Circadian rhythms (approximately 24h) are widely characterized at molecular level and their generation is acknowledged to originate from oscillations in expression of several clock genes and from regulation of their protein products. While general entrainment of organisms to environmental light–dark cycles is mainly achieved through the master clock of the suprachiasmatic nucleus in mammals, this molecular clockwork is functional in several organs and tissues. Some studies have suggested that disruption of the circadian system (chronodisruption (CD)) may be causal for manifestations of the metabolic syndrome. This review summarizes: (1) how molecular clocks coordinate metabolism and their specific role in the adipocyte; (2) the genetic aspects of and scientific evidence for obesity as a chronobiological illness; and (3) CD and its causes and pathological consequences. Finally, ideas about use of chronobiology for the treatment of obesity are discussed.
Los ritmos circadianos (aproximadamente 24 horas) están ampliamente caracterizados a nivel molecular y su generación se realiza gracias a la expresión de varios «genes reloj» y mediante la regulación de sus productos proteicos. Mientras que la adaptación general de los organismos a los ciclos ambientales de luz-oscuridad se lleva a cabo principalmente por el reloj central del núcleo supraquiasmático, este mecanismo de reloj molecular es funcional en varios órganos y tejidos. Algunos estudios muestran que un fallo del sistema circadiano (cronodisrupción [CD]) puede ser la causa de las manifestaciones del síndrome metabólico. Esta revisión resume, (1) cómo los relojes moleculares coordinan el metabolismo y su papel específico en el adipocito; (2) aspectos genéticos y evidencias científicas de la obesidad como enfermedad cronobiológica, y (3) CD, sus causas y consecuencias patológicas. Finalmente, se discuten ideas sobre el uso de la cronobiología en el tratamiento de la obesidad.
Life is a rhythmic phenomenon. When any life activity is studied over time, oscillations suggesting that these activities do not occur continuously are found. Circadian (from the Latin term circa diem, approximately one day) rhythms are such an innate part of our lives that we rarely pay attention to them.1 The adequate function of these endogenous circadian rhythms allows organisms to predict and anticipate environmental changes, and to temporarily adapt their behavioral and physiological functions to such changes. In humans, current social habits such as sleep time reduction and increasingly irregular day-to-day sleep-wake rhythm (jet lag, shift work, and increased exposure to bright light during the night) or high snack consumption act upon the brain to induce a loss of “perception” of internal and external rhythms.2
There are currently studies suggesting that interruption or internal desynchronization of the circadian system (chronodisruption (CD)) may contribute to manifestations of metabolic syndrome (MS) and complications occurring in obesity such as dyslipidemia, glucose intolerance, endothelial dysfunction, hypertension, type 2 diabetes mellitus, and cardiovascular disease, among others.3,4 Chronobiology (the science that studies changes in individuals over time) is implicated in most of these changes.4 In fact, circadian control of both cardiovascular function5 and hormones involved in metabolism (insulin, glucagon, growth hormone, and cortisol) and obesity (leptin and ghrelin)6 is well known. All of these daily rhythms may be involved in hunger and satiety signals, meal times and finally, the degree of obesity. In a not too distant future, dietary recommendations will maybe include not only what should be eaten and how, but also when it should be eaten. In this report, an attempt will be made to summarize recent discoveries concerning the relationship between chronobiology, etiology, and the pathophysiology of obesity.
Organization of the circadian systemThe circadian system of mammals consists of a network of hierarchically organized structures responsible for the generation of circadian rhythms and for their synchronization with the environment. The main component of this circadian system is a central pacemaker located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Since the endogenous oscillation period of the SCN is not exactly 24h, when subjects are artificially maintained under constant environmental conditions, free-running circadian rhythms with a period slightly different from 24h occur. Under natural environmental conditions, however, the SCN is “readjusted” every day by a periodic light/dark signal thanks to the existence of a nonvisual pathway based on ganglion cells containing the pigment melanopsin and the retinohypothalamic tract. Although light (light/dark changes) is the main input signal to the SCN, there are other periodic inputs, such as meal times (intake/fast) and programmed exercise (activity/rest), which may set the clock of the mammal circadian system. The central pacemaker in turn synchronizes the activity of several peripheral clocks outside the SCN through cyclic hormone secretion and the activity of the autonomous nervous system. The difference between the maintenance of a healthy and unhealthy (CD) temporary internal order depends, among other effects, on the production by peripheral clocks of rhythms ordered by the central pacemaker.
