Patients with type 1 diabetes (T1DM) treated with continuous subcutaneous insulin infusion (CSII) have the availability of several specific features of these devices. The aim of this study was to evaluate the relationship between real use of them and the degree of glycemic control in patients using this therapy.
Patients and methodsForty-four T1DM patients on CSII therapy with or without real-time continuous glucose monitoring (CGM) were included. Data from 14 consecutive days were retrospectively collected using the therapy management software CareLink Personal/Pro® and HbA1c measurement performed at that period. The relationship between the frequency of use of specific features of insulin pumps (non-sensor augmented or sensor-augmented) and glycemic control was analyzed.
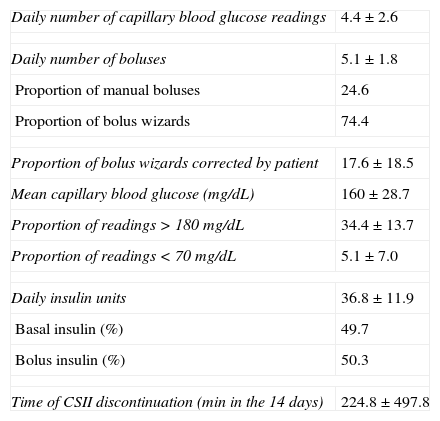
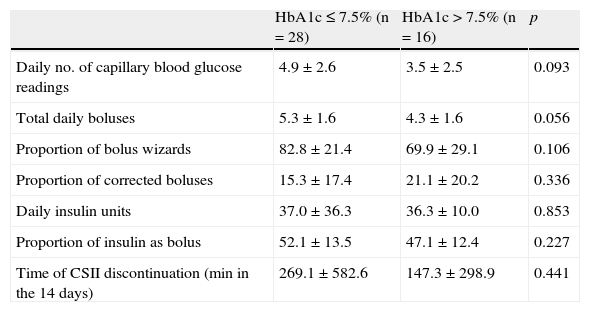
ResultsMean HbA1c in the group was 7.5±.8%. Mean daily number of boluses administered was 5.1±1.8, with 75.4% of them being bolus wizards (BW). Daily number of boluses was significantly greater in patients with HbA1c<7.5% than in those with HbA1c>7.5% (5.3±1.6 vs 4.3±1.6, P=.056). There was a trend to greater use of BW in patients with better control (82.8±21.4% vs 69.9±29.1%, P=.106). HbA1c was lower in patients using CGM (n=8) as compared to those not using sensor-augmented pumps (7.6±.8 vs 7.1±.7, P=.067), but the difference was not statistically significant.
ConclusionsMore frequent use of BW appears to be associated to better metabolic control in patients with T1DM using pump therapy. In standard clinical practice, augmentation of insulin pump with CGM may be associated to improved glycemic control.
Los pacientes con diabetes tipo 1 (DT1) en tratamiento con infusión subcutánea de insulina (ISCI) tienen a su alcance diversas prestaciones específicas para estos dispositivos. El objetivo del estudio fue valorar la relación entre la utilización real de las mismas y el grado de control glucémico en pacientes habituados a este tipo de terapia.
Pacientes y métodosIncluimos 44 pacientes con DT1 en tratamiento con ISCI con o sin monitorización continua de glucosa (MCG). Recogimos retrospectivamente los datos de 14 días consecutivos mediante la plataforma CareLink Personal/Pro® y la determinación de HbA1c realizada en ese periodo. Posteriormente, analizamos las diferencias en el uso de las prestaciones del dispositivo en función del grado de control glucémico y las diferencias entre los grupos con o sin MCG.
ResultadosLa HbA1c media del grupo fue de 7,5±0,8%. El número de bolus/día administrados fue de 5,1±1,8, siendo el 75,4% de ellos en forma de bolus ayuda (BA). Los pacientes con mejor control (HbA1c<7,5%) se administraban más bolus/día (5,3±1,6 vs. 4,3±1,6; p=0,056) que aquellos con peor control. Existía una tendencia a una mayor utilización de BA en los pacientes con mejor control (82,8±21,4% vs. 69,9±29,1%; p=0,106). Sin alcanzar la significación estadística, los pacientes portadores de MCG (n=8) tenían una HbA1c inferior a aquellos sin esta prestación (7,6±0,8 vs. 7,1±0,7; p=0,067).
