There is a controversy among different scientific societies in relation to the recommendations on whether universal screening for the detection of thyroid dysfunction during gestation should be performed or not. Although various studies have shown an association between subclinical hypothyroidism or hypothyroxinemia with obstetric problems and/or neurocognitive impairment in the offspring, no evidence on the possible positive effects of treatment of such conditions with thyroxin has been demonstrated so far. However, there is a general agreement about the need for treatment of clinical hypothyroidism during pregnancy and the risks of not doing so. Because it is a common, easily diagnosed and effectively treated disorder without special risk, the working Group of Iodine Deficiency Disorders and Thyroid Dysfunction of the Spanish Society of Endocrinology and Nutrition and Spanish Society of Gynaecology and Obstetrics recommends an early evaluation (before week 10) of thyroid function in all pregnant women. Given the complex physiology of thyroid function during pregnancy, hormone assessment should be performed according to reference values for each gestational trimester and generated locally in each reference laboratory. Thyrotropin determination would be sufficient for screening purposes and only if it is altered, free thyroxin or total thyroxin would be required. Adequate iodine nutrition is also highly recommended before and during pregnancy to contribute to a normal thyroid function in the pregnant women and fetus.
Existe una conocida controversia entre distintas sociedades científicas respecto a la recomendación o no de que se realice un cribado universal para la detección de disfunción tiroidea (DT) durante la gestación. Aunque varios estudios asocian el hipotiroidismo subclínico o la hipotiroxinemia con problemas obstétricos y/o con alteraciones neurocognitivas de la prole, no hay evidencia sobre los posibles efectos positivos de su tratamiento con tiroxina. Sin embargo, existe un acuerdo generalizado sobre la necesidad del tratamiento del hipotiroidismo clínico durante la gestación y los riesgos que podría ocasionar la abstención terapéutica. Por tratarse de una enfermedad frecuente, de fácil diagnóstico y para la que se dispone de un tratamiento efectivo y exento de riesgos, la Sociedad Española de Endocrinología y Nutrición (Grupo de Trabajo de Trastornos por Deficiencia de Yodo y Disfunción Tiroidea) y la Sociedad Española de Ginecología y Obstetricia recomiendan que se evalúe precozmente (antes de la semana 10) la función tiroidea a todas las mujeres embarazadas. Dada la compleja fisiología de la función tiroidea durante la gestación, la valoración de las hormonas debe realizarse utilizando valores de referencia para cada trimestre y para cada zona con las técnicas de laboratorio propias. Para el cribado, bastaría con la determinación de tirotropina y solo si esta está alterada, debería analizarse también la tiroxina libre o total. Debe recordarse también que una adecuada nutrición de yodo desde antes y durante el embarazo es fundamental para contribuir a la normalidad de la función tiroidea materno-fetal.
Because of the potential significance of maternal thyroid disease for the mother and fetus, the working group of the Spanish Society of Endocrinology and Nutrition (SEEN) on Iodine deficiency disorders and thyroid dysfunction and the Spanish Society of Gynecology and Obstetrics (SEGO) considered appropriate, in addition to emphasizing the importance of adequate iodine intake during pregnancy, to address the controversial subject of the universal screening of thyroid function and autoimmunity in the pregnant population.
In the past 20 years there has been a significant increase in our understanding of thyroid disorders and pregnancy with relevant clinical implications, including:
- -
A better understanding of the significant adaptive effects of maternal thyroid status as the result of pregnancy.1
- -
The impact of even mild to moderate iodine deficiency on the mother, pregnancy outcome and, particularly, the developing fetus.2
- -
A better understanding of the tests that assess thyroid function/autoimmunity in pregnancy in order to measure normal values during pregnancy.
- -
The adverse effects of different degrees of maternal thyroid hypofunction and the time of their detection during pregnancy on pregnancy outcome and the neuropsychological development of the child.3
- -
The optimal treatment for pregnant women who are administered levothyroxine (LT4) before conception and the need for increased doses from the first weeks of pregnancy.4,5
- -
The influence of positive thyroid autoimmunity, even without thyroid dysfunction (TD), on fertility and the course of pregnancy.6
These advances in our understanding of the thyroid gland, TD, and thyroid autoimmunity have represented the scientific basis upon which the need to consider the convenience of conducting a comprehensive study of thyroid function and autoimmunity status in all women of childbearing age, either when they want to conceive or in an early stage of pregnancy, has arisen.
In the absence of unequivocal evidence, other considerations need to be taken into account in any discussions concerning the establishment of a universal screening strategy:
- -
Most common disorders (autoimmunity/dysfunction) affecting the thyroid and pregnancy do not usually cause symptoms or signs. Diagnosis is made by chemical tests.
- -
Subclinical hypothyroidism (SCH), excluding asymptomatic positivity of antithyroid antibodies, is the most common thyroid disorder in pregnancy, and any consideration of thyroid function screening during pregnancy will depend on the clinical impact of this condition and on the potential benefits of its treatment.
- -
Maternal thyroid status experiences significant physiological and adaptive changes. Peripheral levels of thyroid hormones and thyroid-stimulating hormone (TSH) change during pregnancy, and their specific normal limits depending on gestational age should be established for adequate interpretation, particularly of subclinical thyroid disease.
The following dichotomy exists in TD screening strategy for the pregnant population: universal screening versus selective screening. The different relevant positions are discussed below:
- 1.
