To assess in a growth retardation (GR) model the impact of different propranolol (P) doses on anthropomorphometric and biomechanical variables of the appendicular skeleton.
Materials and methodsTwenty-one day-old male Wistar rats were divided into the following groups: control (C), C+P3.5 (CP3.5); C+P7 (CP7); C+P10.5 (CP10.5); C+P14 (CP14); ED, ED+P3.5 (EDP3.5); ED+P7 (EDP7); ED+P10.5 (EDP10.5), and ED+P14 (EDP14). Control animals with/without P were fed a rodent diet ad libitum. GR rats with/without P were given 80% of the same diet per 100g body weight for 4 weeks (T4). Propranolol 3.5, 7, 10.5, and 14mg/kg/day was intraperitoneally injected 5 days/week for 4 weeks to the CP3.5 and EDP3.5; CP7 and EDP7; CP10.5 and EDP10.5, and CP14 and EDP14 groups respectively.
ResultsAt T4, energy restriction had negative effects upon overall growth, femur, and its mechanical competence. Propranolol improved bone rigidity in GR animals at doses of 7 and 10.5mg/kg/day, with a maximum response at 7mg/kg/day.
ConclusionsPropranolol 7mg/kg/day would be the most effective dose for modeling incorporation of bone, as shown by the increased skeletal structural and mechanic efficiency in this animal model of growth retardation. Such effect may result from maintenance of mechanosensor viability, changes in its sensitivity and the biomechanical reference point and/or effector response in GR rats.
Evaluar en un modelo de retraso del crecimiento (enanismo por desnutrición [ED]) el efecto de diferentes dosis de propranolol (P) sobre las variables antropo-morfométricas y biomecánicas del esqueleto apendicular.
Materiales y métodosRatas macho Wistar de 21 días se dividieron en grupos: control (C), C+P3,5 (CP3,5); C+P7 (CP7); C+P10,5 (CP10,5); C+P14 (CP14); ED, ED+P3,5 (EDP3,5); ED+P7 (EDP7); ED+P10,5 (EDP10,5) y ED+P14 (EDP14). Los animales controles con/sin P recibieron una dieta para roedores ad libitum; las ratas ED con/sin P recibieron por cada 100g de peso corporal un 80% de la misma dieta durante 4 semanas (T4). Propranolol 3,5; 7; 10,5 y 14mg/kg/día fue inyectado intraperitonealmente 5 días/semana durante 4 semanas en CP3,5 y EDP3,5; CP7 y EDP7; CP10,5 y EDP10,5 y CP14 y EDP14, respectivamente.
ResultadosA T4, la restricción energética produjo efectos negativos sobre el crecimiento global, el fémur y su competencia mecánica. Propranolol mejoró la rigidez ósea en los animales ED con dosis de 7 y 10,5mg/kg/día, con un máximo de respuesta a 7mg/kg/día.
ConclusionesEl propranolol 7mg/kg/día sería la dosis más efectiva en la incorporación modelatoria de hueso con incremento de su eficiencia estructural y mecánica en el presente modelo animal de retraso del crecimiento. Dicho efecto podría ser el resultado del mantenimiento de la viabilidad del mecanosensor, de modificaciones de su sensibilidad, del punto de referencia biomecánico y/o de la respuesta de los efectores en las ratas ED.
The quality of the axial and appendicular skeleton is determined by many factors, of which one of the most important is nutritional status. During the development of an individual, calorie-protein malnutrition, mainly during the critical growth periods, contributes to growth impairment, with the resultant risk of osteoporosis and bone fragility in advanced age.1–6 While osteoporosis, the most common condition affecting bone remodeling, occurs more frequently in adulthood, an individual's bone quality is directly related to peak bone mass, which is in turn influenced by endogenous and exogenous factors which condition growth and development.7,8
Certain beliefs and practices imposed by parents on their children may restrict the nutrition of the latter up to the point of inducing a nutritional disease called nutritional dwarfism (ND). ND is characterized by a slowing of body growth with delayed pubertal development without evidence of organ failure or changes in biochemical markers of malnutrition.9–12
Our laboratory has reported a model of nutritional stress in growing rats which mimics human ND, a form of malnutrition causing a deficiency in weight- and height-for-age indices, with a weight-for-height index within normal limits. This animal model of nutritional dwarfism was achieved through chronic administration of a balanced diet to newly weaned rats. Global restriction was 20% as compared to the energy requirements of animals of the same age.13
Since neuroendocrine changes secondary to nutritional stress occur in ND,14,15 bone acquisition during the critical growth stages may be substantially affected. Indeed, prior studies conducted at our laboratory showed an impaired architectural design with a decreased mechanical capacity to withstand deforming forces in femora from rats with ND.2,3,16,17
In vivo and in vitro studies have demonstrated the participation of the sympathetic nervous system (SNS) in the modulation of bone metabolism. The existence of skeletal sympathetic nerves, the presence of sympathetic fibers in bone marrow, and increased osteoclastogenesis with increased bone resorption and/or decreased bone formation due to increased SNS activity have also been shown.18–23 In addition, the presence of β-adrenergic receptors in bone cells was shown, and the activation of such receptors resulted in an increased RANKL expression with increased bone resorption.24
However, chemical or surgical sympathectomy studies provided conflicting results about the role of SNS in bone metabolism, suggesting that the former has an anabolic effect on the latter.25,26
While β-blockers are known to be widely used in cardiovascular diseases, many findings suggest their probable preventive and/or therapeutic impact through a protective effect of bone mass against various stress situations in both humans27,28 and experimental animals.29,30 In fact, in male adult mice of the C57BL/6 strain, chronic propranolol (P) administration was shown to prevent bone volume reduction caused by intake of a diet with a 40% calorie restriction with calcium and phosphate compensation.31 Studies conducted by Bonnet et al.30 showed that low P doses improved bone biomechanical competence in ovariectomized rats with no hemodynamic changes.
In previous studies conducted at our laboratory, an increased bone quality was seen in rats with ND given chronic P treatment.32 There are however some inconsistencies as regards the effect of β-blockers such as P on bone mass increase and fracture risk reduction as reported by other experimental26,33 and clinical34,35 studies in both young individuals and adults.
Because of the relationship between nutritional status and the biomechanical fitness of bone, and evidence of a β-adrenergic control of bone mass, the objective of this study was to assess, in an animal model of nutritional dwarfism (ND), the effect of different doses of a β-blocker, propranolol, on anthropomorphometric and biomechanical bone variables. The dose which would most effectively allow for the achievement of a structural rigidity adequate to withstand standard and/or maximal mechanical stimulation while preserving bone integrity was also determined.
Materials and methodsExperimental animalsWistar male rats were used from weaning to 21–23 days of age (baseline weight: 48.90±1.60g; mean±SE), supplied by the Department of General and Oral Biochemistry of the School of Dentistry of Buenos Aires University. The animals were housed in galvanized cages under adequate hygienic conditions to prevent coprophagia. Light–dark 12:12h cycles were respected. Room temperature was kept at 21±1°C, and relative humidity at 50–60%. Experimental animal use, care, and treatment complied with standards of the National Institutes of Health36 and were approved by the ethics committee of the School of Dentistry of Buenos Aires University.
DietExperimental animals were fed a special rodent diet (Purina pellets) with the following composition (g/100g of diet): protein: 22.70; lipids: 7.09; fiber: 6.00; Ca: 1.30; P: 0.80; ash: 6.50; water: 7.60; dextrin qs 100.
Experimental designEighty rats were randomized to 10 groups of 8 animals each to received vehicle or P (P; Laboratorios Richmond, Argentine) at doses of 3.5 (P3.5), 7 (P7), 10.5 (P10.5), and 14 (P14) mg/kg/day as follows: control+vehicle (C); control+P3.5 (CP3.5); control+P7 (CP7); control+P10.5 (CP10.5); control+P14 (CP14); experimental ND+vehicle (ND); experimental ND+P3.5 (NDP3.5); experimental ND+P7 (NDP7); experimental ND+P10.5 (NDP10.5); and experimental ND+P14 (NDP14). Propranolol was intraperitoneally injected 5 days a week for 4 weeks at the abovementioned doses. The control and ND groups were given saline with the same regimen. The control groups with/without P were fed ad libitum a standard diet, and ND groups with/without P received 80% of the diet taken by C groups on the previous day corrected for body weight (consumption in g/100g body weight/day), both for 4 weeks. Feed intake was recorded daily, and body weight and length every 2 and 4 days respectively. The animals were killed by intramuscular injection of ketamine hydrochloride, 0.1mL/100g body weight (Holliday Lab.) and xylazine, 0.02mL (Konig Lab., Buenos Aires, Argentina) at 4 experimental weeks (T4). Femora from each of the animals were immediately dissected, avoiding damage to the periosteum. Both bones were weighed and measured with a digital caliper and used to study bone biomechanical properties.
