Adrenal insufficiency (AI) is a rare endocrine disease, associated to increased mortality if left untreated. It can be due to a primary failure of the adrenal glands (primary AI) or malfunctioning of the hypothalamic-pituitary-adrenal axis (HPA) (secondary AI). The lack of data on incidence/prevalence of adrenal insufficiency in Spain complicates any evaluation of the magnitude of the problem in our country. Initial symptoms are non-specific, so often there is a delay in diagnosis.
Current therapy with available glucocorticoids is associated with decreased quality of life in patients with treated AI, as well as with increased mortality and morbidity, probably related to both over-treatment and lack of hydrocortisone, associated with non-physiological peaks and troughs of the drug over the 24hours. The availability of a new drug with a modified dual release (immediate and retarded), that requires one only daily dose, improves and simplifies the treatment, increases compliance as well as quality of life, morbidity and possibly mortality. This revision deals with the knowledge on the situation both globally and in Spain, prior to the availability of this new drug.
La insuficiencia suprarrenal (IS) es una enfermedad poco frecuente pero con riesgo vital si no se trata. Puede ser por fallo primario de las glándulas suprarrenales (IS primaria) o por mal funcionamiento del eje hipotalámico-hipófiso-adrenal (HPA) (IS secundaria). La carencia de datos sobre la incidencia/prevalencia de la IS en España dificulta apreciar la magnitud del problema en nuestro país. Los síntomas iniciales son inespecíficos, por lo que con frecuencia se retrasa el diagnóstico.
Con las pautas actuales sustitutivas de glucocorticoides en los pacientes con IS tratados la calidad de vida está disminuida y existe una mayor morbimortalidad, probablemente en relación tanto con la sobredosificación como con la falta de glucocorticoides con picos y nadires no fisiológicos a lo largo de las 24h. La disponibilidad de un nuevo fármaco con liberación modificada dual (inmediata y retardada) que requiere una sola dosis diaria mejora y simplifica el tratamiento, incrementa la adherencia, mejora la calidad de vida, la morbilidad y posiblemente la mortalidad. Esta revisión repasa la realidad y conocimientos sobre el tema tanto globalmente como en España ante la situación previsible de disponer en el futuro de este nuevo fármaco.
Adrenal insufficiency (AI), the clinical manifestation of deficient glucocorticoid production or action, is an uncommon condition which may be fatal if left untreated. The most common causes of AI are the primary failure of the adrenal glands (mostly due to autoimmune adrenalitis of Addison's disease) or impaired function of the hypothalamic-pituitary-adrenal axis (HPA). In both situations, cortisol secretion is deficient.
The overall prevalence of AI in Spain is unknown because there are no specific registers. The prevalence of autoimmune adrenalitis, according to series from other European countries, is estimated at 93–140 per million inhabitants,1,2 with an annual incidence of 5–6 new cases per million inhabitants in Caucasian populations.3 The most common etiology of secondary deficiency is pituitary lesion of chronic exogenous glucocorticoid administration, which induces atrophy of the corticotropic cells. The latter is only relevant when exogenous corticoid treatment is discontinued. Few Spanish data are also available about the number of patients with corticotropin (ACTH) deficiency, which in most cases is associated with several other pituitary deficiencies (hypopituitarism). A global prevalence ranging from 150 to 280 per million is estimated.3 A single study on the prevalence of hypopituitarism in the region of Galicia reported 45 cases/100,000 inhabitants and an annual incidence of 4.2/100,000 inhabitants in 2001. Approximately 62% of patients have associated ACTH deficiency.4
The cardinal symptoms of AI were described by Thomas Addison in 1885, and include weakness, fatigue, anorexia, joint pain, orthostatic hypertension, and skin hyperpigmentation (specific to primary AI but absent in ACTH deficiency). These symptoms are easily overlooked because of their low specificity, and diagnosis is therefore often delayed. In an acute condition, AI represents a medical emergency that leads to severe arterial hypotension, decreased consciousness, and even death if left untreated.
The identification of people at risk or with a predisposition to experience clinical or subclinical AI would allow for earlier adequate diagnosis. In addition, understanding of the etiology of adrenal failure is of interest for its monitoring and treatment. Personal and pathological history and a good anamnesis may suggest a suspected diagnosis. When adrenal failure is suspected, hormonal diagnosis should be confirmed, the cause should be sought and, above all, replacement therapy should be started with hydrocortisone. Table 1 summarizes the different causes of AI.