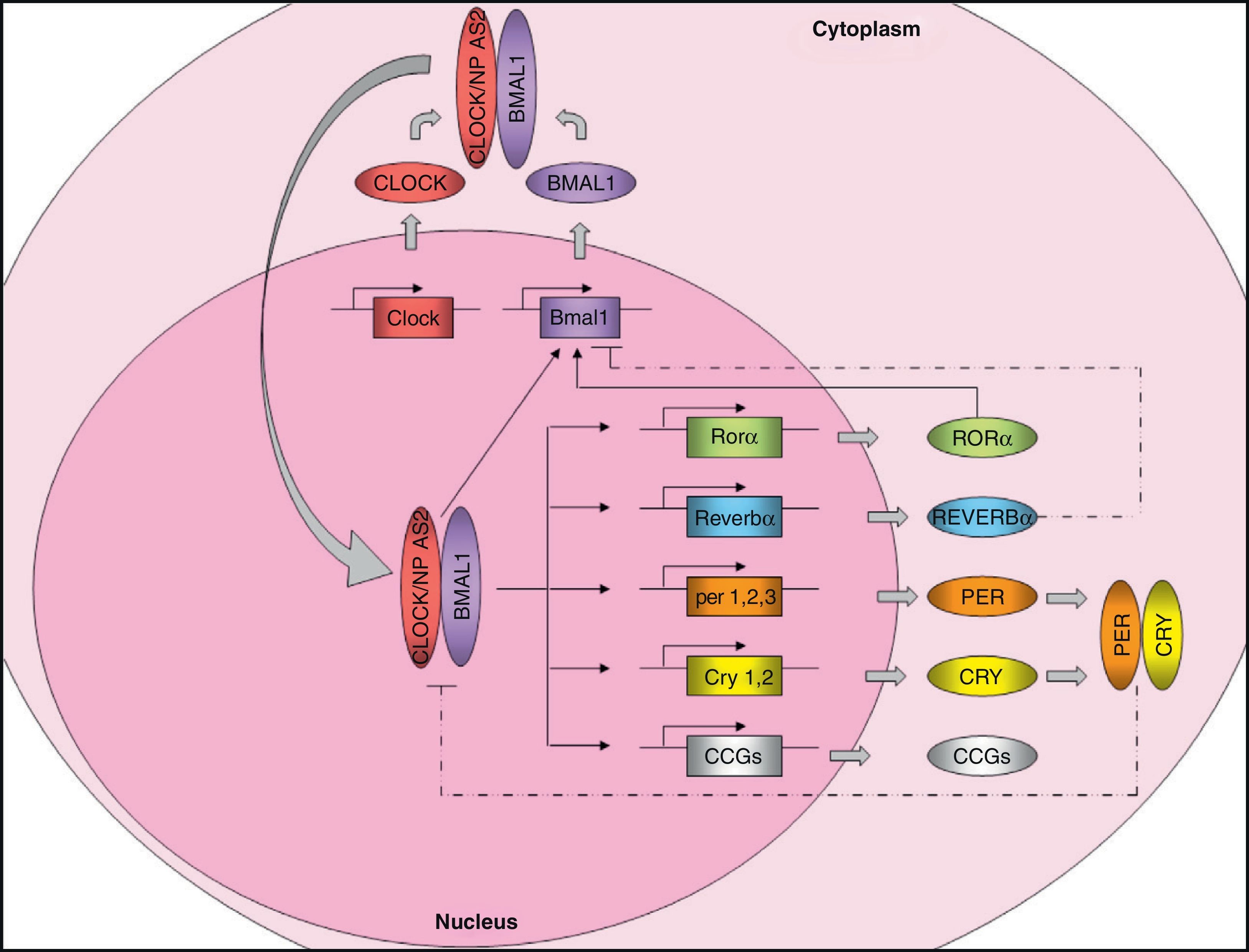
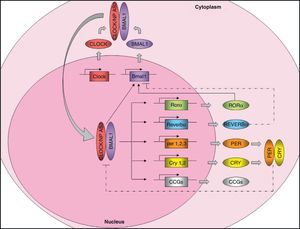
Molecular organization of the central pacemakerAs discussed above, the central pacemaker, located in the hypothalamic SCN, controls the expression of circadian rhythms in mammals and generates 24-h cycles in most physiological and behavioral variables. Circadian rhythm expression is controlled through the so-called “clock genes”. These genes encode for a number of proteins that generate self-regulatory mechanisms using positive and negative transcription feedback loops7 (Fig. 1). On the one hand, there are positive elements, namely CLOCK (circadian locomotor output cycles kaput) and BMAL1 (brain and muscle ARNT-like protein 1), two proteins with a helix-loop-helix structure containing PAS domains (Per-ARNT-Sim, involved in protein-protein interactions). As an alternative to CLOCK, there is a homolog, NPAS2 (neuronal PAS domain protein 2) which can functionally compensate for the absence of CLOCK. The positive elements form the heterodimer CLOCK/BMAL1, which binds to the promoter of several clock genes such as Per1 (period 1), Per2 (period 2), Per3 (period 3), Cry1 (cryptochrome 1), Cry2 (cryptochrome 2), Rev-Erbα (reverse erythroblastosis virus α), Rorα (retinoid-related orphan receptor-α), and other genes controlled by the clock (CCGs, clock-controlled genes) such as the Pparα gene. The transcription product of Pparα, the PPARα protein, induces transcription of Bmal1 and Rev-Ervα by binding to the PPRE domain (PPAR-response elements) present in its promoters and is able to regulate BMAL1 and CLOCK activity. Heterodimer CLOCK/BMAL1 also stimulates Bmal1 transcription, generating a positive feedback loop. Nuclear receptors REV-ERBα and RORα participate in the regulation of Bmal1 expression, inhibiting or activating its transcription, respectively. On the other hand, the negative elements CRY1 and PER2 form the heterodimer CRY1/PER2, which translocates to the nucleus to inhibit CLOCK/BMAL1 activity.8,9 In addition, CKI¿ (casein kinase I epsilon) enhances its instability and promotes its degradation through the phosphorylation of PER.
Molecular machinery of circadian clock. The positive elements CLOCK and BMAL1 heteromerize in cytoplasm to form a protein complex. The heterodimer translocates to the nucleus and binds onto the promoter of certain genes (Per1, Per2, Per3, Cry1, Cry2, Reverbα, Rorα), and many clock-controlled genes (CCGs), controlling their expression. The CLOCK/BMAL1 heterodimer also stimulates Bmal1 expression, forming a positive feedback loop. As an alternative to CLOCK, there is a homolog, NPAS2, which could functionally compensate for the lack of CLOCK. On the other hand, the negative feedback loop is mainly regulated by PER and CRY, which heteromerize in cytoplasm, translocating to the nucleus and inhibiting transcription of CLOCK/BMAL1. Bmal1 expression is also controlled by REVERBα (which inhibits it) and RORα (which stimulates it). Regulation of CCG expression by the circadian clock confers rhythmicity to molecular and physiological processes. Solid lines: stimulation. Dotted lines: inhibition.
