Amyloidosis includes different diseases characterized by the extracellular accumulation of insoluble, toxic protein in different tissues and organs. The most common forms of systemic amyloidosis are primary amyloidosis (PA) of light chains and secondary amyloidosis (SA) caused by chronic inflammatory diseases.1–4
In autopsy studies, amyloid material is found in the thyroid gland of 80% of patients with SA and 50% of patients with PA.5,6
Amyloid goiter (AG) is an uncommon condition that is characterized by thyroid tissue infiltration by amyloid material, which causes thyroid gland enlargement.6,7 In SA, the deposition of amyloid A (AA) protein is associated with atrophy of thyroid follicles.7,8 The clinical picture of these patients is characterized by rapid, painless thyroid gland enlargement which may be associated with dysphagia, dyspnea, or hoarseness. Thyroid function is not impaired in most cases.5–7
A female patient with amyloid goiter associated with amyloidosis secondary to rheumatoid arthritis is reported below.
A 46-year-old female patient diagnosed with rheumatoid arthritis at 21 years of age attended the emergency room of our hospital complaining of gradual, painless thyroid gland enlargement for the previous 8 months. She reported dysphagia, odynophagia, cough, and fever during the three months prior to admission. Her regular treatment consisted of prednisone (15mg/day) and methotrexate (7.5mg/day). The patient had no history of kidney failure or altered thyroid function. Physical examination findings included: BP, 100/60mmHg; HR, 102bpm; RR, 22 breathings/min; and oral temperature, 37°C. Other findings included pale skin, multiple cervical adenopathies, thyroid gland enlargement (grade 1b) with multiple nodules on palpation and an increased consistency, and joint deformity without active synovitis. Thyroid ultrasound examination in the emergency room showed multinodular goiter.
Laboratory test results included: WBC, 16,000/mm3; hemoglobin, 8.3g/dL; albumin, 1.9g/dL; creatinine, 1.7mg/dL; glucose, 90mg/dL; TSH, 1.24μIU/dL (normal range, 0.4–4.0); free T4, 1.61ng/dL (normal range, 0.8–1.9); creatinine clearance, 56mL/min; and 24-h proteinuria, 1.9g/day. The erythrocyte sedimentation rate was 70mm/h, and urine examination revealed WBCs. Intravenous ceftriaxone was therefore added to treatment.
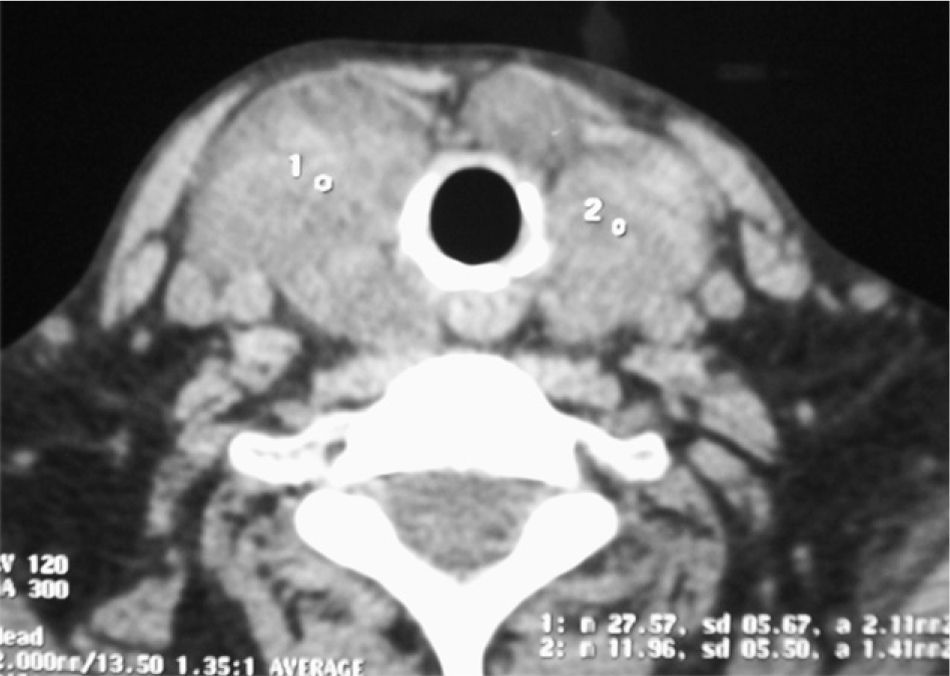
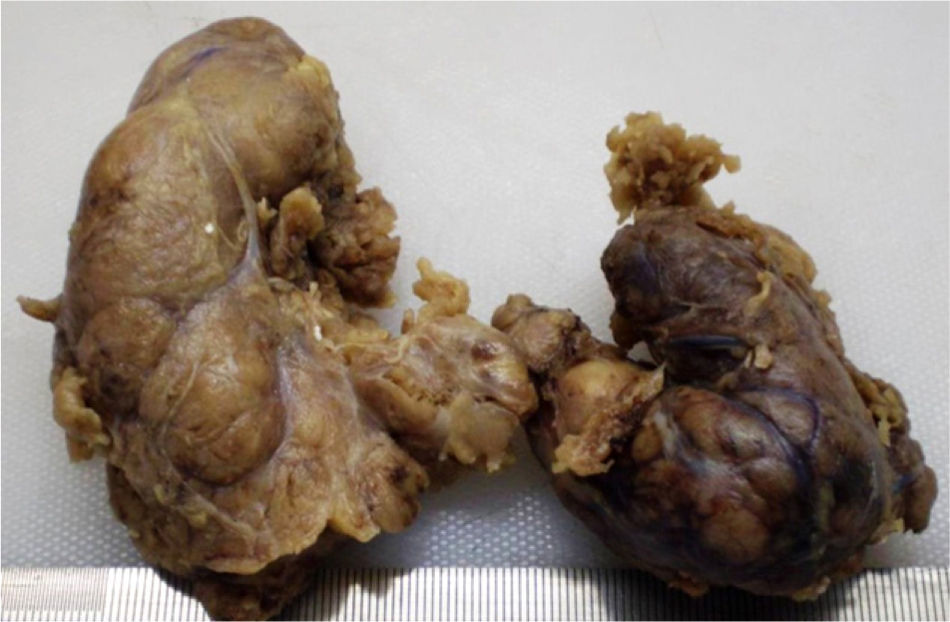
Because of compressive symptoms, a computed tomography (CT) scan was performed before surgery, showing multinodular goiter with no tracheal compression (Fig. 1). Due to dysphagia and progressive dyspnea, the patient underwent total thyroidectomy. A pathological study of the surgical specimen showed an enlarged thyroid gland with multiple nodules, of an increased consistency and with adipose tissue-like areas (Fig. 2). Microscopic study showed extensive infiltration of thyroid parenchyma by an amorphous, eosinophilic material with no neoplastic cells.
Because of kidney involvement, characterized by moderate renal failure and proteinuria, a renal biopsy was performed, showing kidney infiltration by amyloid. The patient was discharged free of symptoms on replacement therapy with levothyroxine 75μg/d and cyclophosphamide 25mg/d. One week later, she was readmitted to hospital for severe epigastric pain, diarrhea, and dehydration. No ulcer was found in an upper gastrointestinal endoscopy, but a biopsy of gastric mucosa showed amyloid infiltration.