Universal screening before conception or at the first prenatal visit and the evaluation of women with abnormal thyroid function tests.In clinical hypothyroidism (CH), unanimity exists as to the need and importance of its treatment,5,7 although this was questioned in a recent study by Lazarus et al.8 To date, various authors have thought that although thyroid function screening could be recommended,9 there were no conclusive data showing that the treatment of women with SCH with LT4 was beneficial and free of risk.9,10 Both the American College of Obstetricians and Gynecologists11 and the American Thyroid Association (ATA) guidelines12 agree with this view and state that universal screening of thyroid dysfunction and/or autoimmunity should not be performed in this population. However, in 2009 the SEEN working group on TD issued guidelines that clearly supported thyroid function screening before or in the first trimester of pregnancy.13 A recent editorial agreed with this view.14
- 2.
Screening in cases at high risk for TD only.Various scientific societies, including the ATA and the American Association of Clinical Endocrinologists (AACE), recommend the identification, careful monitoring and treatment, if appropriate, of women at risk of clinical or subclinical TD before conception or in early pregnancy.12,15 Poppe et al. stated that systematic testing could be more efficient in women at high risk, particularly infertile.6 However, if only high risk pregnant women were to be tested, approximately one-third of women with hypothyroidism would be undiagnosed.16 According to the ATA, the risk factors include12: (a) a history of TD, goiter, positive antithyroid antibodies, cervical radiation, or thyroid surgery; (b) age >30 years; (c) a family history of thyroid disease; (d) clinical signs or symptoms of hypothyroidism (although these usually occur in only 30% of cases); (e) type 1 diabetes mellitus or any other autoimmune disease; (f) history of miscarriage or abortion, prematurity, or infertility; (g) morbid obesity; (h) treatment with lithium or amiodarone, or the recent administration of iodinated contrast; and (i) living in an area with moderate to severe iodine deficiency.
Screening is defined as the systematic, active identification of a health problem not recognized by the rapid performance of tests, examinations, or other procedures. It should be stressed that screening tests are performed in apparently healthy people and that they are not intended to be diagnostic. Thus, positive results require subsequent confirmation. Population screening refers to the large scale screening of populations or complete population groups. Screening is a medical investigation that is not the result of the search for help by a patient because of a specific health problem and which assumes that the early detection of disease, or identification or risk, is beneficial for the individual and the community. It is a secondary prevention strategy consisting of the early detection and appropriate management of the health problem in order to reduce its morbidity, mortality and sequelae.
In 1968, the World Health Organization issued a document outlining the principles to be followed by a screening program17 (Table 1) and these continue to be applicable from the viewpoint of public health policies. The 10 principles established were subsequently grouped under different criteria18 which refer to three aspects: the disease or health problem (severity, high prevalence of the preclinical phase, an adequate understanding of its natural history, and the time from the occurrence of the first signs to frank disease), the screening test (valid; simple and inexpensive; safe and acceptable; and reliable), and problem diagnosis and treatment (the availability of adequate resources and accessible, effective, acceptable, and safe treatment). The decision to use screening should be based on the evaluation of each of the above criteria. Disease significance is assessed based on its frequency and severity,19 so that screening for highly prevalent conditions with severe consequences may be considered more appropriate. On the other hand, the early identification of a disease requires the existence of a detectable presymptomatic stage and the availability of tests (biological markers, X-ray examinations, etc.) allowing for such detection. A good understanding of the natural history of the health problem, especially of the extent of its preliminary stages (pathological versus physiological changes, progression), is therefore essential so as to allow for timely intervention. As regards the screening test itself, it has to be valid (sensitive for correct identification of those affected and specific for identification of those not affected), accurate, and reliable. It should therefore have a good capacity to discriminate people with the health problem from those who do not have the problem. In addition to its technical characteristics, the screening test should be accepted by the population. Acceptance depends on the nature of the risk associated with the disease and on the health education level of the population. However, the test should be easy to perform and cause few problems and virtually no discomfort or inconvenience.
Principles of screening.
| 1. The disease must be an important health problem |
| 2. There should be an accepted treatment for patients in whom the disease is identified |
| 3. Facilities for diagnosis and treatment should be available |
| 4. The disease should have a latent stage or detectable early symptoms |
| 5. A valid screening test should be available |
| 6. The screening test should be acceptable to the population |
| 7. The natural history of the health problem, from the latent stage to frank disease, should be known and understood |
| 8. There should be agreement on the treatment of patients |
| 9. The cost of screening (including the diagnosis and treatment of identified patients) has to be balanced with the cost of general health care devoted to the problem |
| 10. Screening should be conceived as a continuous process, rather than as a single project conducted only once |
The ability to adequately manage the health problem once identified is perhaps the most important criterion when screening is considered. Thus, if no treatment is available for the initial stages of the disease that may change the course and prognosis of the condition, screening should not be considered. Timely and adequate management includes a prior agreement on the treatment regimen to be followed by patients with a clear diagnosis and a monitoring strategy for those with borderline diagnosis. Finally, the availability of resources refers to the material and human means needed to perform screening and to appropriate healthcare services in order to verify the findings and treat people in whom a suspected diagnosis is confirmed. This is because early disease detection should lead to a substantial improvement in the health of the population and to a favorable economic balance in both cost–benefit and cost–effectiveness ratios.
Universal screening of thyroid function during pregnancyIt is not easy to reach a consensus in this particular case, because several (rather than one) thyroid-associated diseases occur during pregnancy. Moreover, their potential impact on pregnancy may be very heterogeneous depending on the cause and/or severity of the dysfunction. A simplistic approach would be to state that “thyroid abnormalities are common in women of childbearing age, are easily detected with a simple TSH measurement and, when identified, are adequately treated with a safe and inexpensive thyroxine supplement”.20 However, TD evaluation in pregnant women is a more complex subject. Following the criteria of Beaglehole et al.,18 the following questions may be asked:
Is TD during pregnancy a health problem? Are simple and reliable diagnostic tests available? Is universal screening profitable from the cost-benefit viewpoint? Who should undergo screening for TD and when and how? Is there a simple, no risk treatment for which solid evidence is available?