The direct participation of the circadian clock machinery in metabolism is currently very well understood today. Approximately 10–30% of genes, depending on the tissue, appear to maintain an expression rhythm guided by clock genes. This occurs with metabolic enzymes (e.g., acyl-CoA oxidase, HMG-CoA synthase) and multiple transport systems such as fatty acid transport protein, sirtuin-1 (SIRT-1), and albumin D-site binding protein (DBP), which show circadian rhythms controlled by the molecular clock and are therefore considered clock-controlled genes (CCGs). Clock proteins such as BMAL1 and other CCG proteins (PPARα and REV-ERBα) are involved in lipid metabolism. BMAL1 and CLOCK are also involved in glucose homeostasis, while CLOCK and PER2 appear to be associated with appetite regulation.
To sum up, there is strong evidence to show that the circadian system influences metabolism and that different metabolic signals in turn induce regulation of this system. SIRT-1 plays a significant role in this bi-directional communication because it contributes to in vivo circadian control by acting as an enzymatic rheostat of circadian function and by translating metabolic signs from the cell to the circadian clock.
Peripheral clocks. Adipose tissue as a modelRecent studies have shown that regulation of the intracellular circadian system does not only lie in the brain. Some peripheral tissues such as the heart, liver, and pancreas, among others, have their own clocks and are able to work autonomously through circadian expression of their “clock genes”, although modulated and synchronized by the central clock.10,11
Adipose tissue, as a peripheral tissue, has clock genes that play an essential role in the physiology of tissue itself, regulating the rhythmic expression of secreted bioactive substances such as adipokines (adiponectin, leptin, and resistin, among others) which affect systemic metabolism. Recent research shows the significance of the adequate functioning of adipose tissue clock genes and the effect of their desynchronization on the development of certain diseases, such as obesity.
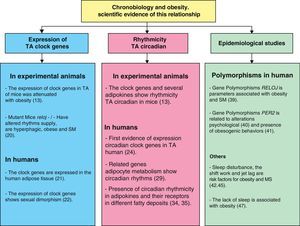
Clock genes in adipose tissue from experimental animal modelsMost studies of clock genes in adipose tissue are currently conducted in experimental animals, especially rodents.12–17 In fact, their expression has been shown to have a circadian rhythmicity in these experimental models, so that the rhythms of positive elements (Bmal1 and Clock) are in anti-phase to the rhythms of negative elements (Per2 and Cry1).12,13,18,19
However, recent studies have shown that circadian rhythms of adipose tissue are altered in different conditions associated with obesity. For instance, several research groups have studied clock genes in adipose tissue from obese mice,13,18–20 and while some authors suggest that the effect of obesity on clock gene machinery is mild,19 others have shown that this condition significantly attenuates or even blocks clock gene expression.13,18 The Ando et al. study,13 which showed that the rhythmic expression of clock genes is attenuated to a different extent depending on the degree of obesity, has been decisive in this regard. Thus, rhythm attenuation is mild in KK (obese) mice, while a significantly higher rhythm flattening occurs in KK-AY mice (with a higher degree of obesity and diabetes). This notion was supported by the Turek et al. study,20 where mutant knockout mice homozygous for Clock had impaired intake rhythms, were hyperphagic and obese, and also developed MS characterized by hyperleptinemia, hyperlipidemia, hepatic steatosis, hyperglycemia, and hypoinsulinemia. This study was crucial for understanding the relationship between obesity and chronodisruption, and has been the basis for subsequent physiological, clinical, and epidemiological studies on obesity, MS, and chronobiology.
Clock genes in human adipose tissueOur research group recently demonstrated, for the first time in humans, that clock genes are expressed at different visceral and subcutaneous adipose locations.21 The associations found between the basal expression of clock genes (PER2, BMAL1, and CRY1) and abdominal fat contents and cardiovascular risk factors suggest that these genes have a relevant role in MS changes and obesity.21 We have also recently noted that sex dimorphism exists in basal clock gene expression in adipose tissue, which is greater in females than males.22 These results agree with those from preliminary studies conducted in mouse liver.23 This sexual dimorphism may account for the different chronotypes of males and females, because various studies have shown that males are more prone to eveningness, while morningness is more common in females.24
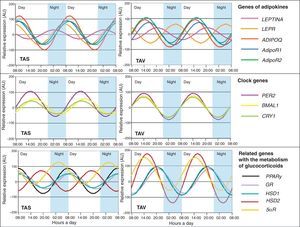
After showing clock gene expression in human adipose tissue at a given time of day, we wondered whether these genes would have circadian behavior and, after a series of cell cultures, we were able to show the periodic nature of these genes in adipose tissue.25 A significant consideration was that the expression rate persisted ex vivo for at least two circadian cycles after surgery. These results agreed with prior studies where circadian expression of clock genes was analyzed in other tissues.26–29 By conducting this study we were also able to show that circadian rhythm may oscillate regardless of SCN and that this oscillating intrinsic mechanism may regulate the clock setting of certain genes implicated in adipocyte metabolism such as PPAR-γ and other genes related to glucocorticoids.25,30 We also showed that these circadian patterns differed between the different fat deposits.25,30 In fact, clock gene expression in visceral adipose tissue was more closely associated with MS characteristics than subcutaneous fat.25,30
Circadian rhythms in adipose tissue metabolismThe involvement of circadian rhythms in glucose and lipid homeostasis is currently well known, and many metabolic factors, including enzymes, transporters, and hormones, are also known to show circadian changes. Recent studies have shown that biological rhythms and metabolism are closely linked. Kennaway et al.31 showed this connection in a study conducted in transgenic mice which showed an interrupted expression of the Clock gene. These animals had non-rhythmic expression of clock genes in liver and skeletal muscle, but preserved rhythmicity in SCN and the pineal gland. Although mice did not develop obesity or increased fatty acid levels, they showed increased plasma adiponectin levels, decreased mRNA of the Glut4 glucose transporter in skeletal muscle, low glucose tolerance, low insulin levels, and decreased gene expression with loss of rhythmicity in enzymes related to hepatic glycolysis and gluconeogenesis. Some studies have also found a strong association between adipose tissue metabolism and circadian rhythm.32,33 During the 24h of the day, the adipocyte must reciprocally adjust triglyceride synthesis (lipogenesis) and storage rates to triglyceride degradation (lipolysis) rates.