Growth assessmentAnthropometricsBody weight (W) and length (L) were measured. Weight was recorded every 4 days, using a PC 4000 Mettler scale with a precision of ±1mg, after fasting of the animals for at least two hours and a maximum of four hours. Body length was recorded as the distance from nose to the hairs of the base of the tails of the animals.
Dietary intakeDietary intake was measured daily on a Mettler scale with a precision of ±1mg. Intake was measured as the difference in feeding box weight between two consecutive days, expressed as 100g rat/day.
Assessment of femoral morphometryBones were ablated and freed from muscle and tendon tissue. Bones were weighted, and their length was measured using a digital micrometer caliper with a precision of 0.05mm.
Assessment of the biomechanical properties of the femoral shaftBone mechanical quality was assessed using a mechanical three-point flexion test with an Instron model 4442.37 The femur was placed in a horizontal position, with its anterior side looking downwards, on two supports equidistant from its ends, separated by a constant distance of 13mm. The shaft was centrally loaded at an increasing rate, at 50N, at a velocity of 5mm/min until fracture. The load/deformation (W/d) curves obtained allowed for determining the following variables, representative of the structural properties of the entire bone: fracture load (Wf, N), maximum limit elastic load (Wy, N), and bone rigidity (Wy/dy, N/mm), where dy represents bone deformation in the sector with elastic behavior. Sections were made at the mid-femoral shaft using an Isomet microsaw with a diamond tip (Buehler, Lake Bluff, IL, USA), and lateral and medial horizontal and vertical diameters of the cross section were calculated in order to assess bone geometric properties: total bone cross-sectional area (CSA, mm2), estimated by the formula: π/4·V·H; axial moment of inertia (Ix, mm4), estimated by the formula: Ix=π(V3H−v3h)/64; and medullary area (MA, mm2), estimated as MA=π/4·v·h, where H and V correspond to the horizontal and vertical lateral diameters respectively, and h and v to the horizontal and vertical medial diameters respectively of the bone cross-section at the mid-femoral shaft. The cortical bone cross-sectional area (A, mm2) was calculated as the difference between the CSA and the MA.
The femur was assumed to be an elliptic cylinder, and mathematical formulas were used to indirectly calculate the following material properties of cortical bone: Young's elastic modulus (E, N/mm2), estimated using the formula: E=Wy·L3/48dy·Ix, where Wy is the limit elastic load, L the bone length, dy the yield deformation, and Ix the axial moment of inertia; y and maximum elastic stress (σy, N/mm2), estimated using the formula: σy=L·V·Wy/8·Ix, where V is the vertical external diameter.
Statistical analysisResults were given as mean±SE. Data were analyzed using analysis of variance (ANCOVA). A Student–Newman–Keuls t test for multiple comparisons was subsequently used. Means differences were considered significant at p<0.05.38 Statistical analysis was performed using Graphpad Prism version 3.0 software (Graphpad Software, San Diego, CA).
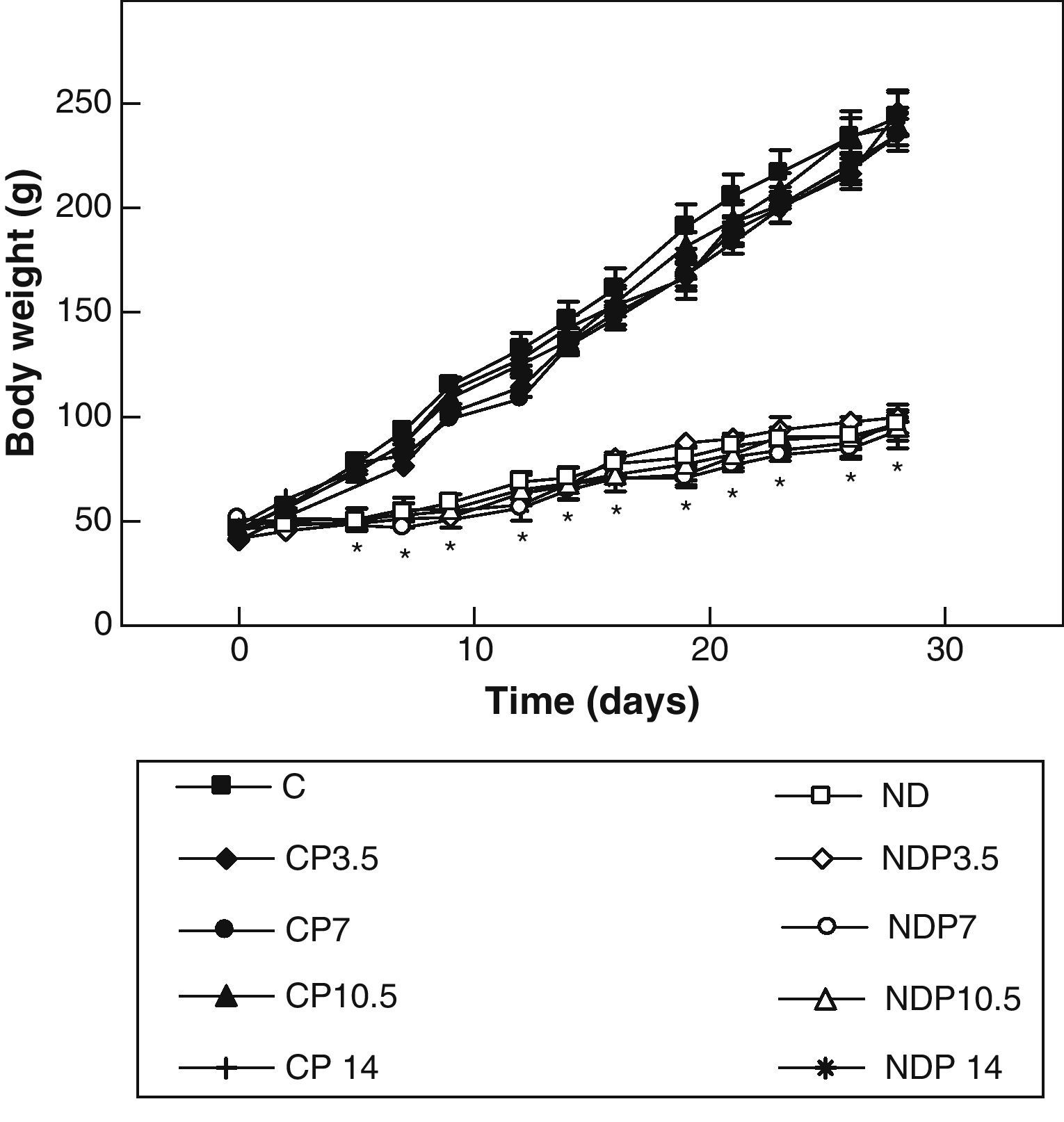
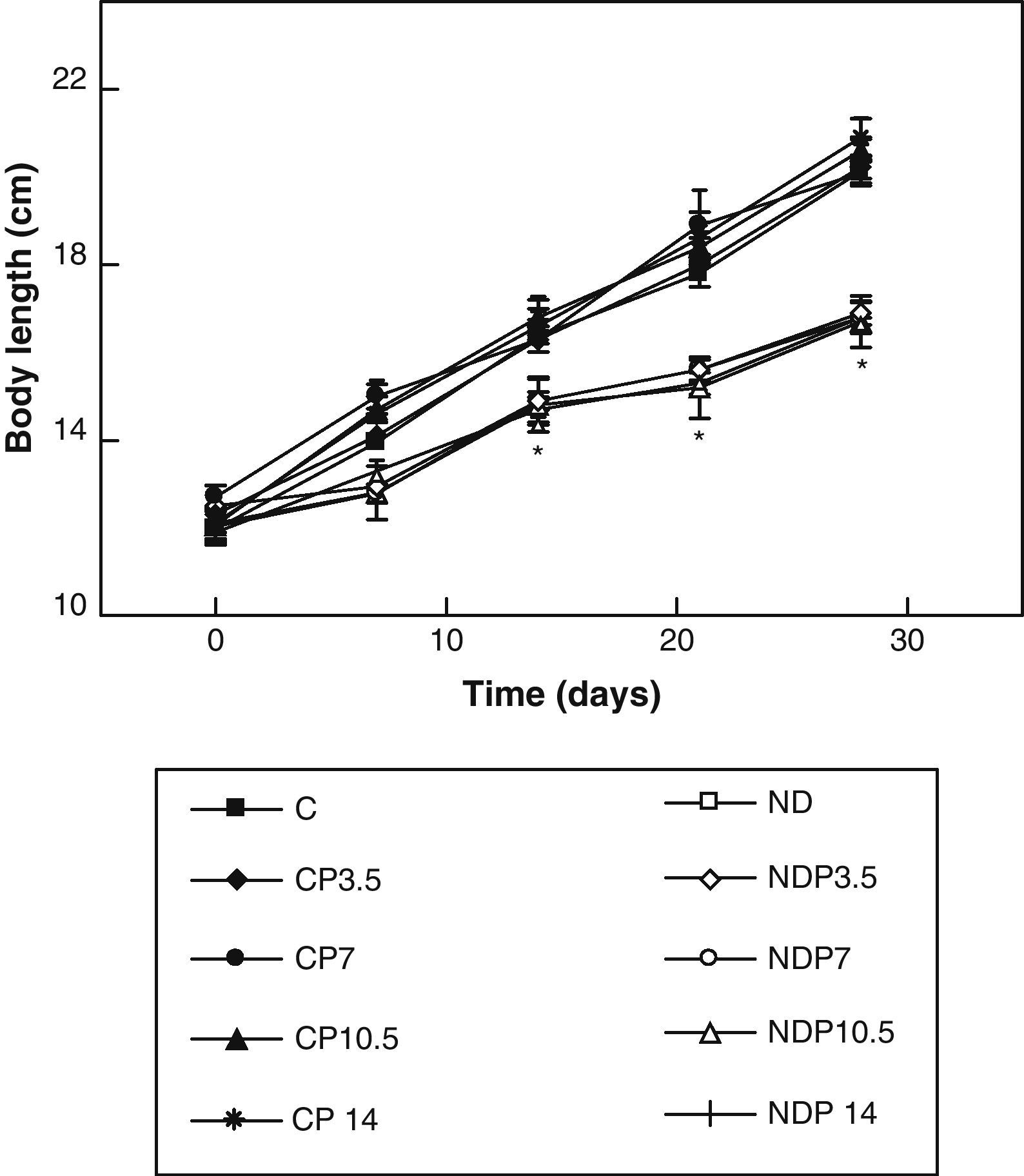
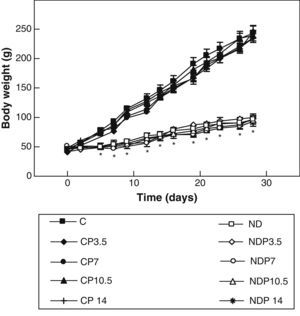
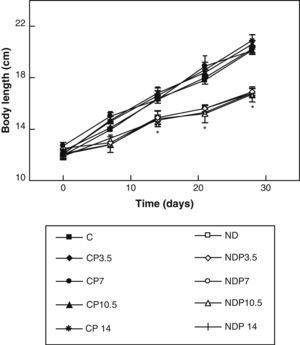
ResultsFigs. 1 and 2 show the body weight and length profiles of control and experimental rats with/without P over time respectively. Energy restriction induced a highly significant (p<0.001) decrease in the growth rate in experimental as compared to control animals, and this was independent of P administration for any dose given.