Arguments in favor of universal screeningThyroid dysfunction during pregnancy as a health problemPrevalence of thyroid dysfunction in pregnancyThe limited data available on the prevalence of hypothyroidism in healthy pregnant Spanish women21–23 show a SCH rate of 4.6–6.4% and a CH rate of 1.6%.24 Another study reported a SCH prevalence ranging from 6.5% to 9.9%.25 In the international medical literature, similar rates of clinical and subclinical TD (hypothyroidism and hyperthyroidism) have been reported,16,26,27 wherein from 5.7% to 11.8% of women will experience some change in thyroid function during pregnancy. According to Stagnaro-Green et al., the prevalence of CH may be up to 0.5% in women of childbearing age,28 but a 2.5% prevalence of CH has recently been reported in a large population of pregnant women in the US.29 Maternal hypothyroxinemia (decreased serum levels of free thyroxine [FT4] in plasma with normal TSH) is approximately 150 times more common than congenital hypothyroidism3 and represents a thyroid hypofunction state whose most common cause is nutritional iodine deficiency.30 On the other hand, 5–14% of healthy pregnant women have positive antiperoxidase (TPO) and/or antithyroglobulin antibodies,6,31 which will be associated with higher TSH and lower FT4 levels.32 As regards hyperthyroidism, the prevalence of Graves’ disease may range from 0.1% to 1%,33 while the prevalence of the syndrome of gestational hyperthyroidism may range from 1% to 13%.34
Obstetric complications associated with thyroid dysfunction- -
Infertility: a known association exists between hypothyroidism and decreased fertility. However, recent studies show that hypothyroidism does not prevent pregnancy.6,35 In addition, a high proportion of women with CH or SCH not treated with thyroxine become pregnant.36
- -
Miscarriage/intrauterine fetal death: a high association has been reported between thyroid autoimmunity and increased miscarriage rate, endometriosis, and ovarian failure.6 Assisted reproduction success rates are reduced in the presence of thyroid autoimmunity with elevated TSH.37 In advanced pregnancy, the risk of intrauterine fetal death is increased in pregnant women with TSH>6mIU/L (odds ratio 4.4),38 and the overall risk of miscarriage clearly increases in the event of HC or SCH.39–41
- -
Restricted intrauterine growth: a positive correlation exists between the degree of maternal hypothyroidism (subclinical or frank) and the restriction of intrauterine fetal growth.42–44
- -
Hypersensitive states in pregnancy: a correlation has been found between TSH and endothelin levels in pregnant women, commensurate with the severity of preeclampsia/eclampsia.45–47
- -
Detachment of a normally situated placenta: relative risk of 3 in SCH.26
- -
Prematurity: strongly associated with clinical and/or subclinical hypothyroidism.48,49
In 1999, Haddow et al.7 noted that children born to women with undiagnosed hypothyroidism (TSH above the 90th percentile) during pregnancy had at 9 years of age significantly lower scores in tests related to intelligence, attention, speech, reading skills, school achievement, and visual-motor performance. Other studies have related SCH, hypothyroxinemia, or thyroid autoimmunity in mothers to poorer results of their offspring in intelligence and psychomotricity tests.50 The effect of maternal hypothyroxinemia has recently generated a wide debate about its implication in the neuropsychological development of the offspring. While various authors51–53 showed that “maternal hypothyroxinemia” (FT4<10th percentile) in the first trimester, with no SCH, was associated with a decreased neuropsychological development in the offspring, a recent study by Craig et al.54 found no difference. However, as far back as 2000, Morreale de Escobar et al. reported epidemiological and experimental evidence which strongly suggested that hypothyroxinemia in the first trimester (with or without increased TSH) increased the risk of poor neuropsychological development in the offspring.3 The delay occurs due to a decreased bioavailability of maternal thyroxine to the developing fetal brain.25,55 The rigorous method used by Berbel et al. to select their study population for the assessment of neuropsychological parameters would clearly support the effect of hypothyroxinemia on such parameters.25
Detectable early stageThe thyroxine requirements in women with known hypothyroidism increase in pregnancy,56 and their thyroxine doses must therefore be increased between 30% and 50% from the time pregnancy is confirmed. A 6-10-week delay in the supplementation required (iodine and/or thyroxine) at the start of pregnancy increases the risk of delayed neurodevelopment in the offspring.25 Thus, it is also important to ensure adequate iodine intake during pregnancy (250μg/day).12,57 In addition, a good nutritional iodine status, related to the use of iodinated salt for long periods before pregnancy, may decrease the risk of TD during pregnancy.58 In our environment, iodinated salt and milk consumption59 may contribute to good iodine intake. In populations with iodine deficiency during pregnancy and lactation, supplementation with 150-200μg of iodine guarantees achievement of the recommended intake.
Considerations about screening for thyroid dysfunction in healthy pregnant womenSociety, and more specifically pregnant women, demands a comprehensive analysis of anything related to fetal development.60 The possibility of detecting pregnant women at risk of developing TD during pregnancy and therefore preventing potential neurological sequelae in their offspring is no longer an exclusive debate of scientific societies and has begun to attract the general interest of the US population.61 Thyroid function testing in the first trimester of pregnancy requires a single blood measurement, which could be done at the same time as all other laboratory tests, and does not require other additional tests or examinations requiring specific training by the obstetrician.