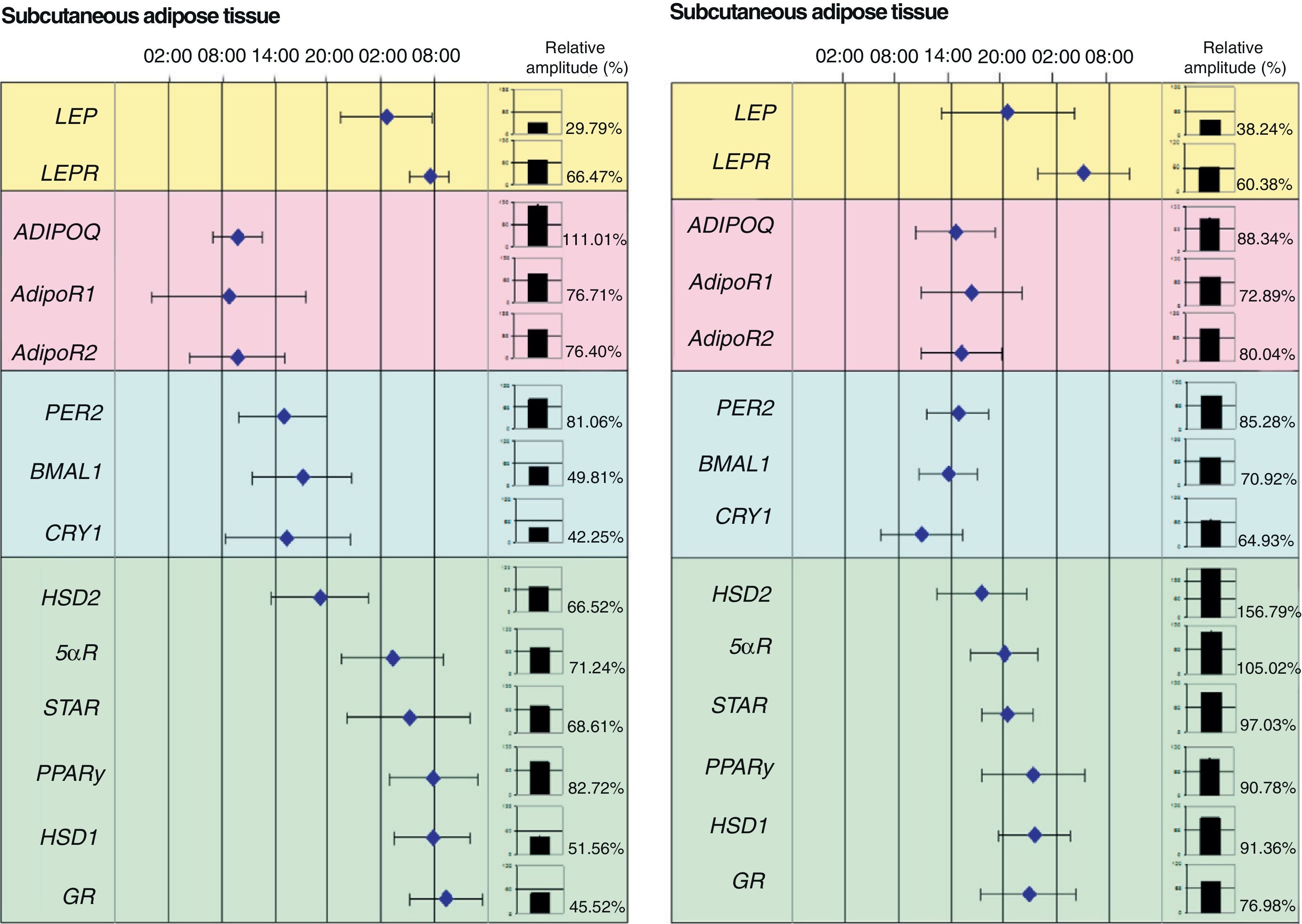
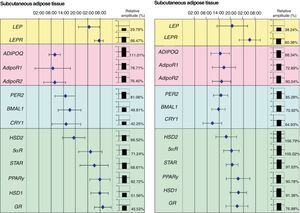
Our research group recently showed the relationship between adipose tissue clock genes and different genes which are involved in metabolism of this tissue. In fact, we were able to show in morbid obese patients that circadian expression of clock genes was related to abdominal fat contents and some cardiovascular risk factors such as plasma LDL (low density lipoproteins), total cholesterol levels and waist circumference. In addition, clock gene expression was directly related to genes involved in adipose tissue metabolism such as PPARγ. In fact, when the acrophases (the time at which gene expression is maximal) of clock genes (PER2, BMAL1 and CRY1), PPARγ, and other genes related to glucocorticoid metabolism (HSD1, HSD2, 5αR, STAR, and GR) were represented on a time scale, they were seen to distribute over time in two markedly different groups: one for the clock genes and the other for genes associated with adipose tissue metabolism (Fig. 2).