Etiology of adrenal insufficiency.
| PRIMARY |
| Autoimmune: sporadic, polyglandular autoimmune syndrome type 1 and type 2 |
| Infectious: tuberculosis, fungal infections, cytomegalovirus, human immunodeficiency virus |
| Metastasis |
| Adrenal infiltration: amyloidosis, hemochromatosis |
| Bilateral intra-adrenal hemorrhage: meningococcal sepsis, trauma, coagulopathy |
| Adrenoleukodystrophy, adrenomyeloneuropathy |
| Congenital adrenal hyperplasia or hypoplasia |
| ACTH-resistance syndromes |
| Bilateral adrenalectomy |
| Drug-induced: ketoconazole, etomidate, aminoglutethimide, mitotane, metopyrone, abiraterone |
| SECONDARY |
| Treatment with exogenous glucocorticoids |
| Hypopituitarism |
| Selective resection of ACTH-secreting pituitary tumor |
| Pituitary apoplexy, Sheehan's syndrome |
| Granulomatous disease: sarcoidosis, tuberculosis |
| Pituitary irradiation |
| Isolated ACTH deficiency: Idiopathic, genetic, lymphocytic hypophysitis |
Diagnosis is confirmed by basal cortisol levels below the reference limits (<165nmol/L or 5mcg/dL) or peak cortisol levels less than 500nmol/L (18mcg/dL) after stimulation with 250mcg of ACTH IV. If secondary AI is suspected, a pituitary stimulation test, such as insulin-induced hypoglycemia, will confirm ACTH deficiency if peak cortisol achieved after hypoglycemia (glucose<2.2nmol/L) is less than 550nmol/L (22mcg/dL).
Once the biochemical diagnosis is confirmed, it is important to ascertain the etiology in order to know whether any specific treatment for the cause is available, in addition to hormone replacement therapy. Long term treatment of these patients requires the collaboration of a specialist with experience, although diagnostic suspicion and acute treatment are the responsibility of all physicians, as this is often a life-threatening medical emergency.
Despite the optimization of replacement therapy with the currently available drugs, associated comorbidities, mortality, and health-related quality of life do not completely normalize in these patients.
Physiological circadian rhythm of cortisolPhysiological cortisol production follows a circadian rhythm. A progressive cortisol increase is seen during the early morning, with peak levels just before waking up in the morning which gradually decrease over the day to minimum concentrations (even to <50nmol/L=1.8mcg/dL) at about midnight.5 Cortisol increase during the early morning is precisely the most marked difference between the normal circadian rhythm of cortisol and that seen in patients with AI,6 as the latter wake up with very low cortisol levels which only increase after they have taken exogenous glucocorticoids. It is therefore important to try and achieve serum cortisol levels as similar as possible to a normal circadian rhythm, bearing in mind that the cortisol production rate in healthy subjects is lower than previously thought.7
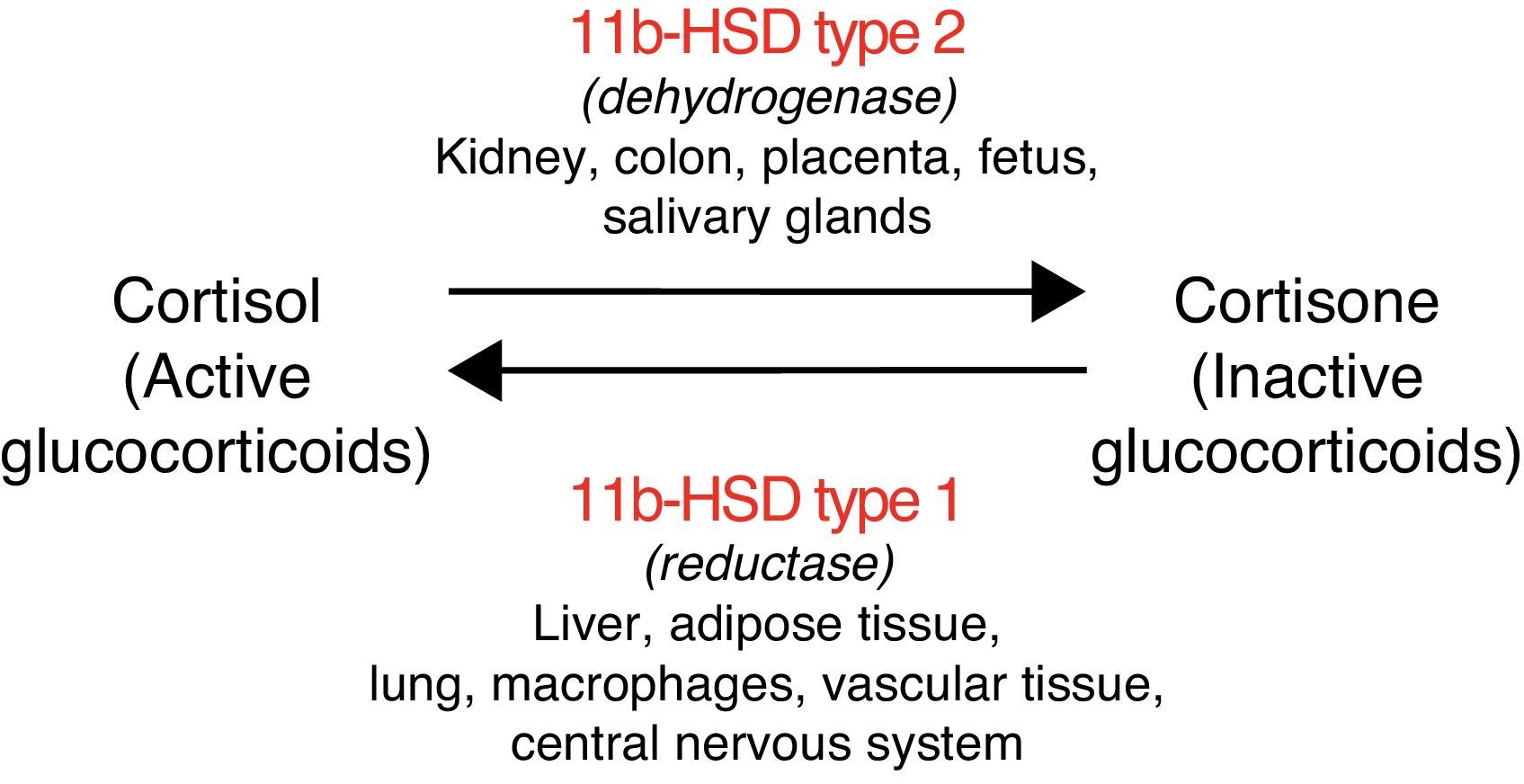
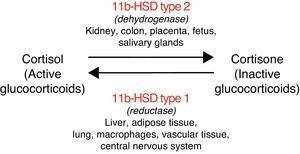