As regards tests that may be used for screening, TSH level continues to be a very sensitive marker of thyroid function during pregnancy,14,62 even taking into account the effect of beta-human chorionic gonadotropin (β-HCG), especially in the first trimester, when a decrease in TSH is induced, and reference values (RVs) are therefore also lower.63 Accurate measurement of maternal FT4 during pregnancy with the commonly used immunoassay methods has some difficulties. This is due to the interference caused by changes in plasma proteins during pregnancy. However, it has recently been shown that some FT4 immunoassays64 may provide a good approximation to the standard provided by tandem mass spectrometry, which has in turn been shown to have a very good correlation to equilibrium dialysis, considered as the gold standard procedure, in the different trimesters of pregnancy.65 The measurement of total T4 (TT4), performed using more robust methodology than that used for the measurement of FT4, has also been used. Changes in TT4 during pregnancy are more predictable, and it appears that RVs established in different populations are more comparable and probably more reliable than those of FT4. A rapid calculation to obtain the RV of TT4 in pregnant women has been proposed, consisting of multiplying by 1.5 the RV of the non-pregnant population.63 The FT4 index, which has fallen into disuse, is a good method in situations where thyroxine binding globulin levels are impaired, as occurs in pregnancy.66
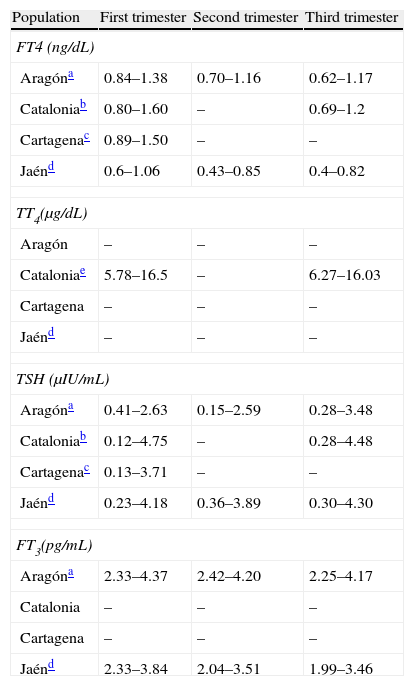
Because of the significant changes in thyroid hormone levels occurring during pregnancy,67 measurement of RVs should not only be performed for each trimester of pregnancy, but also for each area/population68 and using the routine procedures at each center, particularly for FT4. Only four studies reporting the RVs of their respective populations (Table 2) conducted in Aragón,31 Cartagena,69 Catalonia,70 and Jaén71 have been published. The recent ATA guidelines assume that, in the absence of local RVs, the cut-off points for TSH will be established at 2.5μIU/mL for the first trimester and 3μIU/mL for the second and third trimesters.12 The cut-off point of 2.5μIU/mL was selected not only because it approximates to the 97.5th percentile, but also because a greater morbidity has been shown above this value.5,72
References values of thyroid hormones in different Spanish populations.
| Population | First trimester | Second trimester | Third trimester |
| FT4 (ng/dL) | |||
| Aragóna | 0.84–1.38 | 0.70–1.16 | 0.62–1.17 |
| Cataloniab | 0.80–1.60 | – | 0.69–1.2 |
| Cartagenac | 0.89–1.50 | – | – |
| Jaénd | 0.6–1.06 | 0.43–0.85 | 0.4–0.82 |
| TT4(μg/dL) | |||
| Aragón | – | – | – |
| Cataloniae | 5.78–16.5 | – | 6.27–16.03 |
| Cartagena | – | – | – |
| Jaénd | – | – | – |
| TSH (μIU/mL) | |||
| Aragóna | 0.41–2.63 | 0.15–2.59 | 0.28–3.48 |
| Cataloniab | 0.12–4.75 | – | 0.28–4.48 |
| Cartagenac | 0.13–3.71 | – | – |
| Jaénd | 0.23–4.18 | 0.36–3.89 | 0.30–4.30 |
| FT3(pg/mL) | |||
| Aragóna | 2.33–4.37 | 2.42–4.20 | 2.25–4.17 |
| Catalonia | – | – | – |
| Cartagena | – | – | – |
| Jaénd | 2.33–3.84 | 2.04–3.51 | 1.99–3.46 |
Values reflect the 2.5th and 97.5th percentiles respectively.
FT3: fetal triiodothyronine; TSH: thyroid-stimulating hormone; FT4: free thyroxine; TT4: total thyroxine.