Phase map of circadian rhythms of several human genes in subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). The figure shows the acrophases (time at which gene expression is maximal) of the rhythms of several genes involved in metabolism of human adipose tissue. Mean acrophase values are given together with standard deviation. The relative amplitude of each gene is represented in column charts.
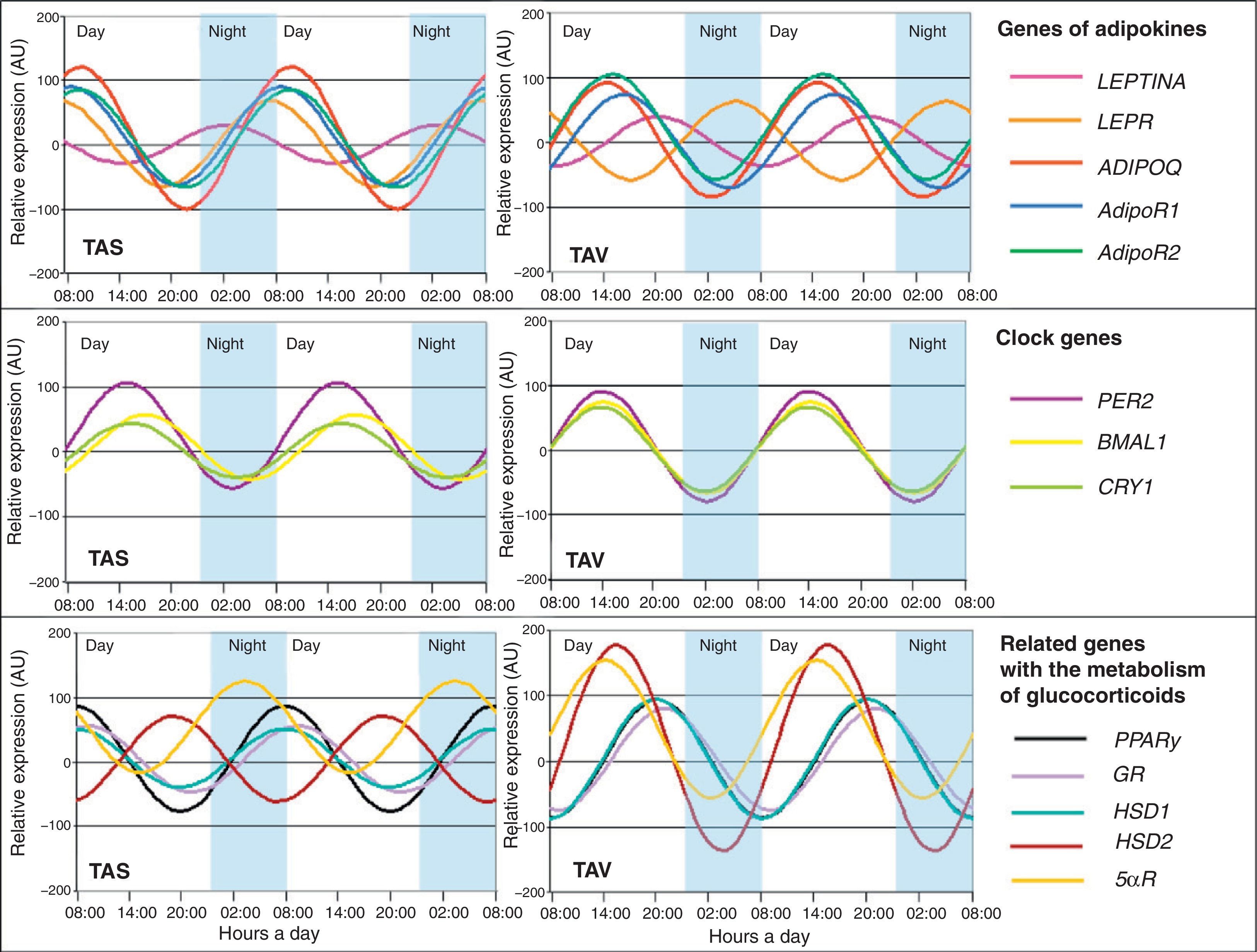
In this regard, we also demonstrated that certain cytokines highly related to adipose tissue show rhythmicity. Adipokine (ADIPOQ), considered to be a protective factor against changes associated with MS, as well as its ADIPOR1 and ADIPOR2 receptors, showed circadian rhythmicity in adipose tissue from patients with severe obesity.34 Adiponectin gene expression fluctuated in the same phase as its receptors (Fig. 3). A correlation analysis of genetic circadian oscillation and MS components showed adiposity and abdominal obesity to be correlated to a decreased rhythm amplitude of adiponectin and its receptors.35 Impaired circadian rhythms of this adipokine could be involved in MS development. We also showed the existence of circadian rhythmicity in leptin, another widely studied adipokine.35 Leptin (LEP), a protein secreted by adipose tissue and strongly involved in intake control, also shows circadian rhythmicity in its expression, as does its LEPR receptor (Fig. 3). Thus, its maximum expression in humans occurs during the night, when there is less appetite and less opportunity for intake.35 These results were to be expected considering that this protein is a potent anorexigenic factor.