The enzyme 11 beta-hydroxysteroid dehydrogenase regulates cortisol metabolism, amplifying the steroid signal when isoform type 1 of the enzyme (11bHSD1) acts, or converting cortisol into cortisone (inactive steroid) when isoform type 2 (11bHSD2) acts.8 The activity of these enzymes is specific to each tissue (Fig. 1). In addition, 11bHSD2 does not inactivate with the same efficacy the different types of synthetic glucocorticoids (e.g. it has little capacity to inactivate dexamethasone). This accounts for the different metabolic consequences with different glucocorticoid formulations.
Morbidity and mortality in patients on glucocorticoid replacement therapy for adrenal insufficiencyBefore glucocorticoid replacement therapy became available (1950), patients with AI had a one-year survival rate lower than 20%. The advent of hydrocortisone replacement therapy markedly improved survival.
However, the few available survival studies in AI show that, despite adequate replacement therapy with glucocorticoids and mineralocorticoids, associated comorbidities, mortality, and health-related quality of life are more significant as compared to the general population.9–16
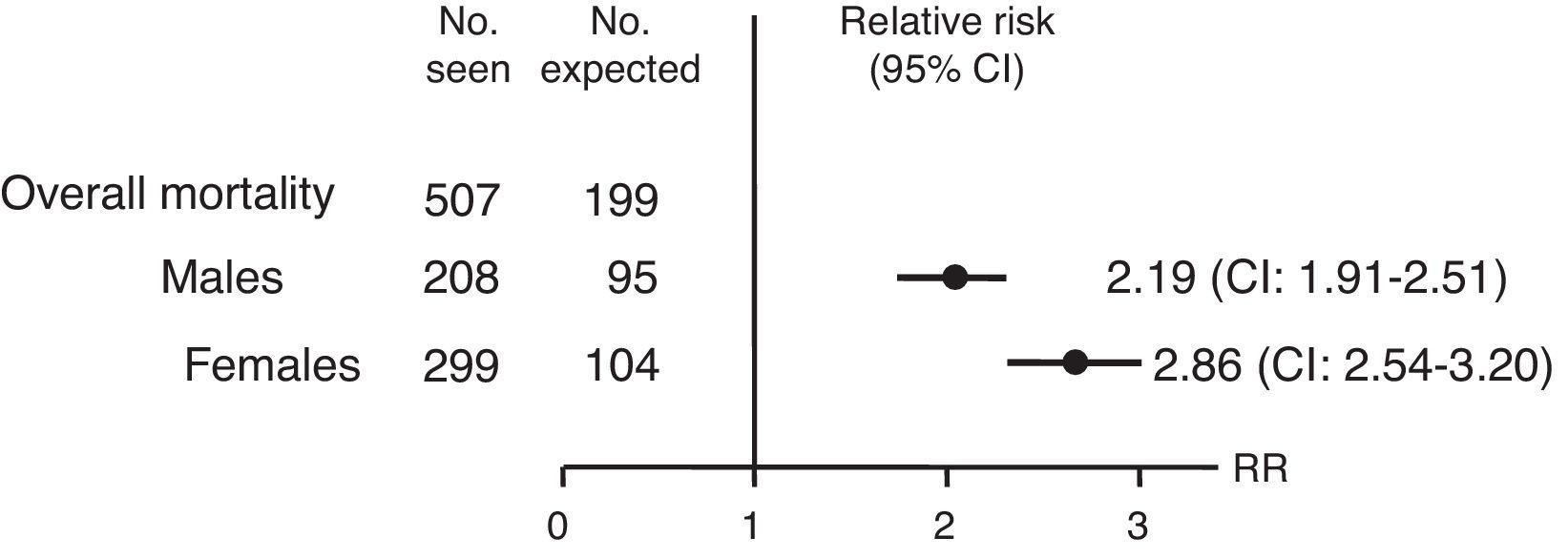
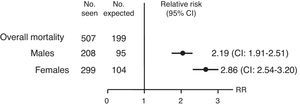
Several studies have reported early mortality in treated AI patients. A retrospective, observational study using data from the National Swedish Hospital and Cause of Death Registers noted in Addison's disease a mortality risk double that of the general population (relative risk [RR]): 2.19 in males (confidence interval [CI]): 1.91–2.51) and 2.86 in females (CI: 2.54–3.20]) (Fig. 2). This increased mortality was mainly attributable to cardiovascular causes, tumors, and infections. An acute adrenal crisis was the cause of 7.1% of these deaths.8
Relative risk and confidence interval (CI) for overall mortality in patients with Addison's disease in Sweden from 1987 to 2001. Source: Bergthorsdottir et al.8
Another more recent and larger Swedish study found a twofold greater overall mortality risk in Addison's disease as compared to the general population, and the risk was even greater in younger patients.9 By contrast, a retrospective, observational Norwegian study on 811 patients only noted an increase in mortality as compared to the reference population if AI was diagnosed before 40 years of age, with a standard mortality rate (SMR) of 1.5 (CI: 1.09–2.01). The most common causes of death were acute AI and infection.10
Mortality is also increased in hypopituitarism, with the risk ranging from 1.2 to 2.17,11–14 but the extent to which this is attributable to ACTH deficiency or other associated pituitary deficiencies is not known.
In the 1990s, Rosén et al. retrospectively analyzed 333 patients with hypopituitarism and found an increased overall mortality in them as compared to the general population, regardless of the cause of hypopituitarism. Cardiovascular death was the most common.11 This increased mortality persisted even after excluding patients who died due to surgical problems and with a history of Cushing's disease or acromegaly, in whom cardiovascular death is known to be increased. The