ConclusionesEl mayor uso del BA en los pacientes con DT1 en tratamiento con terapia ISCI tiende a asociarse con un mejor grado de control metabólico. En la práctica clínica habitual la utilización de MCG combinada con ISCI podría asociarse a un mejor control glucémico.
Multiple studies trying to establish differences between intensive therapy with continuous subcutaneous insulin infusion (CSII)) and standard intensive therapy with multiple dose insulin (MDI) in patients with type 1 diabetes mellitus type 1 (T1DM) have been published. Although prior non-randomized studies showed no differences in the degree of blood glucose control between both types of therapy,1 subsequent studies2 and meta-analyses3,4 have shown the benefit of CSII therapy on blood glucose control in adequately selected patients.
The success of this type of therapy is associated to the need for adjusting insulin doses based on frequent capillary blood glucose monitoring. Accurate dose adjustment requires calculations based on multiple aspects: (a) target blood glucose value; (b) current blood glucose value; (c) amount of carbohydrates (CHs) in the meal; (d) insulin-CH ratio (ICR [amount of insulin needed to cover 10g of CHs]); (e) insulin sensitivity factor (ISF [blood glucose reduction in mg/dL achieved after giving one insulin unit as a bolus]) of the patient; and (f) active insulin remaining (this will depend on the type of insulin administered in the infusion set; with current use of analogs, this is usually fixed at 3–4h, with subsequent adjustment based on patient needs).
Multiple tools have been developed in recent years to optimize use of CSII therapy, including the bolus wizard system, which consists of estimating the amount of insulin needed for the meal and/or as a correction based on capillary blood glucose of the patient, estimated CH intake, and individual parameters previously programmed in the CSII system (blood glucose goals, RIC, ISF, and active insulin time). However, few objective studies have been conducted on the relationship between actual use of these features in daily practice and the degree of metabolic control.
Our study objective was therefore to examine the relationship between actual use of these features of subcutaneous insulin infusion sets in standard clinical practice and the degree of control in patients with T1DM.
Patients and methodsA retrospective, observational study on 44 patients with T1DM monitored at the diabetes unit of the Hospital Clínic i Universitari in Barcelona. All patients had been treated with CSII for at least 12 months before the start of the study and used one of the following systems: 712, 715, 722, and 754 from Medtronic-Minimed (Northridge, USA). Both patients with and without continuous blood glucose monitoring systems were enrolled. Data were regularly downloaded by patients from the devices at home or by health care staff at the consecutive visits using the CareLink Staff/Pro® platforms respectively.
Data from 14 consecutive days were retrospectively collected from each patient, including daily number of blood glucose measurements, values of these measurements and those recorded by the sensor (in patients with a device), data related to insulin amounts administered daily, proportion of these given as a bolus or as basal insulin, proportion of boluses administered using the bolus wizard system, proportions of these boluses corrected by patient, and pump disconnection time. Glycosylated hemoglobin was also measured once by HPLC using Tosoh's G8 Automated HPLC Analyzer (Tosch Bioscience Inc). (San Francisco, USA). calibrated to DCCT standard values (reference values, 4–6%) in each patient during the study period. For data analysis, the cohort was divided into two groups depending on whether HbA1c was ≤7.5% (good [GC] group) or >7.5% (poor control [PC] group). Patients using and not using CBGM devices were also subsequently compared.
The study was approved by the corresponding site committee, and was conduced in compliance with good clinical practice.
Data are shown as mean and standard deviation or as percentages. Means were compared using a Student's t test for non-paired data for normally distributed variables, and a Mann–Whitney U test for non-normally distributed variables. The relationship between capillary blood glucose values and HbA1c was assessed using a correlation test. A value of p≤0.05 was considered statistically significant. Statistical analysis was performed using SPSS 17.0 software (SPSS Inc. Chicago, USA).
ResultsMean patient age was 40.9±10.9 years, and 72.7% (n=33) of the patients were female patients. Mean time since onset of diabetes in this patient group was 21.4±9.8 years. Indications for starting treatment with CSII were as follows: poor blood glucose control (44%), recurrent and/or severe hypoglycemia (25%), concurrent poor blood glucose control and hypoglycemia (25%), intolerance to subcutaneous insulin administration (4.5%), and pregestational control (2.3%) (although patients who had started therapy for this reason were not in the pregestational or gestational control period at the time of the study). Prior duration of CSII treatment before the start of the study was 76.8±52.5 months.
