Type 2 diabetes mellitus (T2DM) is the 9th leading cause of death worldwide, affecting 462 million individuals. To change the natural progression of diabetes, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have emerged as renal and cardioprotective agents capable of achieving stable glycemic control. Currently available literature demonstrates their efficacy profile in preventing MACE-3, mitigating microvascular complications, and improving metabolic profiles, among other benefits. Within this context, an intriguing question arises: can GLP-1 RAs be used to reduce insulin doses?1–3
The aim of our study is to assess whether the addition of subcutaneous semaglutide (OW-semaglutide) once a week to adult patients with T2DM and eGFR >15mL/min results in fewer insulin doses at the 12-month follow-up. We present a prospective study initiated in 2019 on the T2DM population from four Spanish hospitals located in Madrid, Spain. The study protocol was approved by the Fundación Jiménez Diaz Instituto de Investigación Sanitaria Local Ethics Committee. For this purpose, a total of 316 patients, utilizing various insulin regimens received additional OW-semaglutide and were monitored with visits at 6 and 12 months, during which multiple variables including insulin doses were collected.
Forty-four out of 316 initially registered patients discontinued subcutaneous semaglutide (13.9%), and 20 patients were lost to follow-up, mainly due to the pandemic. Ultimately, a total of 252 patients were eligible and included in the study. The baseline characteristics of our study population were: 61% were men, with a mean age of 62.4±9.1 years and a mean course of T2DM of 14.2±7.5 years. Among the comorbidities reported, 88.8% had dyslipidemia; 84.1%, arterial hypertension; and 16.9% were smokers. Microvascular disease was present in 40.8% of patients (29.9% with diabetic nephropathy and 24.5% with diabetic retinopathy). Macrovascular complications included a past medical history of ischemic heart disease in 17.8% of the patients, acute myocardial infarction in 12.2%, stroke in 9.2%, and peripheral artery disease in 8.7%. Although 56.7% of the patients were categorized under primary prevention they had a very high cardiovascular risk, while 32.4% were categorized under secondary prevention. Baseline weight and BMI averaged 96.1±15.4kg/m2 and 34.9±5.2kg/m2, respectively. The mean HbA1c level was 7.97±1.40%; mean eGFR (CKD-EPI), 79.4±22.1mL/min/1.73m2; and mean C-peptide, 2.73±1.6ng/mL. Regarding basal antidiabetic treatment, 84.9% were on metformin, 47.6% on an SGLT2-inhibitor, 53.2% on a GLP-1 RA, 23.0% on a DPP4-inhibitor, 13.9% on repaglinide, 4.8% on a sulfonylurea, and 1.6% on pioglitazone.
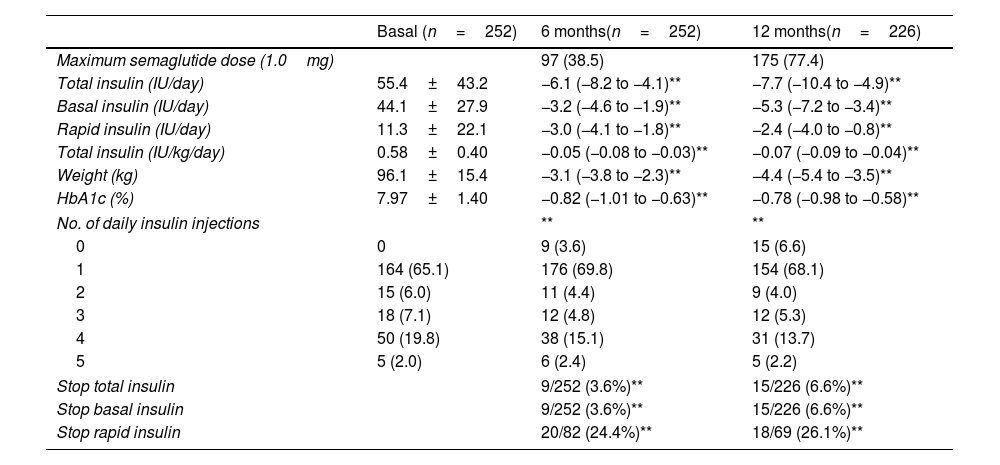
As depicted in Table 1, the results revealed a significant reduction in the total number of insulin doses by 6IU/day at 6 months and nearly 8IU/day at 12 months. Both basal and rapid insulin doses exhibited decreases at both time periods (−3 and −5 for basal insulin at 6 and 12 months, respectively, and −3 and −2 for rapid insulin at the same intervals, respectively). Furthermore, 6.6% of patients discontinued their total insulin regimen, while notably, 26.1% of patients previously on rapid insulin discontinued this regimen by the end of the 12-month period. Only four patients initiated a rapid insulin regimen at the follow-up. The number of patients with ≥3 daily insulin injections decreased. Additionally, weight and HbA1c levels showed significant reductions at the follow-up (−4.4kg and −0.77% at 12 months, respectively). Moreover, the number of patients achieving HbA1c levels <7% increased from 26.1% up to 54.6% at 12 months.