only independent predictors of survival found were age at diagnosis and hypogonadism.12 In another series of 344 patients with hypopituitarism, overall mortality was twofold greater as compared to the general population (SMR: 2.17, CI: 1.88–2.51), with a higher risk in females.13 The most common cause of death was cerebrovascular, and was more common in patients in whom pituitary deficiencies started at a younger age, suggesting inadequate hormone replacement.
In a series of 1014 British patients with hypopituitarism an overall SMR of 1.87 (CI: 1.62–2.16) was also found, which was mainly attributed to cardiovascular and infectious causes. As in the prior series, age at diagnosis, female sex, and untreated hypogonadism were independent predictors associated with mortality.14
In a pediatric series of 5000 patients with hypopituitarism, a mortality risk up to sevenfold greater was seen in secondary hypoadrenalism as compared to the reference population.15 This reflects the importance of the fact that the relatives and caregivers of these children were educated and trained to identify signs of risk of AI and to act in response to them. Table 2 summarizes the early mortality rates.
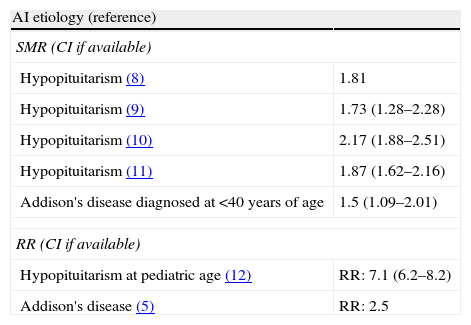
Early mortality in hypopituitarism and Addison's disease.
| AI etiology (reference) | |
| SMR (CI if available) | |
| Hypopituitarism (8) | 1.81 |
| Hypopituitarism (9) | 1.73 (1.28–2.28) |
| Hypopituitarism (10) | 2.17 (1.88–2.51) |
| Hypopituitarism (11) | 1.87 (1.62–2.16) |
| Addison's disease diagnosed at <40 years of age | 1.5 (1.09–2.01) |
| RR (CI if available) | |
| Hypopituitarism at pediatric age (12) | RR: 7.1 (6.2–8.2) |
| Addison's disease (5) | RR: 2.5 |
CI: confidence interval; AI: adrenal insufficiency; SMR: standard mortality rate; RR: relative risk.
This global increase in mortality may partly be due to inadequate glucocorticoid replacement therapy, either by excess or defect, especially in response to stress or intercurrent disease. In fact, it has been shown that patients with treated AI do not achieve a good quality of life or a subjective perception of well-being.8,17 Hahner et al.18 analyzed the quality of life and depression in patients with AI for any cause using questionnaires. They found a poor perception of state of health and a poorer quality of life, particularly in secondary AI, in which other hormone deficiencies with a potential impact on perceived well-being usually coexist. It should be noted that 18% of these patients did not work or were receiving a disability pension, as compared to 4% of controls.18
Another recent study interviewed 1245 patients with AI from several countries. Two-thirds of these patients reported impaired quality of life, which was worse in patients with secondary AI.19
Although the factors determining this poorer quality of life in patients with AI are unknown, they are likely to play a role in the impossibility of achieving, with the medication currently available, a physiological circadian rhythm of circulating cortisol throughout the day and night.