Mean HbA1c of the group was 7.5±0.8%. The daily number of capillary blood glucose measurements done by patients was 4.4±2.6. Mean blood glucose level (160±29mg/dL) was significantly correlated to HbA1c in the group (r=0.71; p<0.000) The daily number of boluses administered was 5.1±1.8; 75.4% of these were as bolus wizards (BWs) and 24.6% as manual boluses. Patients corrected 17.1±18.6% of BWs calculated by the device. Mean daily insulin dose was 36.8±11.9IU. Patients carrying a sensor used it for 76.8% of the study time (Table 1).
Overall cohort data.
| Daily number of capillary blood glucose readings | 4.4±2.6 |
| Daily number of boluses | 5.1±1.8 |
| Proportion of manual boluses | 24.6 |
| Proportion of bolus wizards | 74.4 |
| Proportion of bolus wizards corrected by patient | 17.6±18.5 |
| Mean capillary blood glucose (mg/dL) | 160±28.7 |
| Proportion of readings>180mg/dL | 34.4±13.7 |
| Proportion of readings<70mg/dL | 5.1±7.0 |
| Daily insulin units | 36.8±11.9 |
| Basal insulin (%) | 49.7 |
| Bolus insulin (%) | 50.3 |
| Time of CSII discontinuation (min in the 14 days) | 224.8±497.8 |
CSII: continuous subcutaneous insulin infusion.
A comparative analysis of patients based on their HbA1c values found that more daily boluses (5.3±1.6 vs 4.3±1.6; p=0.056) were administered in patients with GC (n=28) than in those with PC (n=16). A non-statistically significant trend to greater use of BWs was shown in patients with better control (82.8±21.4% vs 69.9±29.1%; p=0.10). No differences were seen in other parameters such as total insulin dose, insulin/CH ratio, percentage of insulin administered as bolus or basal, or percentage of BWs corrected by the patient (Table 2).
Comparison of patients with good and poor metabolic control.
| HbA1c≤7.5% (n=28) | HbA1c>7.5% (n=16) | p | |
| Daily no. of capillary blood glucose readings | 4.9±2.6 | 3.5±2.5 | 0.093 |
| Total daily boluses | 5.3±1.6 | 4.3±1.6 | 0.056 |
| Proportion of bolus wizards | 82.8±21.4 | 69.9±29.1 | 0.106 |
| Proportion of corrected boluses | 15.3±17.4 | 21.1±20.2 | 0.336 |
| Daily insulin units | 37.0±36.3 | 36.3±10.0 | 0.853 |
| Proportion of insulin as bolus | 52.1±13.5 | 47.1±12.4 | 0.227 |
| Time of CSII discontinuation (min in the 14 days) | 269.1±582.6 | 147.3±298.9 | 0.441 |
CSII: continuous subcutaneous insulin infusion.
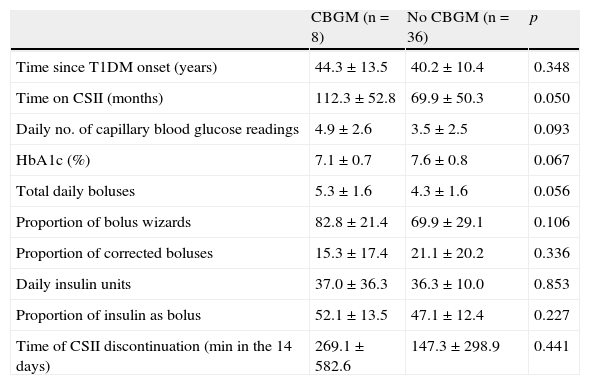
Patients carrying a sensor were seen to have been on CSII for a longer time than patients with no sensor (112.3±52.8 vs 69.9±50.3; p=0.005). Similarly, patients using CBGM had an HbA1c value 2.5% lower than those who did not use it (7.6±0.8 vs 7.1±0.7), but this difference did not reach statistical significance (p=0.06). As regards differences in use of CSII between patients using and not using CBGM, significant differences were only seen in the daily number of blood glucose measurements, which was higher among patients using CBGM (6.13±2.6 vs 4.1±2.5; p=0.04) (Table 3).
