |
| 18. I adjust the insulin dose to my diet in order to ensure good control | 1 | 2 | 3 | 4 | 5 |
| 19. I feel satisfied with my drug treatment, because it helps control my diabetes | 1 | 2 | 3 | 4 | 5 |
| 20. I feel satisfied with my current blood glucose (glycosylated hemoglobin) control | 1 | 2 | 3 | 4 | 5 |
| 21. The management of diabetes is normally integrated into my daily life | 1 | 2 | 3 | 4 | 5 |
| 22. I consider that I have flexibility and freedom in my diet despite having diabetes | 1 | 2 | 3 | 4 | 5 |
| 23. I find it hard to do the daily controls (blood glucose) | 1 | 2 | 3 | 4 | 5 |
| 24. I rest well and my sleep at night is good | 1 | 2 | 3 | 4 | 5 |
| 25. I feel well physically | 1 | 2 | 3 | 4 | 5 |
| 26. I feel well psychologically | 1 | 2 | 3 | 4 | 5 |
| 27. I have other illnesses as a consequence of diabetes that worsen my quality of life | 1 | 2 | 3 | 4 | 5 |
| 28. I feel satisfied with the time I spend doing physical activity | 1 | 2 | 3 | 4 | 5 |
| 29. I consider my quality of life in general to be good | 1 | 2 | 3 | 4 | 5 |
| 30. I am afraid of hypoglycemia (drops in sugar level) | 1 | 2 | 3 | 4 | 5 |
| 31. I often feel concerned about hypoglycemia | 1 | 2 | 3 | 4 | 5 |
| 32. I feel concerned when I have high blood glucose | 1 | 2 | 3 | 4 | 5 |
| 33. I often feel concerned about future complications of diabetes | 1 | 2 | 3 | 4 | 5 |
| 34. I often feel concerned about having to enter hospital because of poor diabetes control | 1 | 2 | 3 | 4 | 5 |
Interference with life: (1–12), self-care (13–23), wellbeing (24–29) and concern about the illness (30–34). The scores corresponding to each subscale are added for correction. Items 12, 23 and 27 are inverted for correct interpretation.
Copyright© 2017 Dácil Alvarado-Martel. ULPGC.
This tool is useful for evaluating fluctuations of the disease in the course of the life of the patient, and for assessing the impact of a specific intervention. The ViDa1 is therefore a good alternative for evaluating the HRQoL of DM1 patients within the current context.
Financial supportD.A.-M. received a predoctorate grant from the Agencia Canaria de Investigación, Innovación y Sociedad de la Información (ACIISI) and the V Ayuda Guido Ruffino for Research in Therapeutic Education for Diabetes of the Sociedad Española de Diabetes in 2015.
The authors wish to thank all the staff members of the Department of Endocrinology and Nutrition of Hospital Universitario Insular Materno-Infantil (Gran Canaria), and of the Department of Endocrinology and Nutrition of Hospital Universitario Dr. Negrín (Gran Canaria), as well as the Diabetics Association of Gran Canaria, and Drs. Isabel Barbero and Rosario Martínez Arias for counseling in the development and validation of the ViDa1 questionnaire. Thanks are also due to all the hospital centers, professionals and patients who participated in the study.
Armando Carrillo. Department of Endocrinology and Nutrition. Complejo Hospitalario Universitario Insular Materno-Infantil de Gran Canaria. Las Palmas de Gran Canaria.
Mauro Boronat. Department of Endocrinology and Nutrition. Complejo Hospitalario Universitario Insular Materno-Infantil de Gran Canaria. Las Palmas de Gran Canaria. Instituto Universitario de Investigaciones Biomédicas y Sanitarias. Universidad de Las Palmas de Gran Canaria. Las Palmas de Gran Canaria.
Ana Expósito-Montesdeoca. Instituto Universitario de Investigaciones Biomédicas y Sanitarias. Universidad de Las Palmas de Gran Canaria. Las Palmas de Gran Canaria.
Maribel Cuadrado-Vigaray. Department of Endocrinology and Nutrition. Hospital Universitario Germans Trias i Pujol. Badalona.
Lía Nattero-Chávez. Department of Endocrinology and Nutrition. Hospital Universitario Ramón y Cajal. Madrid.
Maite Pozuelo-Sánchez. Department of Endocrinology and Nutrition. Hospital Universitario Ramón y Cajal. Madrid.
Pino López-Quevedo. Department of Endocrinology and Nutrition. Hospital Universitario de Gran Canaria Doctor Negrín. Las Palmas de Gran Canaria.
Ana Delia Santana-Suárez. Department of Endocrinology and Nutrition. Hospital Universitario de Gran Canaria Doctor Negrín. Las Palmas de Gran Canaria.
Natalia Hillman. Diabetes Unit. Hospital Universitario La Paz. Madrid.
David Subías-Andújar. Department of Endocrinology and Nutrition. Hospital Parc Taulí. Sabadell.
Pilar Martín-Vaquero. Unit of Diabetes and Metabolism. Clínica D-Médical. Madrid.
Lourdes Sáez de Ibarra. Diabetes Unit. Hospital Universitario La Paz. Madrid.
Didac Mauricio. Department of Endocrinology and Nutrition. Hospital Universitario Germans Trias i Pujol. Badalona. Centro de investigación Biomédica en Red. Diabetes y Enfermedades metabólicas asociadas (CIBERDEM).
Pedro de Pablos-Velasco. Department of Endocrinology and Nutrition. Hospital Universitario de Gran Canaria Doctor Negrín. Las Palmas de Gran Canaria. Instituto Universitario de Investigaciones Biomédicas y Sanitarias. Universidad de Las Palmas de Gran Canaria.
Francisco J. Nóvoa. Department of Endocrinology and Nutrition. Complejo Hospitalario Universitario Insular Materno-Infantil de Gran Canaria. Las Palmas de Gran Canaria. Instituto Universitario de Investigaciones Biomédicas y Sanitarias. Universidad de Las Palmas de Gran Canaria.
Please cite this article as: Alvarado-Martel D, Ruiz-Fernández MÁ, Wägner AM, Equipo ViDa1. ViDa1: un nuevo cuestionario para medir calidad de vida relacionada con la salud en la diabetes tipo 1. Endocrinol Diabetes Nutr. 2017;64:506–509.