On the other hand, changes in metabolic profile have been seen in patients with AI treated with glucocorticoids. In the KIMS database (an international pharmacovigilance study including 2.424 patients with hypopituitarism treated with recombinant human growth hormone), patients with and without ACTH were compared. ACTH-deficient patients treated with hydrocortisone (or equivalent) doses of 20mg/day or higher were seen to have an unfavorable metabolic profile (greater body mass index and higher triglyceride, total cholesterol and LDL levels). No metabolic profile differences were found in patients taking doses lower than 20mg/day.20 Overall, patients treated with glucocorticoids were seen to experience more cardiovascular events and diabetes mellitus, although ACTH-sufficient patients were six years younger on average.
It has also been reported that an attenuated diurnal variation of cortisol profile, i.e. less serum cortisol in the morning and more serum cortisol at night, is associated with abdominal obesity and metabolic syndrome.21
Treatment with glucocorticoids inhibits osteoblastic activity, stimulates osteoclastic activity, and inhibits vitamin D-dependent intestinal calcium absorption, in addition to inducing hypogonadism. All this promotes accelerated bone mass reduction. Indeed, a recent study of patients with Addison's disease (n=292) noted decreased bone mineral density (BMD) in the femoral neck and lumbar spine. An inverse relation was seen between hydrocortisone doses administered and BMD, but no increase was found in the number of fractures.22 Several studies agree with this, reporting decreased BMD, although most patients were receiving glucocorticoid doses of 30mg/day of higher.23,24 Another recently reported study found no differences in BMD in patients treated with low hydrocortisone doses (<20mg/day) as compared to the reference range (in both patients with Addison's disease and with congenital adrenal hyperplasia treated with corticoids). By contrast, patients treated with prednisolone (a longer-acting glucocorticoid) showed lower osteocalcin (a serum bone formation marker) levels and BMD Z-scores as compared to those treated with hydrocortisone,25 in agreement with the findings from another series.26
An additional study reported an increased risk of hip fracture in these patients as compared to controls categorized by age and sex (OR: 1.8, CI: 1.6–2.1), with the risk being greater in women diagnosed with Addison's disease before 50 years of age.27 This has a significant economic impact on the healthcare system and the quality of life of patients.
Current glucocorticoid replacement therapy regimens and their problemsAccording to a recent survey including 1245 patients from several European countries on glucocorticoid replacement therapy, the glucocorticoid drug most commonly used in AI is hydrocortisone two or three times daily (in 75% of cases), followed by prednisone (11%), cortisone acetate (6%), and dexamethasone (4%).19
Because of the short half-life of hydrocortisone, multiple doses are required during the day. Each oral dose administered causes a rapid increase and a high peak in cortisol levels, followed by a rapid decrease. When these patients were asked what factors they considered to be the most important in assessing whether a replacement therapy was optimum, most of them answered that the drug should last for 24h and have few adverse effects.19
Most patients complained of an impaired subjective perception of health with an impact on their social life, physical activity, work or family life due to their disease and its treatment.19 They thought that treatment with multiple daily doses made compliance difficult, which in turn had a negative impact on their well-being.
These data show, from the perspective of the patient, a clear need to improve glucocorticoid replacement therapy in AI, despite the availability of multiple regimens.5 On the other hand, evidence-based treatment guidelines are lacking.
The effectiveness of glucocorticoid therapy is difficult to monitor due to the lack of adequate markers, and is therefore only based on clinical signs, which makes it difficult to identify overtreated or undertreated patients. In addition, most patients report that they feel better with high hydrocortisone doses or taking an excess dose, with the resultant risk of adverse effects. There is currently no valid method of assessing patient adherence to treatment and adaptation of the treatment to physiological cortisol secretion.