Tested by chemiluminescence immunoassay (Architect i2000, Abbott Diagnostics). Calculation made in the population with negative antithyroid antibodies. Iodine intake of the population not provided. Source: Bocos-Terraz et al.31
Tested by chemiluminescence immunoassay (ADVIA Centaur®, Bayer). Calculation made in the population with negative anti-TPO antibodies. Median urinary iodine levels in the first trimester were 163μg/L. This study included the value of the 10th percentile for FT4 (0.86μg/d in the first trimester and 0.80μg/dLin the third trimester). Source: Vila et al.70
Tested by chemiluminescence immunoassay (RocheDiagnostics). Calculation made in the population with negative antithyroid antibodies. Iodine intake of the population not provided. Source: García de Guadiana Romualdo et al.69
Chemiluminescence immunoassay (Beckman Access, Immuno Assay System). Women with positive antithyroid antibodies were excluded. Median urinary levels were 109.85μg/L in the first trimester and, after supplementation, 179μg/L in the second trimester and 181.78μg/L in the third trimester. Source: Santiago et al.71
Screening would provide for: (a) the identification of thyroid disease which would otherwise remain unknown73; (b) the early normalization of maternal thyroid function,5 and (c) the reduction of perinatal complications associated with maternal thyroid hypofunction (prematurity, delayed intrauterine growth, or fetal death).74
The aim of prenatal diagnosis is to allow for therapeutic intervention at crucial stages of development. In this sense, the most ambitious objective of thyroid function screening in the first trimester of pregnancy is to be able to anticipate irreversible situations by providing adequate amounts of iodine and/or thyroxine that will allow for the development of the central nervous system of the fetus under optimal conditions.75 Berbel et al. administered early treatment with potassium iodide to pregnant women with hypothyroxinemia and achieved recovery of thyroxine levels and better results in the psychomotricity tests of their offspring.25 According to the results of the Negro et al. study, approximately 40 women would need to be screened and treated to prevent a single adverse outcome of pregnancy,76 a really favorable situation as compared to other interventional studies in other health problems such as hypercholesterolemia or hypertension.20 The results of the CATS study8 published in February 2012 would suggest that the treatment of pregnant women with CH, SCH, or isolated hypothyroxinemia (IH) with LT4 would not represent any benefit to their children as compared to those of mothers in the same situation but not treated. This publication is undoubtedly of great interest, but in assessing it three aspects should be taken into consideration. First, women start treatment with LT4 in the second trimester, when the most critical period of fetal neurodevelopment has finished, and positive effects are therefore much less likely to occur. Second, the study did not compare populations of children born to hypothyroid mothers (treated and untreated) with a group of children from euthyroid mothers. Haddow et al. made this comparison and found significant changes.7 The absence of this comparison leaves unanswered the question as to whether delayed diagnosis and treatment may have somewhat decreased the neuropsychological potential of children born to hypothyroid mothers. However, 12.1–14.1% of children analyzed had an intelligence quotient (IQ) less than 85. A third aspect to be considered is that the study did not provide data about any obstetric problems in these women and whether treatment with LT4 could have decreased their incidence, as reported in other studies.76,77 Therefore, the Lazarus et al. study does not allow us to rule out the early diagnosis and treatment (<10 weeks) of hypothyroid women as a means of reducing either the risk of children with a lower IQ, or the incidence of obstetric complications.
Few studies assessing the cost–effectiveness of universal screening are available, and those which report that screening is cost–effective. According to the Thung et al. model, screening is cost–effective if it is assumed that treatment with thyroxine of women with SCH would decrease the incidence of children with IQ<85.74 Dosiou et al. concluded that the universal screening for anti-TPO antibodies is effective, even when compared to the screening of women at high risk.73,78
Arguments against universal screeningThyroid dysfunction during pregnancy as a health problemScreening for TD during pregnancy may detect different situations, some of which we cannot be absolutely certain in considering as pathological and whose treatment is therefore at least debatable.
As regards CH, various studies have unquestionably related it to poorer intellectual or neurocognitive results in the offspring,7 increased fetal death,38,79 and other complications such as high blood pressure or delayed intrauterine growth.79 Universal screening aimed at detecting CH may therefore be warranted. The definition and impact of SCH are less clear. In the retrospective study by Casey et al.,26 SCH was associated with preterm delivery (PD), abruptio placentae, more admissions to neonatology, and more frequent respiratory distress. However, in a systematic review of maternal thyroid disease and PD,80 only the Casey et al. study showed an association with PD, and two other studies related it to low birth weight. Thus, an absolute association between SCH and poorer obstetric outcome cannot be considered established yet. Casey et al. stated that universal screening is not justified until it can be shown that treatment with thyroxine may prevent a poorer obstetric outcome.81 Finally, the prospective study by Negro et al.,76 who randomized more than 4000 women to universal or selective screening and started treatment in cases with TSH>2.5μIU/mL in the first trimester and positive anti-TPO antibodies, showed no differences between the universal and targeted screening groups in complicated or unfavorable obstetric and neonatal courses. This conclusion, by itself, would appear to tip the balance in favor of selective screening.
An additional potential finding of screening is IH, which is far from being accepted as a separate thyroid disease in clinical practice.27,82 First of all we should ask what are the limits below which IH should be diagnosed: are RVs based on those measured in the pregnant population and are they specific for each trimester? Studies by Pop et al.51,83 associated IH (<10th percentile) in the first trimester with poorer neuropsychological performance in the offspring. However, these findings were not confirmed in the offspring of mothers in whom FT4 normalized spontaneously in the second and third trimesters or with normal FT4 levels in the first trimester which subsequently decreased.83 Craig et al. also did not report neurocognitive deficiencies in the offspring of mothers with IH in the second trimester of pregnancy.54 This disagreement with the results of Pop et al. may be related to the strict matching criteria used by Craig. Why is TSH not increased in IH, if it is even more elevated in pregnant women with positive anti-TPO antibodies only even with normal FT484,85? It is known that low FT4 levels with no TSH increase may be found in iodine-deficient areas,86 which could mainly be attributed to the effect of an increased T3/T4 ratio.1 What occurs in areas that are not clearly iodine-deficient? The Mitchell et al.87 study reported that with a mean urinary iodine level of 134μg/L in pregnant women, IQ was associated with TSH only in multivariate models (and to FT4 only in univariate models). Moleti et al.88 noted that one third of their pregnant women had had IH at some time during pregnancy, but the reason for this in non-iodine deficient areas is not fully understood. It should be kept in mind that FT4 gradually decreases from the start of the second trimester,84,89 and other interpretations of this decrease more systemic than simple iodine sufficiency should be considered. To sum up, when IH is detected in women, it is currently difficult to interpret with certainty that it is a pathological condition, and doubts arise as to how it should be managed, as it has not been shown to date that treatment with thyroxine improves the slightly poorer psychomotor performance in the offspring, which is the only comorbidity associated with IH reported to date in countries that are not clearly iodine-deficient. Screening for IH does not therefore appear to be warranted for the time being.