Obesity and chronobiology. Genetic aspects and epidemiological evidence for this interactionGenetic evidenceCircadian biology of adipose tissue has been assessed in multiple rodent models having a mutation and/or deficiency in some gene. For instance, mice having a Clock gene mutation are characterized by being obese and not having a rhythmic circadian biology, while Bmal-deficient mice are characterized by having a greater amount of adipose tissue as compared to mice without this deficiency. However, there are conflicting studies in mice suggesting that Bmal1 plays a significant role in the regulation of adipose tissue differentiation, and also in lipogenesis of mature adipocytes.36 As previously noted, the Turek et al. study was the first to show the molecular interaction between clock genes and obesity.20 This study showed that mice with a mutated Clock gene were prone to develop a phenotype similar to MS. Deficiencies in adipokine genes and receptors associated with obesity such as leptin and the melanocortin receptor have also been reported to result in defective circadian rhythms. In addition, interruption of the adipose tissue clock function in ob/ob mice (leptin deficient) was shown to cause in these animals a significant body weight gain, adipocyte hypertrophy, and increased triglyceride and cholesterol levels to a greater extent than in mice with leptin deficiency alone.10
In agreement with these findings, many epidemiological studies in humans show a significant association between various polymorphisms in clock genes and an increased incidence of obesity and MS.37–42 Based on studies by our own and other groups, it may be inferred that some polymorphisms in the CLOCK gene (rs3749474, rs4580704, and rs1801260 (3111T4C)) are of special interest because they are associated with body mass index (BMI), energy intake, and different variables related to obesity.40,43 In fact, our results showed that individuals carrying the gene variants or polymorphisms ate more, slept less, ate more fat, and were more obese. They particularly showed greater abdominal obesity, characterized as being the type of obesity with the greatest metabolic risk. Some of these associations may be functionally explained, such as the CLOCK polymorphism rs3749474, potentially leading to a change in mRNA structure that makes it less viable, so that gene expression loses efficacy. However, one of the most interesting results obtained was perhaps that these associations between gene polymorphism and abdominal obesity or impaired glucose metabolism were only shown in individuals with an unbalanced diet which included a high proportion of saturated fat (factory-made pastries, sausages, and so on) and a low proportion of monounsaturated fat (olive oil). Polymorphism had, however, no effect on subjects with an olive oil (oleic acid) consumption higher than the average. Similar studies with other clock genes have also been conducted. Various studies have shown that the presence of certain PER2 gene polymorphisms was associated with various psychological disturbances, particularly seasonal depression and bipolar disorder.44 This led us to consider whether a sample of overweight or obese patients would have emotional or psychological changes related to obesity and whether such changes would in turn be associated with PER2 gene polymorphisms. Consistent with this idea, our results showed that people carrying the PER2 gene variant showed obesogenic behavior, habits, and emotions, and greater rates of treatment discontinuation, nibbling, diet-induced stress, and food intake as an escape from boredom.45
Epidemiological evidenceMuch greater evidence exists of the relationship between obesity and its chronobiological aspects. One of the most significant findings was that shift work is an independent risk factor for the development of obesity and MS.46 Industrialization has led to continuous 24-h activity in some sectors. This has resulted in an increase in the proportion of the population who routinely does shift work, which is now greater than 20% of all industrial workers. Many epidemiological studies have shown that shift work is associated with a greater prevalence of obesity, hypertriglyceridemia, low HDL levels, abdominal obesity, diabetes, and cardiovascular disease.47 Shift workers also show, as a postprandial metabolic response, increased plasma glucose, insulin, and triglyceride levels which are associated with impaired melatonin circadian rhythmicity.48 In addition to shift work, interesting results have been found by relating sleep disorders caused by jet lag to obesity. Jet lag is not a disease in itself, but is able to alter the normal function of the circadian system. This is why insomnia caused by jet lag and/or shift work is related to obesity and eventually results in significant social problems requiring medical care.49
Other studies show an association between sleep time and metabolic risk. Various clinical studies have shown that healthy subjects who restrict sleep time to 4h for 6 consecutive nights have an impaired glucose tolerance and a decreased insulin response leading to increased plasma glucose levels.50 In addition, people who sleep little have decreased circulating leptin (an anorexigenic hormone) levels and increased ghrelin (anorexigenic hormone) levels.51 This situation is particularly relevant in children who sleep little, and the effects of such sleep shortening appear to vary depending on the day of the week or the season, or are even different in children who have younger siblings. This lack of sleep in children has been reported to be an independent risk factor for obesity.52
When the circadian system fails: chronodisruptionA new term, “chronodisruption” (CD) or circadian interruption, has been introduced in recent years in the science of chronobiology. Chronodisruption may be defined as a significant disturbance in the internal temporal order of physiological, biochemical, and behavioral rhythms. It may also be defined as a rupture of synchronization between internal circadian rhythms and environmental 24-h cycles.53
In our modern society (24h/7 days), CD occurs as the result of not only several already discussed conditions such as jet lag and shift work, but also because of other conditions such as light contamination at night or the preference for doing leisure activities at night. The effect of CD on human health has become relevant in recent years. Current evidence suggests that CD is closely associated with an increased risk of developing certain diseases or of worsening preexistent conditions such as premature ageing, cancer, and cardiovascular diseases and, as shown in previous sections, with obesity and MS.
Premature ageingCircadian system function is affected by age. In the elderly, circadian rhythms have been shown to be characterized by an anticipated phase, a reduced amplitude, an impaired resynchronization ability following a time change, and an internal desynchronization between different rhythms.54 Some studies also suggest that CD has a direct effect upon the acceleration of ageing. For example, it has been noted that a change of 6h/week in the light–dark cycle caused in aged mice a significant shortening of their lives. Circadian rhythm interruption thus appears to reduce life expectation, while adequate circadian system function allows for an increased longevity.