Changes in body weight of animals from the control (C), control+propranolol 3.5mg/kg/day (CP3.5); control+propranolol 7mg/kg/day (CP7); control+propranolol 10.5mg/kg/day (CP10.5); control+propranolol 14mg/kg/day (CP14); experimental ND (ND), experimental ND+propranolol 3.5mg/kg/day (NDP3.5); experimental ND+propranolol 7mg/kg/day (NDP7); experimental ND+propranolol 10.5mg/kg/day (NDP10.5); and experimental ND+propranolol 14mg/kg/day (NDP14) groups during the experimental period. Mean values±standard error of 8 animals per group.
*Significant differences between ND and C groups with/without propranolol (p<0.05).
Changes in body length of animals from the control (C), control+propranolol 3.5mg/kg/day (CP3.5); control+propranolol 7mg/kg/day (CP7); control+propranolol 10.5mg/kg/day (CP10.5); control+propranolol 14mg/kg/day (CP14); experimental ND (ND), experimental ND+propranolol 3.5mg/kg/day (NDP3.5); experimental ND+propranolol 7mg/kg/day (NDP7); experimental ND+propranolol 10.5mg/kg/day (NDP10.5); and experimental ND+propranolol 14mg/kg/day (NDP14) groups during the experimental period. Mean values±standard error of 8 animals per group.
*Significant differences between ND and C groups with/without propranolol (p<0.05).
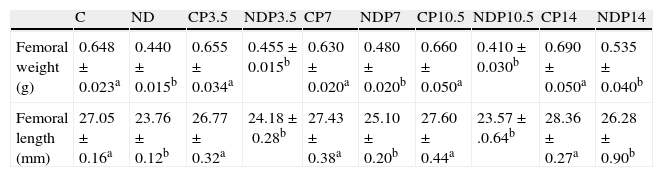
Table 1 shows femur morphometry in control and experimental animals with/without P at T4. Femoral growth was adversely affected in undernourished rats as compared to their respective controls (p<0.001). However, treatment with P did not cause obvious changes in the morphometric parameters assessed at any of the doses used and regardless of nutritional status.
Femoral weight and length at the end of the study (T4).
| C | ND | CP3.5 | NDP3.5 | CP7 | NDP7 | CP10.5 | NDP10.5 | CP14 | NDP14 | |
| Femoral weight (g) | 0.648±0.023a | 0.440±0.015b | 0.655±0.034a | 0.455±0.015b | 0.630±0.020a | 0.480±0.020b | 0.660±0.050a | 0.410±0.030b | 0.690±0.050a | 0.535±0.040b |
| Femoral length (mm) | 27.05±0.16a | 23.76±0.12b | 26.77±0.32a | 24.18±0.28b | 27.43±0.38a | 25.10±0.20b | 27.60±0.44a | 23.57±.0.64b | 28.36±0.27a | 26.28±0.90b |
Animals from the control (C); control+propranolol 3.5mg/kg/day (CP3.5); control+propranolol 7mg/kg/day (CP7); control+propranolol 10.5mg/kg/day (CP10.5); control+propranolol 14mg/kg/day (CP14); experimental ND (ND), experimental ND+propranolol 3.5mg/kg/day (NDP3.5); experimental ND+propranolol 7mg/kg/day (NDP7); experimental ND+propranolol 10.5mg/kg/day (NDP10.5); and experimental ND+propranolol 14mg/kg/day (NDP14) groups at the end of the study. Mean values±standard error of 8 animals per group. Different letters indicate significant differences between groups (p<0.05).
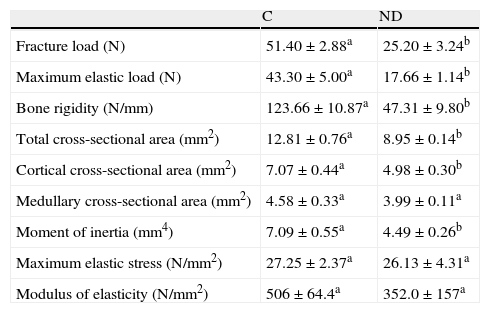
Table 2 shows the biomechanical properties of the femoral shaft of ND versus C animals. In agreement with prior studies at our laboratory,2 biomechanical femoral data showed that structural properties (maximum fracture load, limit elastic load, and bone rigidity) of the midshaft were negatively affected after 4 weeks of food restriction in ND versus C animals (p<0.01). However, the resistance of the appendicular skeleton of ND animals after nutritional stress was adequate for the body size reached, as was shown by the normalization of the femoral fracture load by body weight. In fact, Wf/p (N/g) for ND versus C rats was 0.091±0.02 versus 0.097±0.01 respectively (p>0.05).