Systemic amyloidosis may be a serious complication of inflammatory diseases, such as rheumatoid arthritis, usually occurring 8–10 years after the start of joint involvement.9,10 In our patient, kidney, thyroid gland, and gastrointestinal tract involvement were found.
AG was first reported in 1858 by Beckman. It is an uncommon complication of both primary and secondary amyloidosis.6 AG is also associated with medullary thyroid carcinoma, and may occur in 50–70% of patients with this tumor.5 In these cases, however, the primary constituent of amyloid is calcitonin, which is located in the tumor area and does not infiltrate the rest of the gland.1,7,11
Patients with AG usually show a rapid, diffuse, and painless growth of the thyroid gland. This growth may be associated with compressive symptoms such as dysphagia, odynophagia, dyspnea, or hoarseness. Palpation most often reveals diffuse gland enlargement, but individual nodules may also be felt. If rapid thyroid gland enlargement occurs, a primary thyroid neoplasm such as primary lymphoma or anaplastic carcinoma should be ruled out.6,7
AG evaluation usually includes a thyroid ultrasound examination, which shows amyloid deposits as hypoechogenic masses.5 In our patient, ultrasound revealed multinodular goiter with hypoechogenic areas.
Fine needle aspiration (FNA) is another helpful tool for the diagnosis of AG.13 However, because of the heterogeneous distribution of amyloid within the gland, FNA results may not be definitive in some cases.7 Overall sensitivity of FNA is greater than 90%.12,13
Thyroid function test results are usually normal, as occurred in our patient, but hypothyroidism, hyperthyroidism, or low T3 syndrome have been reported.3,11,14
AG histology is characterized by perifollicular amyloid deposits surrounded by adipose tissue which distorts the normal architecture of thyroid follicles.6,7 Congo red staining was not performed in our patient, but crystal violet staining showed extensive infiltration of parenchyma by an eosinophilic material consistent with amyloid. Congo red staining is the procedure of choice for the diagnosis of amyloidosis, showing a typical green birefringence under polarized light. However, staining with crystal violet or thioflavin T is also helpful.5 These stains cannot differentiate the proteins forming amyloid. Immunohistochemistry methods with anti-AA amyloid antibodies are therefore required for final diagnosis.6
Most patients undergo total thyroidectomy because of compression symptoms, but elective thyroidectomy may be performed in some patients with extensive goiter.6,7
Preoperative diagnosis of AG should be considered in patients with a history of long-standing systemic amyloidosis or inflammatory disease who report rapid thyroid gland enlargement. FNA will allow for ruling out a primary neoplasm in most cases.
Please, cite this article as: Pinto Valdivia M, et al. Bocio amiloide secundario a artritis reumatoide. A propósito de un caso. Endocrinol Nutr. 2012;59:69–83.