CancerStudies conducted on both animals and humans have documented that one of the consequences of CD is cancer start and development.55 In fact, a relationship between CD and cancer diagnosis has been studied in humans. In patients with colorectal cancer, clearly marked rhythms were associated with better quality of life, improved response to chemotherapy, and greater survival.56
Cardiovascular diseaseThe normal (dipper) circadian blood pressure pattern is characterized by lower blood pressure levels during the night and a peak value in the morning upon waking up. Thus, we now know that the best indicator for predicting the risk of myocardial infarction is the presence of elevated blood pressure values during the night.57 In fact, hypertensive patients with a normal blood pressure reduction during the night have a risk of cardiovascular mortality similar to normotensive subjects.58 There are cases where blood pressure values do not decrease as much as expected during the night and are similar to daytime levels. This is the so-called non-dipper blood pressure pattern. This pattern has been shown to be characteristic of shift workers or elderly people with altered circadian rhythms.
Obesity and MSOne of the well-known effects of CD on human health is the development of obesity and MS. As discussed in this review, epidemiological studies have shown that shift work, sleep deprivation, and a shift to nighttime feeding are associated with a high risk of suffering obesity and MS.4 Many of the circadian system functions related to metabolism, such as metabolic regulation of lipids and glucose, or insulin response, may be impaired by CD, so contributing to the pathophysiology of obesity.
Causes of chronodisruptionChronodisruption may be the result of changes at different levels. It may result from impairment in inputs to the central oscillator or synchronizers, such as light–dark cycles or meal times; central oscillator (SCN) failures; or problems in outputs related to melatonin and glucocorticoids.
InputsIt has been noted that light deficiency or a light intensity or spectrum below optimal ranges may contribute to the occurrence of pathological signs related to CD.59 Changes in meal times, considered as one of the most important external synchronizers, are also a significant factor in CD. For example, subjects with a nocturnal lifestyle, characterized by late dinner and nibbling during the night, have hyperglycemia and low leptin and melatonin levels during the night.60 A nocturnal lifestyle is currently considered to be one of the main risks for health in modern society, in addition to night eating syndrome, obesity, and diabetes.
Central oscillatorCD, typical of conditions such as jet lag or shift work, is caused by differences in the synchronization rates of the different biological variables. In fact, such desynchronization results from a phase difference between SCN rhythms and rhythms produced by peripheral tissues. CD may, however, also be caused by a change in the molecular machinery of the central clock, such as the impaired Clock gene in mutated mice associated with obesity or, for instance, BMAL1 changes which have been related to obesity or PER2 changes associated with various cancers and psychological conditions.
OutputsThe third element that may cause CD is impairment in circadian system outputs including, among others, the abovementioned melatonin.61,62 Melatonin is secreted in response to the sympathetic action of the SCN. It is also a known antioxidant which causes the release of free radicals.63 The antioxidant action of melatonin is associated with the induction of antioxidant enzyme expression/activity and to the improved function of the mitochondrial electron transport chain.64,65 It has also been associated with changes related to obesity, by acting as a protective hormone. In fact, melatonin reduces blood pressure and improves glucose metabolism.66
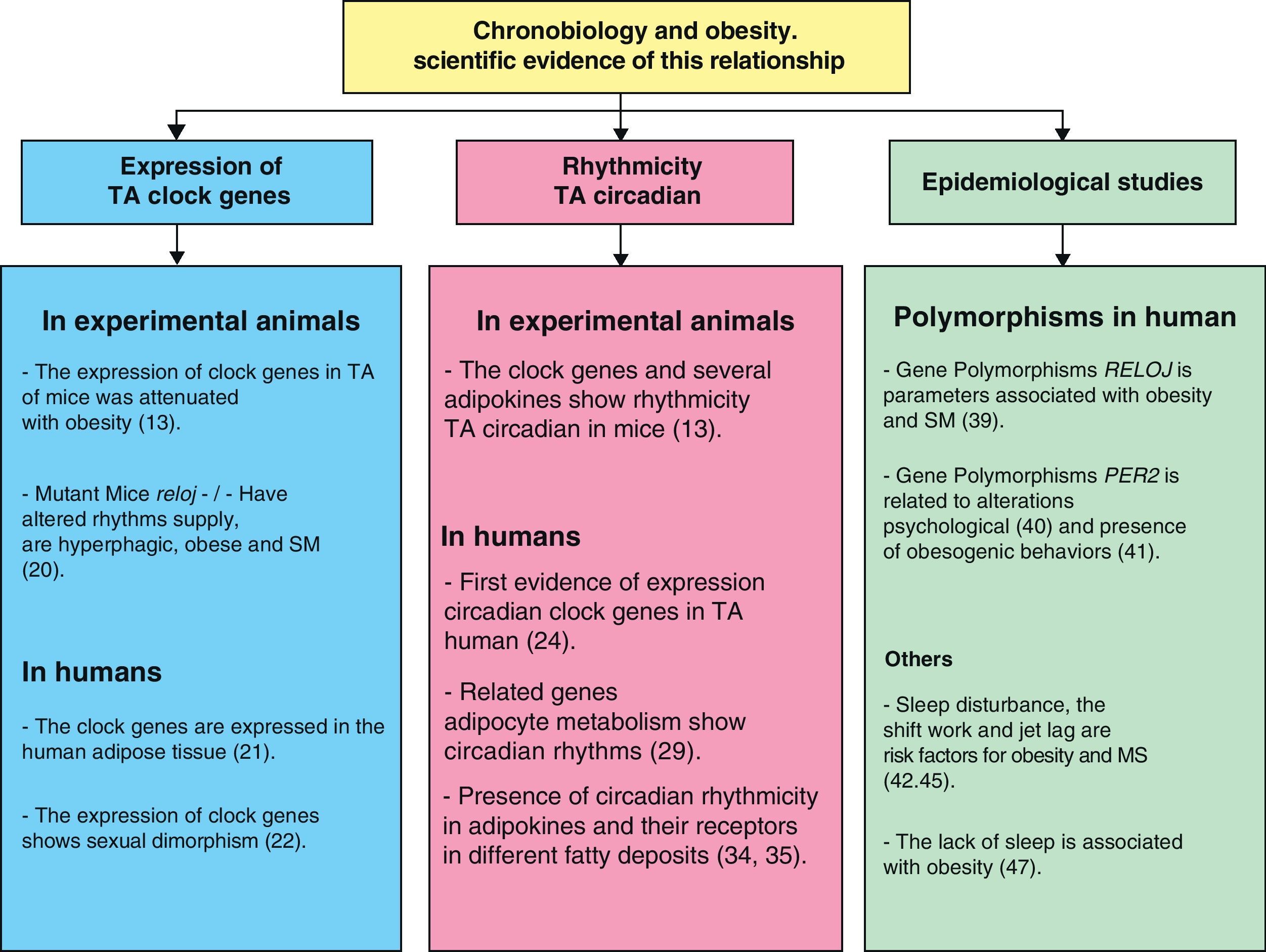
Chronobiology in management of obesity and metabolic syndromeScientific evidence showing the relationship between chronobiology and obesity (Fig. 4) has been discussed in this review. We may thus infer that chronobiology could be a valuable tool for the future management of obesity and MS using chronotherapy. Based on the foregoing, three intervention levels regarding the functional organization of the circadian system need to be taken into consideration in order to establish a treatment based on this science: inputs to the central oscillator, the central oscillator itself, and outputs from it.