Structural (fracture load, maximum elastic load, bone rigidity), geometric (total cross-sectional area, cortical cross-sectional area, medullary cross-sectional area, and moment of inertia), and material properties (maximum elastic stress and modulus of elasticity) of femora from control (C) and experimental ND animals at the end of the study.
| C | ND | |
| Fracture load (N) | 51.40±2.88a | 25.20±3.24b |
| Maximum elastic load (N) | 43.30±5.00a | 17.66±1.14b |
| Bone rigidity (N/mm) | 123.66±10.87a | 47.31±9.80b |
| Total cross-sectional area (mm2) | 12.81±0.76a | 8.95±0.14b |
| Cortical cross-sectional area (mm2) | 7.07±0.44a | 4.98±0.30b |
| Medullary cross-sectional area (mm2) | 4.58±0.33a | 3.99±0.11a |
| Moment of inertia (mm4) | 7.09±0.55a | 4.49±0.26b |
| Maximum elastic stress (N/mm2) | 27.25±2.37a | 26.13±4.31a |
| Modulus of elasticity (N/mm2) | 506±64.4a | 352.0±157a |
Mean values±standard error of 8 animals per group. Different letters indicate significant differences between groups (p<0.05).
Analysis of the total cross-sectional area and the axial moment of inertia showed that such geometric variables were significantly lower in the ND as compared to the C group at the end of the experimental period (p<0.01) (Table 2). The cortical cross-sectional area of the femoral midshaft was significantly less in ND as compared to C rats (p<0.01). No significant between-group differences were seen in the medullary cross-sectional area (p<0.05).
As regards the intrinsic quality of the bone material, represented by the variables of maximum elastic stress and modulus of elasticity, no significant between-group differences were found at the end of the study. (p<0.05) (Table 2).
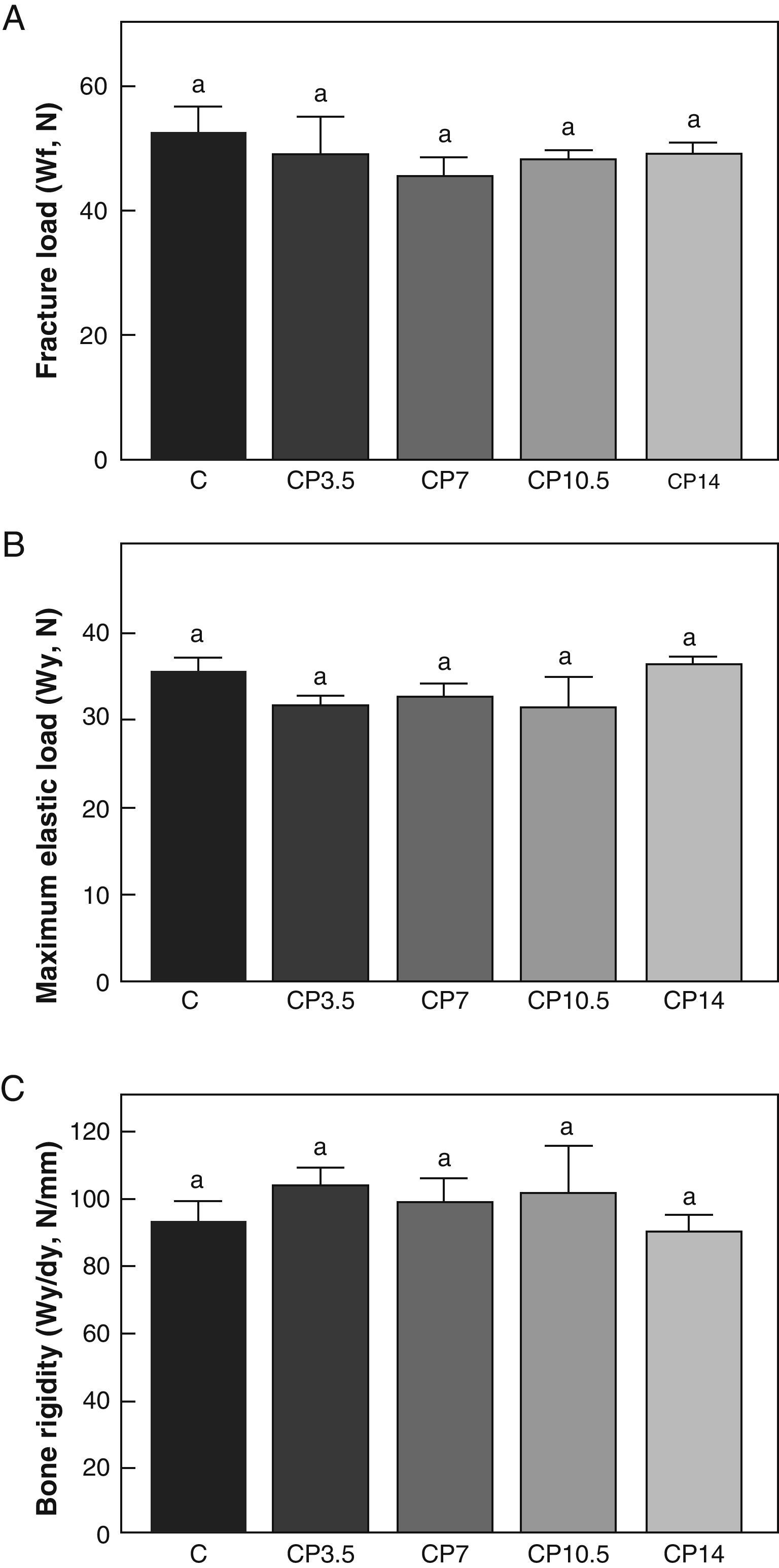
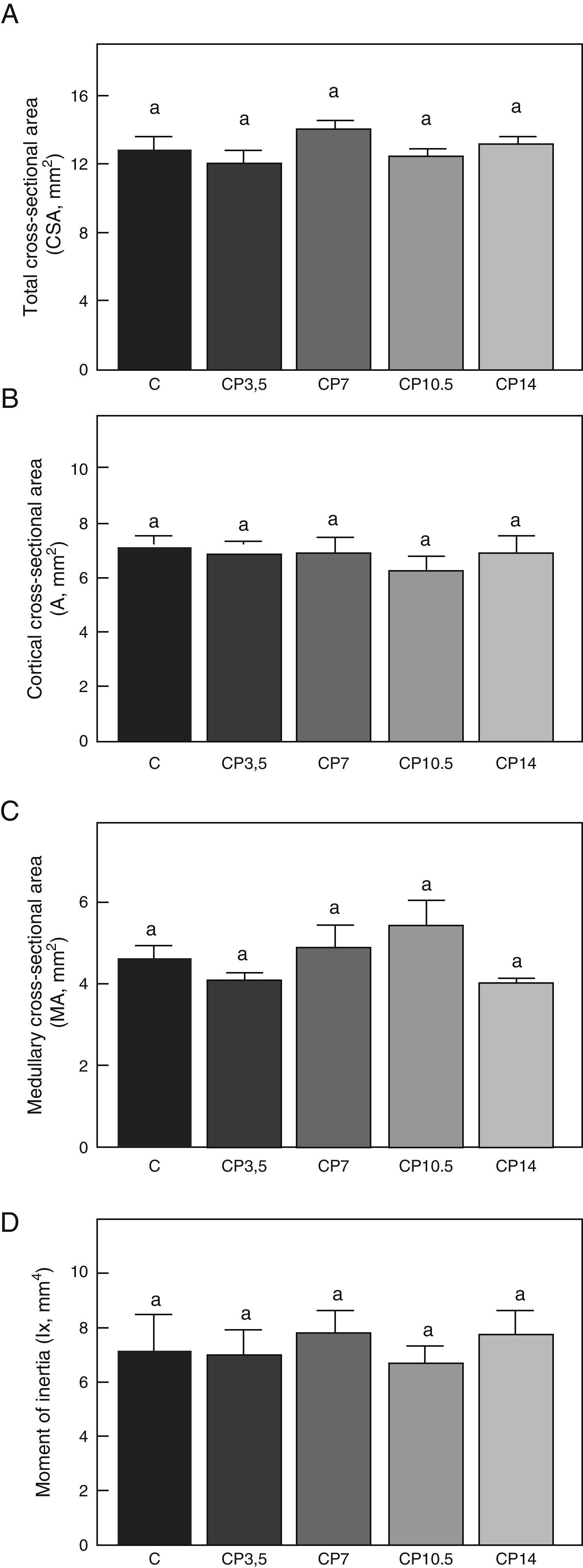
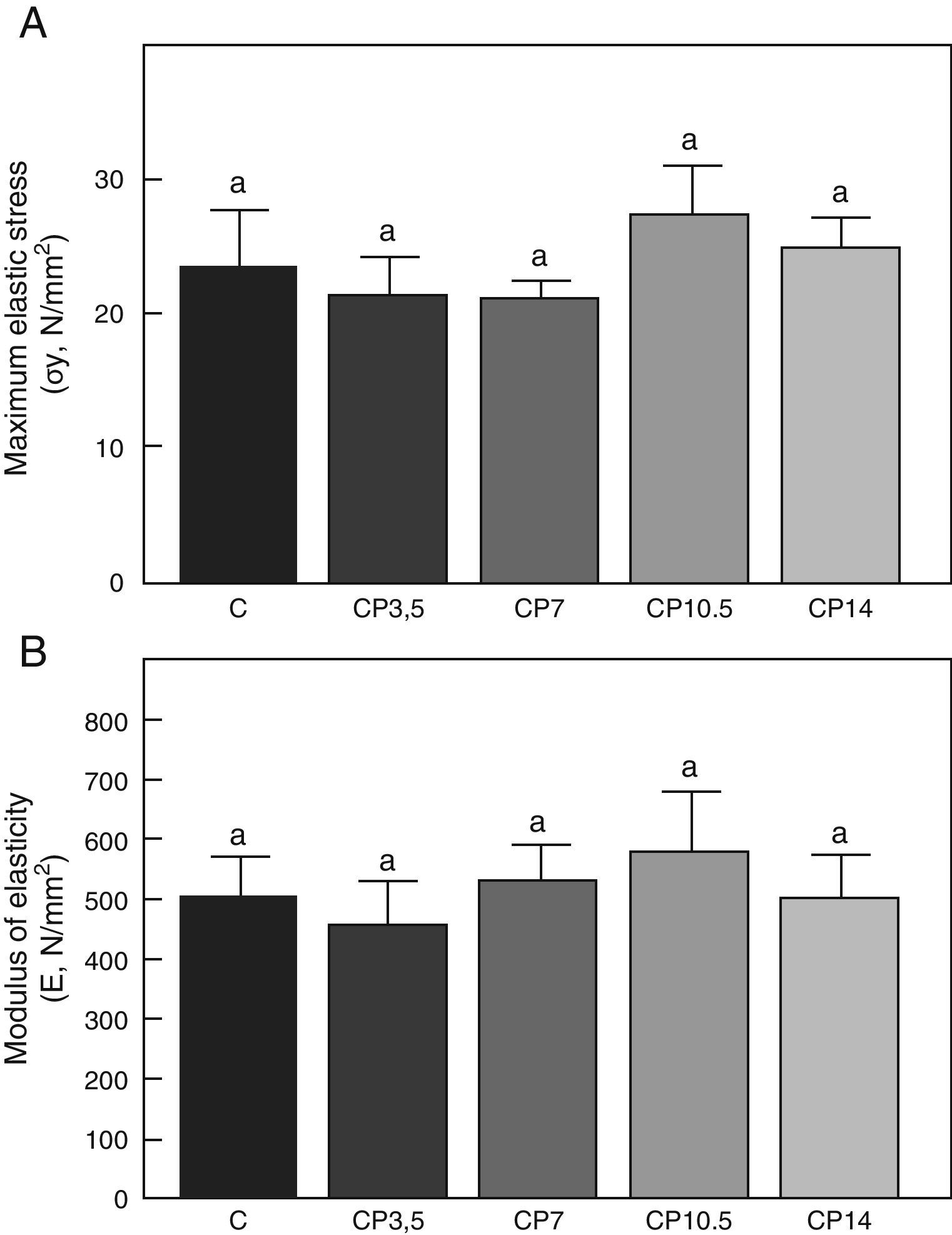
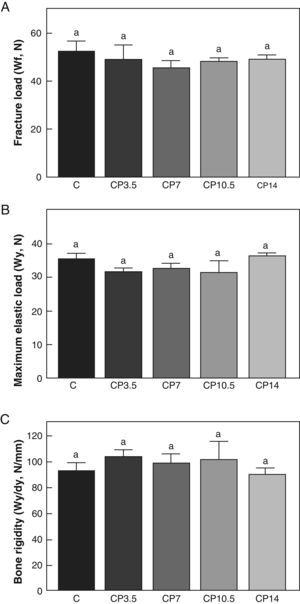