Comparison of patients with and without continuous blood glucose monitoring.
| CBGM (n=8) | No CBGM (n=36) | p | |
| Time since T1DM onset (years) | 44.3±13.5 | 40.2±10.4 | 0.348 |
| Time on CSII (months) | 112.3±52.8 | 69.9±50.3 | 0.050 |
| Daily no. of capillary blood glucose readings | 4.9±2.6 | 3.5±2.5 | 0.093 |
| HbA1c (%) | 7.1±0.7 | 7.6±0.8 | 0.067 |
| Total daily boluses | 5.3±1.6 | 4.3±1.6 | 0.056 |
| Proportion of bolus wizards | 82.8±21.4 | 69.9±29.1 | 0.106 |
| Proportion of corrected boluses | 15.3±17.4 | 21.1±20.2 | 0.336 |
| Daily insulin units | 37.0±36.3 | 36.3±10.0 | 0.853 |
| Proportion of insulin as bolus | 52.1±13.5 | 47.1±12.4 | 0.227 |
| Time of CSII discontinuation (min in the 14 days) | 269.1±582.6 | 147.3±298.9 | 0.441 |
T1DM: type 1 diabetes mellitus; CSII: continuous subcutaneous insulin infusion; CBGM: continuous blood glucose monitoring.
This retrospective study conducted with data collected in standard clinical practice from patients with T1DM using CSII systems, which provide use of BWs, allows for assessing if a relationship exists between actual use of some features incorporated into the new insulin infusion sets and the degree of metabolic control.
A previously reported study by Cukierman-Yaffe et al.5 showed that parameters associated for better metabolic control in patients with D1TM on CSII included both a lower daily number of capillary blood glucose measurements and a greater use of BWs. Our study data show, on the one hand, a great acceptance of the BW system by patients carrying infusion sets incorporating this feature, because this was used for the vast majority (75%) of boluses administered by patients during the study period. These data are similar to those recently reported by Lepore et al.6
On the other hand, as capillary blood glucose data directly downloaded from patient glucometers through the CareLink Pro/Personal platforms perfectly correlate to HbA1c data found during the period, patients with better metabolic control were also seen to make a greater use of BW in this study. In addition, and also in agreement with the abovementioned study, better blood glucose control in this study was associated to a higher daily number of capillary blood glucose measurements.
Several studies previously assessed the specific impact of use of BW on post-prandial blood glucose in both adults7,8 and children,9 showing decreases in post-prandial blood glucose, glycemic variability, and the need for post-prandial correcting boluses. Those data could not be analyzed in this study, but improved glycemic control may be expected to be mainly due to this post-prandial improvement.
An additional study finding was the observation that patients on CBGM concomitant with CSII have a better blood glucose control than those with no CBGM. The HbA1c values were 0.5% lower in patients on CBGM as compared to those not using CBGM. Although this difference was not statistically significant, probably because of the low number of patients with CBGM in the study (n=8), it is consistent with the data recently reported by a multicenter study specifically focused on this issue.10
This study has several limitations. First, it was a retrospective, observational study, and causal relationships cannot therefore be inferred; associations may only be established. Second, the study was conducted at a single site, and extrapolation of data to other sites could therefore be controversial. Moreover, patient sample was small (n=44), and observation time was short (14 days). Finally, availability of data about the impact of BW on the frequency of mild hypoglycemia would have allowed for a better data interpretation (no severe hypoglycemic episodes occurred during the study period). However, the fact that data were directly downloaded from the device and were collected in standard clinical practice conditions allows for a direct vision of the extent of use of these new tools provided by CSII devices and the actual impact they may have on the daily life of patients with T1DM.
To conclude, this study shows that greater use of BW in patients with T1DM treated with CSII tends to be associated to a better metabolic control, and that use of CBGM combined with CSII in standard clinical practice could be associated to improved glycemic control, as seen in previously reported controlled trials.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Quirós C, Patrascioiu I, Giménez M, Vinagre I, Vidal M, Jansà M, et al. Evaluación de la utilización de las prestaciones específicas de los sistemas de infusión subcutánea de insulina y su relación con el control metabólico en pacientes con diabetes tipo 1. Endocrinol Nutr. 2014;61:318–322.