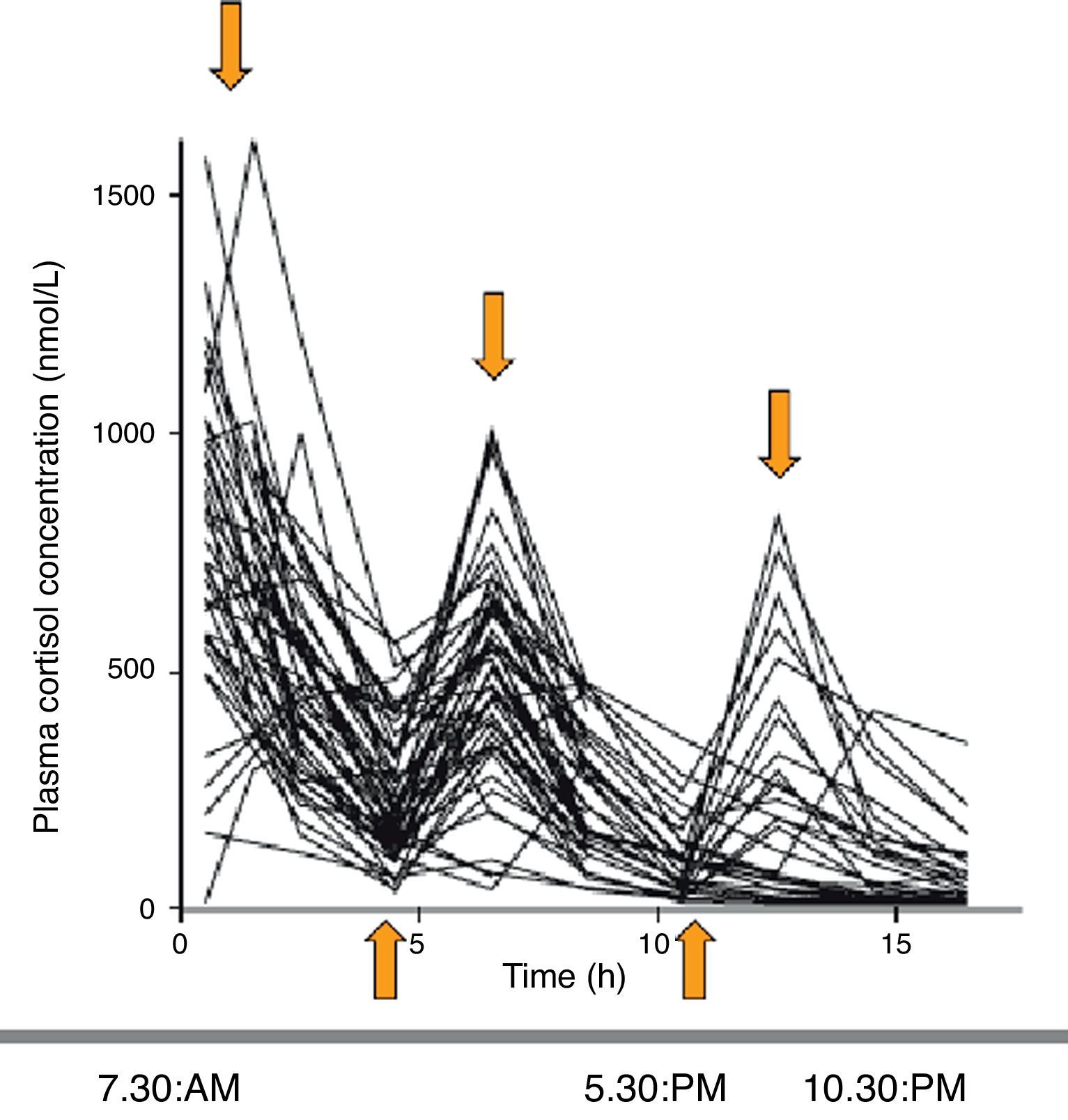
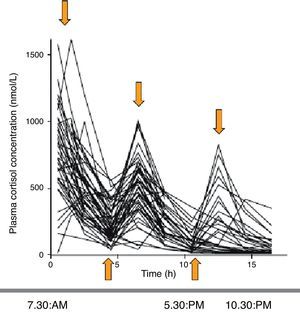
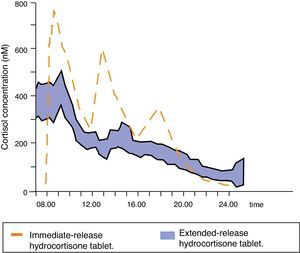
The assessment of circulating cortisol pharmacokinetics with the different treatment regimens shows that, as compared to physiological cortisol limits (obtained from healthy controls), cortisol levels show overtreatment and undertreatment at 8, 16, and 24h in 79%, 55%, and 45% of patients, respectively. The hydrocortisone regimen that best mimicked the physiological pattern was shown to be the one consisting of 10mg (at 7h 30min), 5mg (at 12h), and 5mg (at 16h 30min). However, 54%, 44%, and 32% of patients continued to be overtreated or undertreated at 8, 16, and 24h respectively (Fig. 3).5
Serum cortisol profiles over the day in patients with AI treated with hydrocortisone (short half-life) as 2–3 doses daily. Supraphysiological peaks and infraphysiological nadirs are found. Source: Simon et al.5.
The best physiological profiles, with less underdosing and symptom improvement (particularly fatigue), were achieved with three hydrocortisone doses (10-5-5mg),28,29 which is considered the best currently available treatment.