Changes in main insulin treatment variables at the follow-up.
| Basal (n=252) | 6 months(n=252) | 12 months(n=226) | |
|---|---|---|---|
| Maximum semaglutide dose (1.0mg) | 97 (38.5) | 175 (77.4) | |
| Total insulin (IU/day) | 55.4±43.2 | −6.1 (−8.2 to −4.1)** | −7.7 (−10.4 to −4.9)** |
| Basal insulin (IU/day) | 44.1±27.9 | −3.2 (−4.6 to −1.9)** | −5.3 (−7.2 to −3.4)** |
| Rapid insulin (IU/day) | 11.3±22.1 | −3.0 (−4.1 to −1.8)** | −2.4 (−4.0 to −0.8)** |
| Total insulin (IU/kg/day) | 0.58±0.40 | −0.05 (−0.08 to −0.03)** | −0.07 (−0.09 to −0.04)** |
| Weight (kg) | 96.1±15.4 | −3.1 (−3.8 to −2.3)** | −4.4 (−5.4 to −3.5)** |
| HbA1c (%) | 7.97±1.40 | −0.82 (−1.01 to −0.63)** | −0.78 (−0.98 to −0.58)** |
| No. of daily insulin injections | ** | ** | |
| 0 | 0 | 9 (3.6) | 15 (6.6) |
| 1 | 164 (65.1) | 176 (69.8) | 154 (68.1) |
| 2 | 15 (6.0) | 11 (4.4) | 9 (4.0) |
| 3 | 18 (7.1) | 12 (4.8) | 12 (5.3) |
| 4 | 50 (19.8) | 38 (15.1) | 31 (13.7) |
| 5 | 5 (2.0) | 6 (2.4) | 5 (2.2) |
| Stop total insulin | 9/252 (3.6%)** | 15/226 (6.6%)** | |
| Stop basal insulin | 9/252 (3.6%)** | 15/226 (6.6%)** | |
| Stop rapid insulin | 20/82 (24.4%)** | 18/69 (26.1%)** | |
Data are expressed as mean (95% CI) for continuous variables and n (%) for categorical variables. A paired t-test and a symmetry test were performed for continuous and categorical variables, respectively, to compare baseline and follow-up variables.
We conducted a multiple regression analysis to identify predictors of insulin dose reduction at 12 months following the initiation of OW-semaglutide. The independent variables included age, sex, course of T2DM, total insulin dose, baseline HbA1c levels (<7% vs ≥7%), BMI, C-peptide, and eGFR (<60 vs ≥60). Additionally, we considered the semaglutide dose achieved at 12 months (1.0mg vs ≤0.5mg), reductions in HbA1c levels and weight at the follow-up, the number of oral antidiabetic drugs used, basal antidiabetic treatment, and changes in antidiabetic treatment at the follow-up. Our analysis revealed that higher baseline insulin doses (beta −0.44), baseline HbA1c levels <7% (beta −0.17), greater reductions in weight at the follow-up (beta −0.04), eGFR ≥60mL/min (beta −0.26), GLP-1 RA-naïve patients (beta −0.21), and initiation of metformin (beta −0.20) were independent predictors associated with greater reductions in insulin doses at 12 months.
As evidenced by randomized controlled trials (RCTs) and real-world data (RWD) studies in the literature, our investigation confirms that the addition of a GLP-1 RA leads to a significant reduction in the daily insulin requirements of our patients. Regarding basal insulin, we saw that the addition of a GLP-1 RA can reduce insulin over-basalization in up to 46.6% of patients. In cases of patients on intensive therapy (>100IU/day), numerous studies have explored the effect of adding liraglutide or exenatide, revealing notable reductions in HbA1c, weight, insulin dose, and glycemic variability (GV) as measured by continuous glucose monitor (CGM). We also observed a reduction in daily insulin injections, which could be associated with a decrease in lipodystrophies and improved glycemic control.3–6
The significance of these studies lies in improving the quality of life, managing symptoms, mitigating organ damage, reducing the risk of hypoglycemia, and enhancing the overall prognosis of patients with T2DM by reducing insulin doses and improving disease control. Several investigations, such as the one conducted by Gamble et al. with 6072 patients have indicated that although high insulin doses may be associated with higher mortality rates, the risk also varies depending on the duration of insulin exposure, glycemic control, weight gain, and occurrences of cardiovascular events and hypoglycemia. It is obvious that the addition of GLP-1 RA to these patients led to better glycemic control while reducing insulin doses, thereby ameliorating disease comorbidities and mitigating adverse effects associated with insulin therapy.4–9
In conclusion, our findings suggest that the addition of OW-semaglutide to a Spanish population of T2DM patients led to fewer daily insulin dosage requirements, thereby contributing to improved metabolic profiles when combined with insulin deprescription. These results highlight the potential of insulin deprescription as a therapeutic strategy, underscoring the necessity for further trials to elucidate its efficacy profile and clinical applicability.