Positive thyroid autoimmunity may be found in approximately 10% of pregnant women.90,91 Two meta-analyses have associated it with PD or miscarriage,90,92 although this association has not been confirmed by other meta-analyses.80 In a Japanese study93 where seven types of autoantibodies were measured, excluding anticardiolipin antibodies, PDs were similar in the control group and in women with some positive antibody or with positive antimicrosomal antibodies. A recent review94 reported that, in addition to antiphospholipid antibodies, other antibodies such as antiprothrombin, antinuclear, antilaminin, antigliadin, antitransglutaminase, and anti-TPO antibodies, and combinations thereof, have been associated with recurrent pregnancy losses. The mechanism by which thyroid autoimmunity may induce obstetric problems is not known with certainty, but it has been suggested that anti-TSH receptor antibodies that would block the action of β-HCG may coexist.95 However, it cannot be ruled out that this is simply an association of obstetric problems with other autoantibodies which have not been taken into account in many studies, or to the older age of women with positive thyroid autoimmunity.92
Frank hyperthyroidism is associated with miscarriage, placental detachment, preeclampsia, PD, malformation, delayed intrauterine growth, goiter, and fetal and neonatal hyperthyroidism.96 However, gestational hyperthyroidism (low TSH with normal or slightly elevated FT4 and negative antibodies) is not associated with any fetal morbidity.81 In a setting of universal screening, from 1.7%81 to 3%84 of pregnant women could be diagnosed as having hyperthyroidism in the first trimester, and although it may be associated with hyperemesis gravidarum, its treatment with antithyroid agents may cause fetal complications because of the risk of inducing an iatrogenic decrease in thyroxine. Clinical hyperthyroidism may therefore be treated with antithyroid agents,33 but always with the precaution of maintaining FT4 levels in the upper normal range and avoiding the induction of a deficiency situation. Subclinical hyperthyroidism should not be treated.5
Considerations regarding screening for thyroid dysfunction in healthy pregnant womenBecause of the absence of RVs of TSH which are specific for the pregnant population, and especially of FT4, caution should be taken before assuming that healthy pregnant women from countries not clearly iodine-deficient have a pathological condition, even more considering the RVs usually given in the commercial kits routinely used in clinical practice. A French review97 used different criteria to those proposed in the medical literature to define hypothyroxinemia: 41% of their pregnant women would have had a pathological condition using a cut-off value of FT4 <12pmol/L, as compared to 10% using a FT4 value <10.3pmol/L, and if SCH had been considered with a cut-off value of TSH >2.5μIU/mL, 26.3% of their pregnant population would have had hypothyroidism. As in other series of pregnant women, no association was found between thyroid function tests and urinary iodine levels. The authors concluded that the wide range of hypothyroxinemia and the prevalence of SCH should trigger a critical reflection on diagnostic levels. The fact is that RVs for TSH in the first trimester of pregnancy are not clearly established and show relevant changes depending on the week in which they are measured62,85,98 and the type of population, even when the same test method is used.68 According to different studies, in the first trimester the 2.5th percentile may range from 0.02 to 0.05μIU/mL98,99 and the 97.5th and 98th percentiles may range from 2.15-2.3 to 3.61-4μIU/mL.85,98–100
As regards maternal FT4, it should be added that interference with the routinely used immunoassay procedures may occur in situations where transporter proteins and their ligand properties change, as occurs during pregnancy, as discussed above. FT4 levels are particularly low in the third trimester of pregnancy as compared to non-pregnant women, and immunoassay procedures may overestimate hypothyroxinemia in such cases.66 Some authors state that there is no absolute FT4 value that may define hypothyroxinemia using these procedures.101 The most accurate method for testing FT4 in these situations has been shown to be equilibrium dialysis, which directly measures the hormone.102 However, this is a very cumbersome and expensive method, which makes its use in daily clinical practice difficult. Tandem mass spectrometry, a procedure developed in recent years, has been shown to have a very good correlation to equilibrium dialysis during pregnancy,65 unlike immunoassay techniques, which also show considerable individual variability.102
Potential benefits of thyroid function screening and management of thyroid dysfunction in pregnant womenThe benefits of treatment of CH with thyroxine have already been discussed.7 By contrast, no conclusive studies exist for SCH. A recent Cochrane review103 concluded that in view of the limited evidence regarding the treatment of these cases, implementation of a universal screening program to detect TD in the pregnant population is difficult to justify.