At this level, it is essential to achieve regular light exposure and optimal sleep regulation to achieve an adequate rhythm of the sleep-wake cycle. Certain studies have shown that sleep regulation for 4 weeks in a group of adult people significantly improves quality of life.67 Regular meal times are also important. It has been noted that individuals with nighttime feeding have a greater body weight increase as compared to those with an adequate intake distributed all over the day.68 Regular physical activity is also a good measure to be considered for the treatment of obesity. Epidemiological studies show that regular, long-term exercise is associated with improved sleep quality during the night and with decreased fatigue during the day.68
Central pacemakerThe chronobiological characterization of an individual in this regard could be of interest for predicting susceptibility to obesity and weight loss. This would consist of the identification of various polymorphisms in clock genes, which would give information regarding response capacity to strategies for achieving weight loss using low-calorie diets. In this regard, our research group has shown that a CLOCK gene polymorphism (rs1801260) may help predict the resulting body weight reduction in patients attending a nutrition clinic for overweight or obesity.40 These new strategies may represent a step towards individualized medical care and nutrition aided by the chronobiological characterization of the subject.
OutputsPharmacological solutions, such as the administration of melatonin agonists (ramelteon and agomelatine), may exist. This effect is possible because these agents improve melatonin circadian rhythmicity by inducing sleep and improving depressive states without the immune system stimulation that would cause melatonin administration.69 There may be, however, other non-pharmacological solutions, characterized by cortisol rhythm regulation. Circadian glucocorticoid rhythms are considered a key factor in the synchronization of peripheral clocks. Normal cortisol rhythm is characterized by a peak upon waking up and a decrease during the rest of the day. According to Van Someren et al.,70 a normal cortisol rhythm may be achieved by establishing regular waking up and meal times and regular and adequate light exposure and physical exercise. Such behavioral interventions may have a direct effect on obesity and particularly on abdominal obesity, both of them related to an altered glucocorticoid rhythmicity.71
Although no experimental studies expressly focusing on the chronobiological treatment of obesity are available, the close relationship between obesity, MS, and chronodisruption suggests that any pharmacological, dietary, and behavioral treatment that improves circadian system function may help to reduce the risk of obesity and improve treatment success.
Overall conclusionThe circadian system and its relationship to obesity have been discussed in this review, emphasizing the main aspects of this new, relevant area of knowledge. We have reported the bases for understanding the circadian system, and have discussed how the inadequate function of this complex system (chronodisruption) increases the risk of developing pathological conditions such as obesity, cardiovascular disorders, and metabolic syndrome. Modern society and its inherent characteristics, such as stress, disordered times, lack of sleep, among other factors, cause circadian system disruptions which are associated with the recent increase in metabolic diseases. An interesting aspect is that these circadian rhythms are unique to each individual and are modulated by genetic factors. Recent studies on clock gene polymorphisms and their interactions with diet open a new door to the development of new therapies based on chronotherapy and nutrigenetics for the treatment of obesity and other associated conditions. An important application would therefore be based on individual chronobiological characterization in order to implement adequate treatment. In addition, the use of drug therapies intended to restore normal circadian rhythms, as well as regular exposure to environmental synchronizers such as light, meal times, and physical exercise could be a solution to desynchronization of the circadian system.
FundingThis study was funded by project BIO/FFA 07/01-0004 of the Autonomous Community of Murcia and project AGL2008-01655/ALI of the Spanish Ministry of Education and Science.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please, cite this article as: Gómez-Abellán P, et al. Aspectos cronobiológicos de la obesidad y el síndrome metabólico. Endocrinol Nutr. 2012;59:50–61.