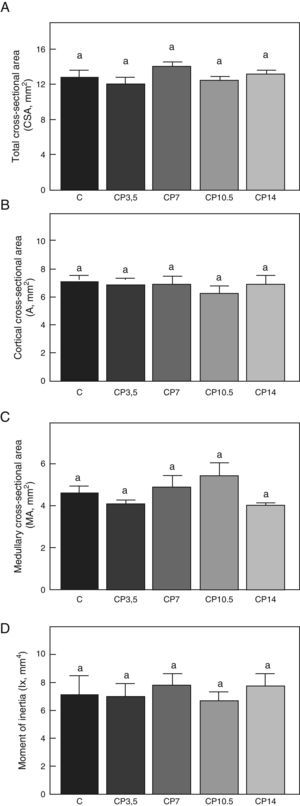
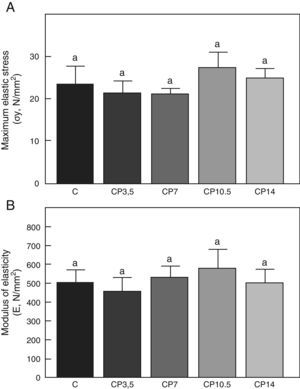
Figs. 3–5 respectively show the structural, geometric, and material properties of femora from control animals with/without P. The results of this study showed that the β-blocker, at the doses used, had no effects on the biomechanical competence of bone within groups of animals fed ad libitum and treated with P or as compared to untreated control animals (Figs. 3–5).
Structural properties. (A) Fracture load (Wf), (B) maximum elastic load (Wy), and (C) bone rigidity (Wy/dy) of femora from control (C), control+propranolol 3.5mg/kg/day (CP3.5); control+propranolol 7mg/kg/day (CP7); control+propranolol 10.5mg/kg/day (CP10.5); and control+propranolol 14mg/kg/day (CP14) animals at the end of the study (T4). Mean values±standard error of 8 animals per group. Different letters indicate significant differences between groups (p<0.05).
Geometric properties. (A) Total cross-sectional area (CSA), (B) cortical cross-sectional area (A), (C) medullary cross-sectional area (MA), and (D) moment of inertia (Ix) of femora from control (C), control+propranolol 3.5mg/kg/day (CP3.5); control+propranolol 7mg/kg/day (CP7); control+propranolol 10.5mg/kg/day (CP10.5); and control+propranolol 14mg/kg/day (CP14) animals at the end of the study (T4). Mean values±standard error of 8 animals per group. Different letters indicate significant differences between groups (p<0.05).
Material properties. (A) Maximum elastic stress (σy), and (B) modulus of elasticity (E) of femora from control (C), control+propranolol 3.5mg/kg/day (CP3.5); control+propranolol 7mg/kg/day (CP7); control+propranolol 10.5mg/kg/day (CP10.5); and control+propranolol 14mg/kg/day (CP14) animals at the end of the study (T4). Mean values±standard error of 8 animals per group. Different letters indicate significant differences between groups (p<0.05).