In thyroid autoimmunity without hypothyroidism, only one study77 has reported to date that the use of thyroxine may decrease the risk of PD. However, another recent study found no changes in women with positive anti-TPO antibodies treated with thyroxine, although this study included no control group.104 Moreover, in another study conducted in women with positive anti-TPO antibodies undergoing assisted reproduction techniques, these obtained no benefits from treatment with FT4.105
As regards IH, could values be normalized with iodine supplementation? According to the NHANES III data, FT4 shows no association with isolated or creatinine-adjusted urinary iodine in the non-pregnant adult population.106 In addition, in women of childbearing age and pregnant women, urinary iodine levels had decreased as compared to prior surveys (from 320 to 145μg/L). TSH and FT4 also showed no association with changes in urinary iodine levels.107 In four prospective studies, no differences were seen in FT4 levels with iodine supplementation as compared to placebo.108 Only two studies25,109 reported lower FT4 levels in the non-supplemented control group as compared to the treatment groups in the third trimester. In another intervention study, by contrast, lower FT4 levels and higher neonatal TSH levels were found in the third trimester in women given supplements of 300μg of iodine as compared to controls.55 Although other outcome parameters such as those obtained in the psychomotor development index may provide arguments in favor of supplementation,25,55 it is questionable whether FT4 is a good marker of response to iodine supplementation in pregnant women, at least with the measurement techniques currently available in clinical practice. By contrast, a recent observational study reported an association between lower FT4 levels and poorer results in neurocognitive tests of girls born to mothers who received potassium iodide supplements during pregnancy.110 Finally, based on the arguments discussed in this section, the benefit of screening does not appear to outweigh its potential problems. Situations of doubtful pathological value that would create diagnostic, therapeutic, and prognostic uncertainty to the mother, obstetrician, and endocrinologist could be detected. Because of this, during the first trimester a cautious approach should be taken to avoid treatments not yet proven to be effective for pregnancy and fetal morbidities, such as the treatment of IH with FT4 or the indication of antithyroid agents due to the inadequate interpretation of inhibited TSH levels, which could induce iatrogenic hypothyroxinemia with a poorer pathological outcome than those intended to be prevented by screening. To sum up, once prior reservations have been discussed, even if universal screening was decided upon based on the expected benefits for the treatment of CH, it would be mandatory to first obtain RVs and to develop a specific training program directed to primary care physicians, obstetricians, internists, and endocrinologists not familiar with TD in pregnancy, mainly aimed at achieving therapeutic abstention in situations with unproven pathological value.
It should also be noted that there are no clinical epidemiological studies related to the cost–benefit issues of universal screening. Studies published to date are theoretical models73,74,78 which have been questioned because of the absence of controlled, prospective studies assessing the effectiveness of thyroxine treatment.111,112
Final considerationsAs a summary of the arguments stated, and following the criteria of Beaglehole et al.,18 the following considerations may be made:
Thyroid dysfunction during pregnancy as a health problem- 1.
There is evidence and general agreement that CH not diagnosed or poorly controlled may have a negative impact on fertility, the course of pregnancy, and neurocognitive results in the offspring, among other aspects.
- 2.
As regards SCH, greater controversy exists with regard to increased prematurity and abruptio placentae. There is evidence that SCH may also affect intellectual capacity in the offspring.
- 3.
To date, harmful effects of hypothyroxinemia have been reported in the offspring of mothers with this condition during pregnancy. However, a recent study found no effects related to hypothyroxinemia in the second trimester.
- 4.
Literature data support the theory that thyroid autoimmunity could also affect fertility and prematurity, and increase the risk of miscarriage. Causality has not been shown, however, because the existence of an overlapping effect with other antibodies or its being related to older age in women who also have a greater prevalence of positive anti-TPO antibodies cannot be ruled out completely.
- 5.
Uncontrolled clinical hypothyroidism may increase morbidity during pregnancy and result in increased prematurity.
- 1.
TSH levels continue to be a very sensitive marker of thyroid dysfunction, even during pregnancy. RVs may be considered to be lower due to the effect of β-HCG, particularly in the first trimester.
- 2.
There are some difficulties regarding the accurate measurement of maternal FT4 with the commonly used immunoassay methods. This is due to changes in transporter proteins which occur during pregnancy. Tandem mass spectrometry is a good alternative procedure in such cases. However, it has been shown that some immunoassays for FT4 may provide a good approximation to the standard provided by that procedure.
- 3.
TT4 may be measured using more robust methodology than that used for measuring FT4. Changes in TT4 during pregnancy are more predictable, and it appears that RVs established in different populations could be more comparable.
- 4.
As regards the cost-effectiveness of screening, few studies are available, and these were based on theoretical models. Prospective, controlled studies are needed to assess whether the diagnosis and subsequent treatment of TD as a consequence of screening are cost-effective.
- 1.
RVs should be measured for each trimester of pregnancy in each area/population and using routine procedures, especially for FT4. Only three studies reporting the RVs of their respective populations are available in Spain (Table 2). The recent ATA guidelines assume that, in the absence of local RVs, the cut-off points for TSH should be established at 2.5μIU/mL for the first trimester and 3μIU/mL for the second and third trimesters.
- 2.
The diagnosis of CH and clinical hyperthyroidism should be understood as in the non-pregnant population, except for the fact that the availability of RVs specific for the pregnant population is indispensable. The same applies to SCH. If RVs are not available, ATA guidelines define SCH as a TSH level ranging from 2.5 to 10μIU/mL with FT4 within RVs. The assessment of TSH decrease in the first trimester and the diagnosis of hyperthyroidism require extreme caution. Caution should also be exercised when diagnosing IH, because of the frequent underestimation of FT4 obtained with some of the commonly used procedures.
- 3.
In these cases, the increased risk of hypothyroidism with positive anti-TPO antibodies mandates thyroid function monitoring during pregnancy.
- 1.
To guarantee normal thyroid function during pregnancy and lactation, the best prophylaxis is adequate iodine intake (250μg/day). Adequate iodine intake long before pregnancy occurs is also very important. This may decrease the risk of TD during pregnancy. Iodinated salt and milk contribute to a good iodine intake.
- 2.
There is evidence that the early treatment of CH (in the first weeks of pregnancy) has an unquestionable beneficial impact on fetal maturation.
- 3.
Despite evidence of the problems associated with SCH, no prospective studies on the potential benefits of treatment with FT4 are available, except for the Negro et al. study. In the recently reported CATS study, treatment was started in the second trimester. This makes it difficult to provide a general recommendation, although potential intervention should be guided by clinical assessment. Treatment may be indicated if SCH is associated with positive anti-TPO antibodies.