Patients treated with hydrocortisone three times daily have higher than physiological cortisol concentrations at dusk, which is associated with poorer glucose tolerance and decreased insulin sensitivity.20,30 These evening cortisol levels have also been associated with abdominal obesity, coronary atherosclerosis,31 insomnia, decreased sleep quality,32 metabolic syndrome, and greater mortality.33 In fact, insomnia in healthy subjects has been related to increased ACTH and cortisol secretion, especially in the evening and during the first half of the night, with more cortisol peaks during the day,31,34 similar to that which occurs with conventional replacement therapy consisting of three daily doses of hydrocortisone.
The fatigue reported by many patients with Addison's disease could be related to poor sleep quality. Undertreated patients with undetectable cortisol levels during the second part of the night have less episodes of rapid eye movements (REM) and a longer latency between REM stages, denoting a non-refreshing, fragmented sleep. This suggests that minimum cortisol doses are needed to start and maintain REM episodes (more important in the second part of the night).35 By contrast, patients with excess treatment and elevated cortisol concentrations at night may experience insomnia or depression, fragmented sleep, and a decreased REM stage.31,34 To sum up, both extremes of activity of the HPA axis lead to sleep fragmentation and, thus, to increased fatigue. It is therefore advisable to achieve as physiological as possible cortisol concentrations throughout the 24h.35
Although a circadian cortisol profile close to the physiological profile has been achieved in selected patients (n=7) using intravenous or subcutaneous hydrocortisone infusion, resulting in significant improvements in vitality and functional performance and better subjective overall perception of health,36 this parenteral route cannot be used in all cases. Thus, the availability of an oral dual (immediate and extended) release hydrocortisone preparation which better mimicked circadian rhythm would be of great value for improving the quality of life and prognosis of these patients.37
Novel formulation of a modified-release, long-acting oral glucocorticoidBecause of the low prevalence of AI, the low cost of glucocorticoid replacement therapy, and the initial belief that both treatment course and consequences were satisfactory, there was previously no interest in developing new drug products for this disease. The recent evidence of increased morbidity and mortality in AI, probably related to supraphysiological hydrocortisone doses as the result of a diurnal exposure profile of insufficient glucocorticoids or inadequate rescue treatment in the presence of intercurrent diseases has led to the development of new drugs.38
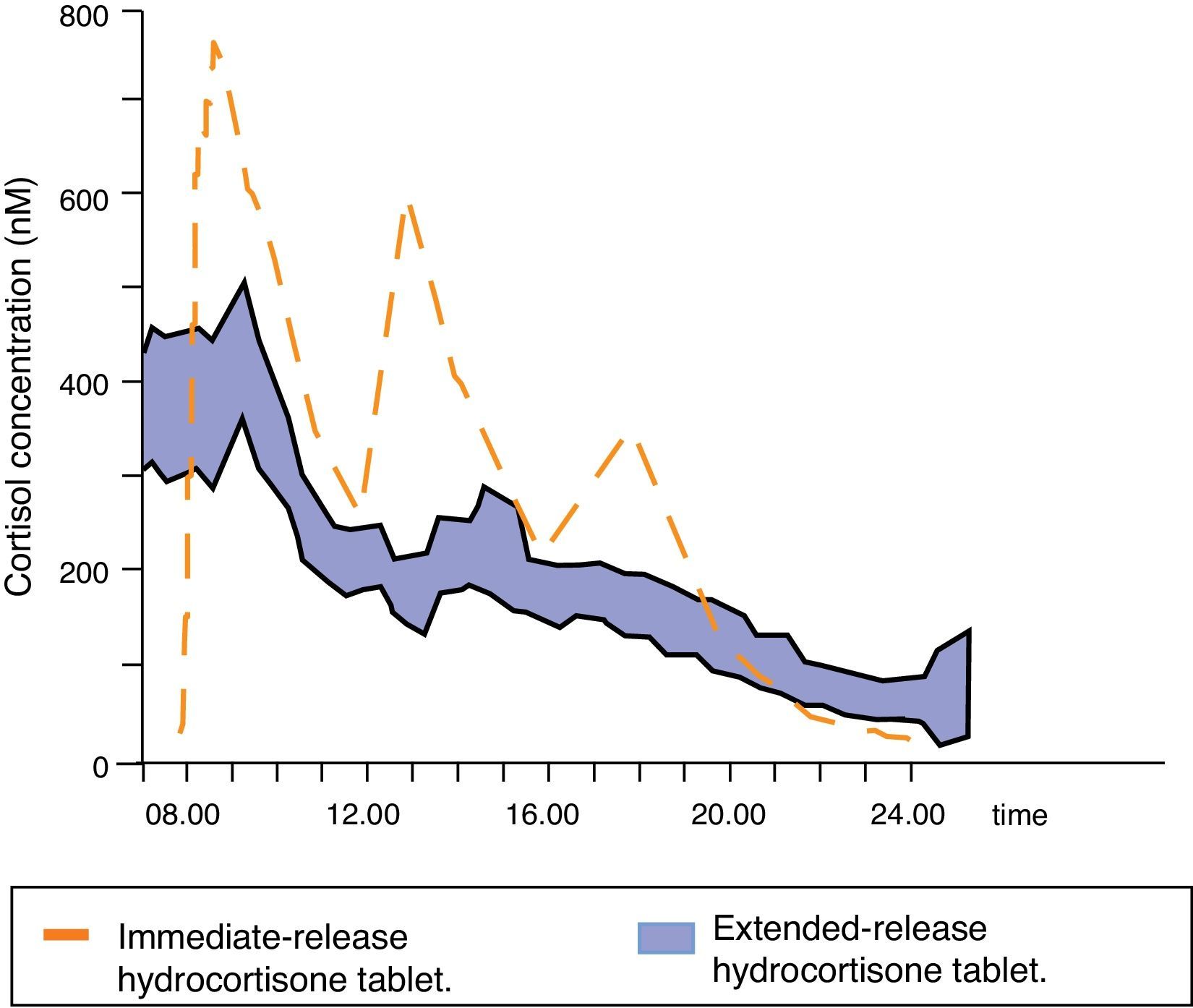
Johannsson et al. developed a new hydrocortisone formulation with modified dual (immediate and extended) release that allows for a much better reproduction of the physiological cortisol profile with a single daily dose of the drug. This new formulation was initially tested in healthy subjects,39 showing high bioavailability, a serum cortisol profile very similar to the physiological profile (except for the morning increase), and adequate intestinal absorption even with food, which led to the conduct of clinical trials. The pharmacokinetics and metabolic consequences of this new treatment were compared to three daily hydrocortisone doses in 64 patients with Addison's disease.37 The pharmacokinetic serum cortisol profile with the new modified formulation was similar to the physiological profile, although the area under the cortisol 24-h time-concentration curve was decreased; in addition, overall serum cortisol concentration over 24h was 20% lower. Cortisol concentrations remained high and stable for the first four hours after drug administration, and cortisol levels during the evening/night decreased by up to 58% (Fig. 4). Such pharmacokinetic differences may explain the decreases in body weight, blood pressure, and glycosylated hemoglobin (even in diabetic patients) seen in patients treated with this modified dual release formulation.