A comparison of bone resistance to fracture relative to body weight showed no significant differences in Wf/p within any of the control groups treated with P or as compared to untreated control animals. Indeed, Wf/p (N/g) values for the C, CP3.5, CP7, CP10.5, and CP14 groups were 0.097±0.01, 0.103±0.01, 0.096±0.03, 0.094±0.01, and 0.097±0.01 respectively (p>0.05).
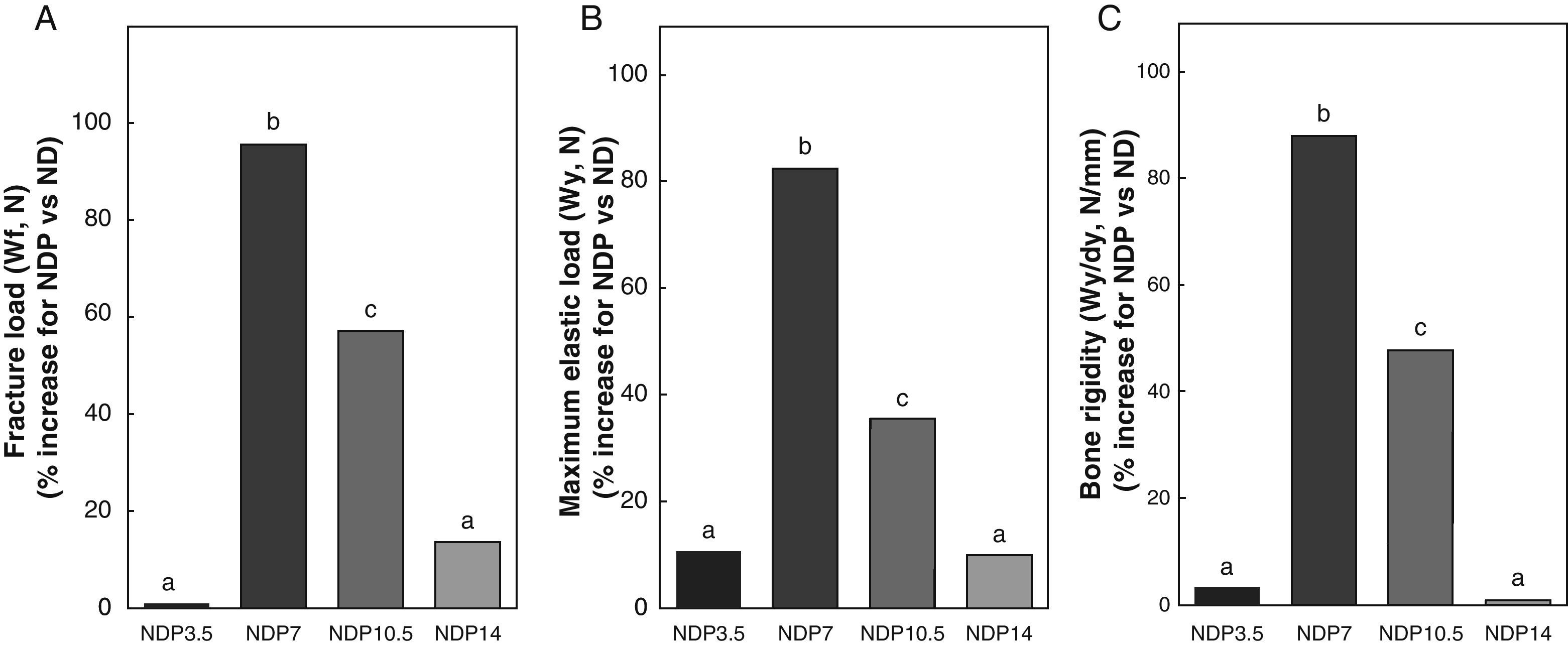
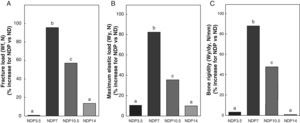
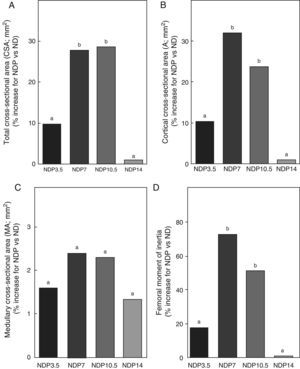
Figs. 6–8 respectively show the structural, geometric, and material properties of femora from ND rats treated with different P doses, expressed as the percent increase in such variables as compared to those found in untreated ND rats. Undernourished animals treated with P doses of 7 and 10.5mg/kg/day had significant increases (p<0.05) in variables representative of the structural properties of the whole bone such as fracture load, maximum limit elastic load, and bone rigidity as compared to untreated ND rats, with a maximum increase at 7mg/kg/day (Fig. 6). P administered to ND rats at doses of 3.5 and 14mg/kg/day caused no significant changes in any of the biomechanical variables assessed (Fig. 6).
Structural properties. (A) Fracture load (Wf), (B) maximum elastic load (Wy), and (C) bone rigidity (Wy/dy) of femora from animals in the experimental ND+propranolol 3.5mg/kg/day (NDP3.5); experimental ND+propranolol 7mg/kg/day (NDP7); experimental ND+propranolol 10.5mg/kg/day (NDP10.5); and experimental ND+propranolol 14mg/kg/day (NDP14) groups expressed as percent increase of such variables as compared to ND values at the end of the study (T4). Different letters indicate significant differences between groups, with ND=a (p<0.05).
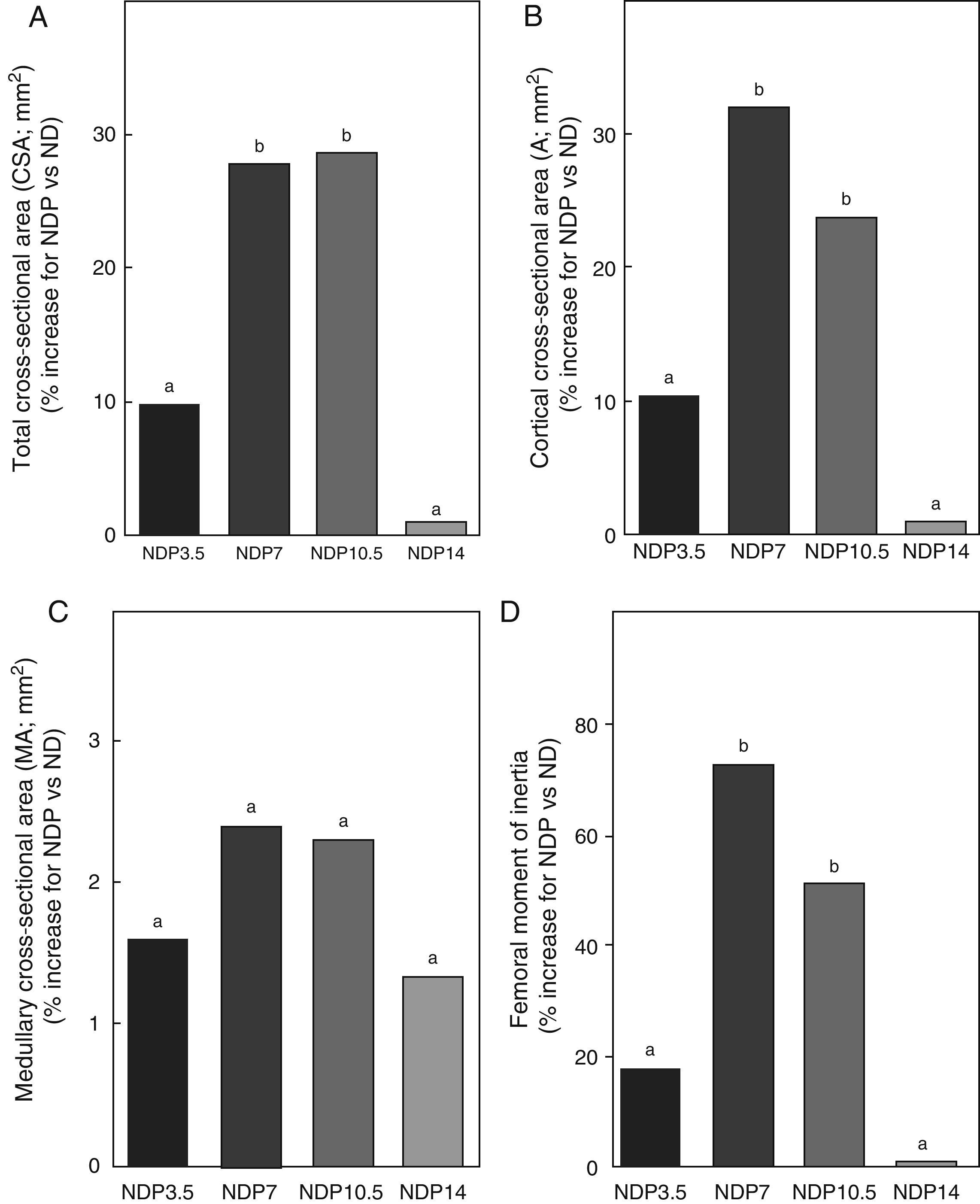
Geometric properties. (A) Total cross-sectional area (CSA), (B) cortical cross-sectional area (A), (C) medullary cross-sectional area (MA), and (D) moment of inertia (Ix) of femora from animals in the experimental ND+propranolol 3.5mg/kg/day (NDP3.5); experimental ND+propranolol 7mg/kg/day (NDP7); experimental ND+propranolol 10.5mg/kg/day (NDP10.5); and experimental ND+propranolol 14mg/kg/day (NDP14) groups expressed as percent increase of such variables as compared to ND values at the end of the study (T4). Different letters indicate significant differences between groups, with ND=a (p<0.05).