- 4.
The difficulty of assessing IH has already been discussed, but if IH is confirmed, in an iodine deficiency setting, patients may benefit from adequate iodine provision.
- 5.
Clinical hyperthyroidism may be treated with antithyroid agents, but with caution and attempting to maintain FT4 levels in the upper normal range and avoiding the induction of a deficiency state. SCH should not be treated.
- 6.
There is no agreement as to whether euthyroid pregnant women with positive anti-TPO antibodies should be treated with FT4. However, if concurrent SCH exists, treatment with FT4 may decrease the risk of obstetric complications.
In 2010 there were 486,575 births in Spain.113 The prevalence of unknown CH in the pregnant population is estimated at 0.3–0.5%,12 but may be much higher.24,29 Assuming that this prevalence also applies to the Spanish population, the number of pregnant Spanish women affected by this condition and not diagnosed during 2010 may have ranged from 1453 to 2420. Of all births, 155,943 were from women under 30 years of age, of whom 20% could have had some criterion that warranted screening.76 Among the remaining 124,754 who would not have required screening according to the ATA,12 from 374 to 624 could have had CH that would not have been diagnosed, a number that could have reached almost 2000 if the prevalence found by Menéndez et al.24 were to be considered. There is complete unanimity and agreement regarding the need to treat such women with FT4, as recommended by the ATA12 with the maximum level of evidence. In 2010, failure to screen that population may have left from 500 to 2000 women without diagnosis and treatment of CH. While additional studies analyzing the cost–benefit or cost-effectiveness of SCH screening are needed, in the case of CH, a relatively common disease with well-known risks (both obstetric and for the offspring) which is diagnosed using simple and inexpensive methods and for which safe and inexpensive treatment is available, it may easily be assumed that a cost-benefit study would provide positive results, and in the absence of such studies, failure to screen appears to be an unethical decision difficult to assume by a clinician. In this regard, population screening for CH in pregnant women, followed by appropriate treatment, would prevent the abovementioned risks and would improve the health of a not insignificant number of women and their children. The SEEN (Working Group on Iodine Deficiency Disorders and Thyroid Dysfunction) and SEGO therefore think that thyroid function screening during pregnancy is justified for the early detection and treatment of CH.
Recommendations of the Spanish Society of Endocrinology and Nutrition (Working Group on Iodine Deficiency Disorders and Thyroid Dysfunction) and the Spanish Society of Gynecology and ObstetricsGrades of recommendation based on the Scottish Intercollegiate Guidelines Network (SIGN).114
- 1.
Screening for TD in the pregnant population would be justified in order to detect CH and start early treatment (<10 weeks). This should preferably be done before conception, if possible, or at the start of pregnancy (SIGN: B).
- 2.
Screening would not be justified to detect SCH or IH, conditions in which no adequate scientific evidence showing the benefit of subsequent treatment with FT4 is as yet available (SIGN: C).
- 3.
Adequate iodine intake should be guaranteed to the whole population, and especially to women of childbearing age and pregnant and lactating women. In the latter groups, the recommended iodine intake is 250μg/day. In iodine-deficient populations, supplementation with 150-200μg of iodine would allow for the recommended intake to be achieved. Ideally, adequate iodine provision would be warranted in the preconceptional state (SIGN: B).
- 4.
Screening at the start of pregnancy only requires the measurement of TSH levels. Testing of FT4 or TT4 would only be justified if TSH was altered (SIGN: B).
- 5.
The availability of RVs of these hormones for each trimester and population, measured using the routine laboratory procedures at the center, is indispensable (ν).
- 6.
Because of the often difficult interpretation of the results of thyroid hormone tests during pregnancy, it would be interesting to devise programs for training primary care physicians, obstetricians, internists, and endocrinologists not familiar with TD in pregnancy, placing particular emphasis on the need for therapeutic abstention in situations with unproven pathological value. The multidisciplinary work of these groups of professionals is indispensable for improving the approach to thyroid disease in the pregnant population (ν).
The authors state that they have no conflicts of interest.
Arena, Jose; Ares, Susana; Arrizabalaga, Juan José; Arrobas, Teresa; Bandrés Nivela, María Orosia; Berbel, Pere; Bezanilla López, Carolina; Caballero, Águeda; de la Vieja Escolar, Antonio; Donnay Candil, Sergio (coordinator of the Working Group on Iodine Deficiency Disorders and Thyroid Dysfunction); Espada, Mercedes; García Fuentes, Eduardo; Gonzalez Mateo, Carmen; Gentil, Alfonso; Iglesias Reymunde, Teresa; Lucas, Anna; Muñoz Marquez, Jose; Menendez, Edelmiro; Millon Ramirez, Maria del Carmen; Moll Mascaró, Gracia; Morales, Francisco; Moreno, Jose Carlos; Pineda Arrivas, Jose Javier; Puig-Domingo, Manel; Riestra, María; Santiago, Piedad; Santiesteban, Pilar; Soriguer, Federico; Torres Costa, María Teresa; Tortosa, Frederic; Velasco, Inés; Vich Sastre, Francisca; Vila, Lluís; Wengrowicz, Silvia.
Please, cite this article as: Vila L, et al. Detección de la disfunción tiroidea en la población gestante: está justificado el cribado universal. Endocrinol Nutr. 2012;59:547-60.
The names of the members of the group are listed in Annex 1.
Endorsed by the Sociedad Española de Endocrinología y Nutrición (SEEN) and the Sociedad Española de Ginecología y Obstetricia (SEGO).
In agreement with authors and editors, this article is simultaneously published in full in Medicina Clínica (Med Clin [Barc] with the corresponding doi).