Mean serum cortisol concentrations following administration of a dose of extended-release hydrocortisone to 64 patients with Addison's disease. Adapted from: Johannsson et al.37.
Better results were also found in psychological function and well-being tests, and 92% of patients chose to continue on this new treatment regimen after study completion.37 Metabolic improvements persisted during the 27-month study extension.40
Recommendation to physicians and patients based on the availability of the novel, modified dual release hydrocortisone formulationAdequate therapeutic education of patients and treatment adherence are important to prevent problems in critical situations. Despite this, the mortality rate from adrenal crisis even after mild infections is worrying. Glucocorticoid replacement therapy must therefore provide an optimum dose and a physiological circadian distribution; it should also contemplate the higher dose required during intercurrent conditions to minimize the risk of acute adrenal crisis.
Although cortisol secretion and concentration increase during severe stress situations (major surgery, infection, etc.), no studies detailing cortisol concentrations during these intercurrent conditions are available. The usual procedure in such cases is to double the hydrocortisone dose in each intake. This approach may not be sufficient because the bioavailability of hydrocortisone is not linearly related to the dose administered, irrespective of the type of hydrocortisone used.
A recent prospective study where approximately 300 episodes of intercurrent disease were documented found that if the number of daily doses of modified dual release hydrocortisone was increased (every 8±2h), a more linear relationship was achieved between the dose administered and cortisol exposure. Less fluctuations and better coverage of 24-h cortisol concentrations as compared to doubling the dose in a single daily dose, thus minimizing the risk of adrenal crisis, were also seen.41
Thus, in patients with non-severe conditions, where the administration of parenteral hydrocortisone is not indicated, modified dual release hydrocortisone, administered as two or three daily doses, may be a better treatment than doubling the standard dose.
Therapeutic education of the patient using simple and safe treatment self-modification schemes should help prevent most cases of acute adrenal crises in patients with AI.
ConclusionsGeneral well-being and quality of life are decreased in patients with AI treated with current corticoid regimens with increased morbidity and mortality.
Such increased morbidity and mortality is probably related to both overdosing and lack of hydrocortisone associated with non-physiological peaks and nadirs.
The availability of a new drug with modified dual release (both immediate and delayed) administered as a single daily dose improves and simplifies treatment, increases adherence, and improves quality of life, morbidity and, possibly, mortality.
This new drug is also useful during intercurrent conditions although, instead of doubling the dose, a second dose should be administered after 8±2h in order to cover the full 24-h period, minimizing the risk of acute adrenal crisis.
The lack of data concerning the incidence and prevalence of AI in Spain makes it difficult to assess the magnitude of the problem in our country.
Conflicts of interestThe authors are members of the Spanish Advisory Board of Viropharma.
Please, cite this article as: Aulinas A, et al. Insuficiencia suprarrenal y su tratamiento sustitutivo. Su realidad en España. Endocrinol Nutr. 2013;60:136–43.