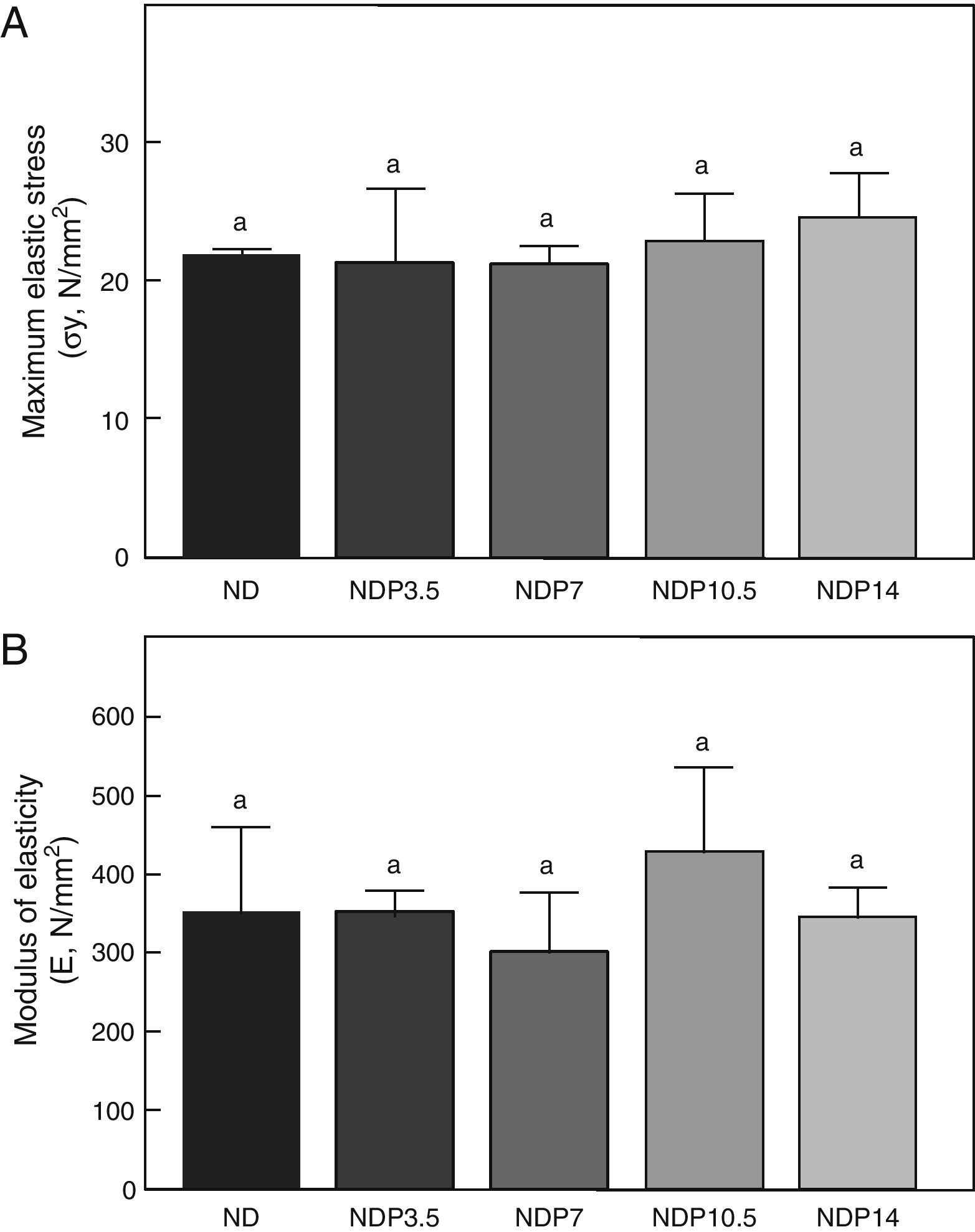
Material properties. (A) Maximum elastic stress (σy), and (B) modulus of elasticity (E) of femora from animals in the experimental ND+propranolol 3.5mg/kg/day (NDP3.5); experimental ND+propranolol 7mg/kg/day (NDP7); experimental ND+propranolol 10.5mg/kg/day (NDP10.5); and experimental ND+propranolol 14mg/kg/day (NDP14) groups expressed as percent increase of such variables as compared to ND values at the end of the study (T4). Different letters indicate significant differences between groups, with ND=a (p<0.05).
Bone resistance to fracture relative to weight (Wf/p; N/g) in undernourished animals treated with P was 0.087±0.02, 0.176±0.02, 0.146±0.01, and 0.092±0.02 in the NDP3.5, NDP7, NDP10.5, and NDP14 groups respectively. Wf/p was significantly higher in the NDP7 and NDP10.5 groups as compared to the ND group (p<0.05). Although fracture resistance relative to body weight was higher in NDP7 as compared to NDP10.5 animals, no significant differences were shown between the groups (p<0.05). There were no significant differences in Wf/p in the ND, NDP3.5, and NDP14 groups (p<0.05).
Regarding the geometric properties of bone, the results of this study showed that undernourished animals treated with 7 and 10.5mg/kg/day of P, NDP7 and NDP10.5 respectively had significant increases in CSA, A, and Ix as compared to ND rats (p<0.01) (Fig. 7). No significant differences were seen between CSA, A, and Ix in the femoral midshaft of NDP7 as compared to NDP10.5 animals. The medullary area was not affected during treatment with any dose of P used (p>0.05).
P administered to ND rats at doses of 3.5 and 14mg/kg/day caused no changes in CSA, A, MA, and Ix (p>0.05) (Fig. 7).
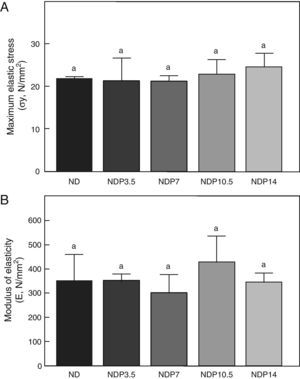
As regards the intrinsic quality of the bone material, no differences were seen in maximum elastic stress and modulus of elasticity within the group of undernourished animals treated with P or between these and the untreated ND group (p<0.05) (Fig. 8).
DiscussionAccording to modern concepts of bone biology, skeletons are live structures that constantly change in response to various stimuli, including mechanical, pharmacological, environmental, and nutritional stimuli; the latter have a great impact on bone quality.2,4–6,17 Indeed, the nutritional factor is one of the determinants in bone acquisition during growth, peak bone mass in adulthood, and the extent of bone loss in old age.
Because of the relationship between the integral development of individuals, health-disease balance, nutritional status, and the biomechanical fitness of bone, and the evidence of β-adrenergic control of bone, the objective of this study was to assess, in an animal model of growth retardation, the effect of different doses of a β-blocker, P, on anthropomorphometric and biomechanical bone variables. Potential changes in bone quality related to mechanical use were assessed in order to infer the most adequate dose that would make it possible to achieve the appropriate structural rigidity to withstand the usual and/or maximal mechanical stimulation to which control animals of the same chronological age could be subjected.
The restriction imposed in this study was sufficiently severe as to negatively affect body growth in both groups of undernourished animals treated with P and in untreated ND animals. Similar behavior was seen in femoral growth in malnourished animals after restriction for 4 weeks. Regardless of nutritional status and the drug dose administered, no significant differences were shown in the above-mentioned parameters between treated groups and their respective controls, C and ND that suggests that the drug used would not induce per se changes in global body weight and length and particularly in bone. Such results agree with those reported by other authors who recorded bone mass changes without anthropometric changes upon administration of the β-blocker.19
As regards the structural properties of the midshaft recorded through analysis of the load/deformation charts, the results of this study showed that they were negatively affected after 4 weeks of food restriction in undernourished animals as compared to the corresponding controls. It may thus be stated that, based on the concepts proposed by Frost for the self-control mechanism of bone mechanical quality,39 an imbalance in the mechanism regulating the bone's mechanostat could be the result of a reduced mechanical stimulation due to a decreased body weight in ND animals as compared to the control group of the same chronological age. It may be stated that the lower the body weight, the less the bone deformation, which would be interpreted by the osteocyte as a signal of biomechanical error, leading to increased osteoclastic activity and bone resorption, which would cause the mechanostat to adjust bone deformation to the new body weight reached by ND animals. It may be noted, however that there were no significant differences between ND rats and their respective controls in femoral fracture load related to body weight, which suggests that the effective resistance of the femur to losing its integrity as a single body is preserved in malnourished animals.
In addition, the relatively lower bone rigidity of ND rats as compared to C rats of the same age could be sufficient for the bone structure of undernourished animals to withstand poor daily mechanical stimulation, but insufficient to withstand the usual and/or maximal physiological efforts of a healthy, well-nourished individual.
It is known that whole bone structural properties are determined by the physico-chemical nature of calcified bone matrix (material properties focused on tissue rigidity) and by the architectural arrangement of that matrix in space (geometric properties focused on macroarchitecture).37 Any change in bone structural properties may and should be explained by changes in material and/or geometric properties.
In agreement with the mechanical properties analyzed, total and cortical areas, as well as the moment of inertia of the cross-section, were significantly lower in ND animals as compared to their respective controls, which may reflect a lower bone mass with an altered spatial distribution of such mass in the animal model of ND.
Analysis of the cross-sectional area of the femoral midshaft showed a cortical wall width approximately 30% smaller in ND versus control animals. Width significantly increased with administration of P at doses of 7 and 10.5mg/kg/day, which suggests an increase in bone mass due to periosteal apposition and/or decreased resorption in the presence of β-blocker in this experimental model. Similar results were seen after the administration of low doses of the β-blocker for a longer time to ovariectomized adult rats.30,40
The lack of significant differences in the medullary width of the cross section between the ND and C groups suggests that the lower bone mass seen in undernourished animals could be the result of decreased bone formation and/or increased subperiosteal resorption with no changes in endosteal surface.
While no significant differences were seen in the cortical cross-sectional area or the moment of inertia between the GRP7 and GRP10.5 groups, the values of those variables tended to be higher in the first than in the second group, thus suggesting a better architectural design of mineralized bone material in undernourished rats treated with 7mg/kg/day of P.
The indicators of the material properties of cortical bone, i.e. modulus of elasticity (a marker of the intrinsic rigidity of bone material) and maximal elastic stress (an indirect marker of bone tissue resistance), were not affected by food restriction, as shown in prior studies conducted at our laboratory,2 or drug administration. The absence of differences in material properties in undernourished animals treated with P suggests that increased resistivity of the femoral shaft would be directly related to increased cortical bone mass and greater efficiency of the architectural model.
According to current concepts, the skeleton is dynamically regulated by deformations caused by regional muscle use for the purpose of optimizing the mechanical efficiency of the architectural design as a function of the load supported by bone regions, with muscle contraction representing the greatest physiological load on bone.41–44
Although, as previously stated, the bone resistivity of ND animals relative to body size reached may be sufficient to withstand low intensity daily mechanical forces, this condition of bone structure would not be adequate to withstand the maximal mechanical stimulation to which animals of the same chronological age could be subjected. Increased resistivity in NDP7 and NDP10.5 animals as compared to ND rats suggests that the mechanical fitness of undernourished animals treated with P 7 and 10.5mg/kg/day for potential maximum physiological effort has been optimized or improved, with the resultant maintenance of a bone deformability far from the critical level at which fracture may occur. However, an analysis of the effect of P dose at which the greatest femoral resistivity was achieved, 7mg/kg/day for 4 weeks, on muscle mass of ND animals showed that this mass relative to body weight did not change as compared to ND and C. Indeed, quadriceps/body weight (mg/g) in the NDP7 versus ND versus C groups was 2.70±0.09 versus 2.63±0.06 versus 2.80±0.11 respectively (p>0.05). These results suggest that P could have an effect on the functional status of bone mechanostat that would not be exerted through a change in the mechanical environment. The incorporation of bone modeling in NDP7 animals could result from a change in the perception of the extent of the deformation, a biomechanical reference point due to a decreased mechanical remodeling threshold and/or the response of osteoclast–osteoblast effectors in the present model of nutritional stress.
Although Frost characterized bone modeling and remodeling as two dynamic effector processes with the participation of osteoclasts and osteoblasts in bone resorption and formation,39 controversy still exists about the role of such mechanisms in mechanical conditions such as the disuse mode seen in ND animals. Research on the behavior of osteocytes as mechanosensor cells would allow us to understand the operational mechanism of the mechanostat in the functional adaptation of bone to the load to be supported.
There is evidence to suggest that osteocyte apoptosis is required for resorption to be started in situations of bone deformation below the remodeling mechanical threshold.45 While the mechanism by which the mechanosensor cell dies in a mechanical disuse situation is unknown, inadequate nutritional supply, as well as the removal of osteocyte metabolic products, may cause, at least partly, the decrease in cell survival. In this regard, it may be stated that P, administered at doses of 7mg/kg/day to NDP7 animals, would have the most adequate antiapoptotic effect, being responsible for the viability of the mechanosensor with the resultant increase in bone quality in ND rats.
From a pharmacodynamic viewpoint, the lower structural rigidity seen in NDP10.5 versus NDP7 animals could be the result of a dose-dependent differential intrinsic efficacy of P related to ligand–receptor-intracellular signaling cascade interaction and/or to regulation of β-adrenergic receptors. In fact, P may behave as a partial inverse agonist when administered at doses of 10.5mg/kg/day under the regimen used here. This is a phenomenon closely related to drugs recognized by G protein-coupled receptors. Indeed, recent studies show the participation of many β-adrenergic antagonists, including P, which would exert a partial agonist activity to decrease cAMP levels and would increase MAPKinase signaling through the recruitment of β-arrestins.46
The results of this study show that P doses of 3.5mg/kg/day do not induce changes in the mechanical quality of the femur in ND rats. This response could be the consequence of a dosage inadequate to produce a beneficial effect of P on the appendicular skeleton. The lack of response seen in NDP14 versus NDP7 animals could be due to the fact that the β-blocker used, administered at a dose of 14mg/kg/day, behaved as a total inverse agonist and/or to adrenoceptor sensitization events.
It may be concluded that P 7mg/kg/day would be the most effective dose for the incorporation of bone modeling, leading to the increased structural and mechanical efficiency of appendicular skeleton in this animal model of growth retardation. Such an effect could result from the maintenance of mechanosensor viability, changes in its sensitivity to deformation, the biomechanical reference point and/or the effector response in ND rats.
FundingThis study was funded by Buenos Aires University (UBACyT O004 Project).
Conflicts of interestThe authors state that they have no conflicts of interest.
Authors thank Graciela Champin for her technical assistance.
Please cite this article as: Lezón CE, et al. Efecto de diferentes dosis de propranolol sobre la eficiencia estructural y mecánica esquelética en un modelo animal de retraso del crecimiento. Endocrinol Nutr. 2012;59:9–20.