To assess blood glucose in patients with uncontrolled type 2 diabetes mellitus treated with oral antidiabetic drugs in primary care at the time of referral to specialized endocrinologists, and the degree of implementation of the national consensus guidelines of the Spanish Society of Diabetes by evaluating steps one (S1), two (S2), and three (S3) of the escalating therapy.
Material and methodsRetrospective, observational study where 81 endocrinologists evaluated patients ≥40 years of age referred from primary care between July 2012 and July 2013, treated with 1–2 oral antidiabetic drugs but no insulin therapy, and with glycosylated hemoglobin (HbA1c) levels ≥6.5%. Patients also had to have HbA1c levels and both fasting and postprandial plasma glucose measurements from the previous three months.
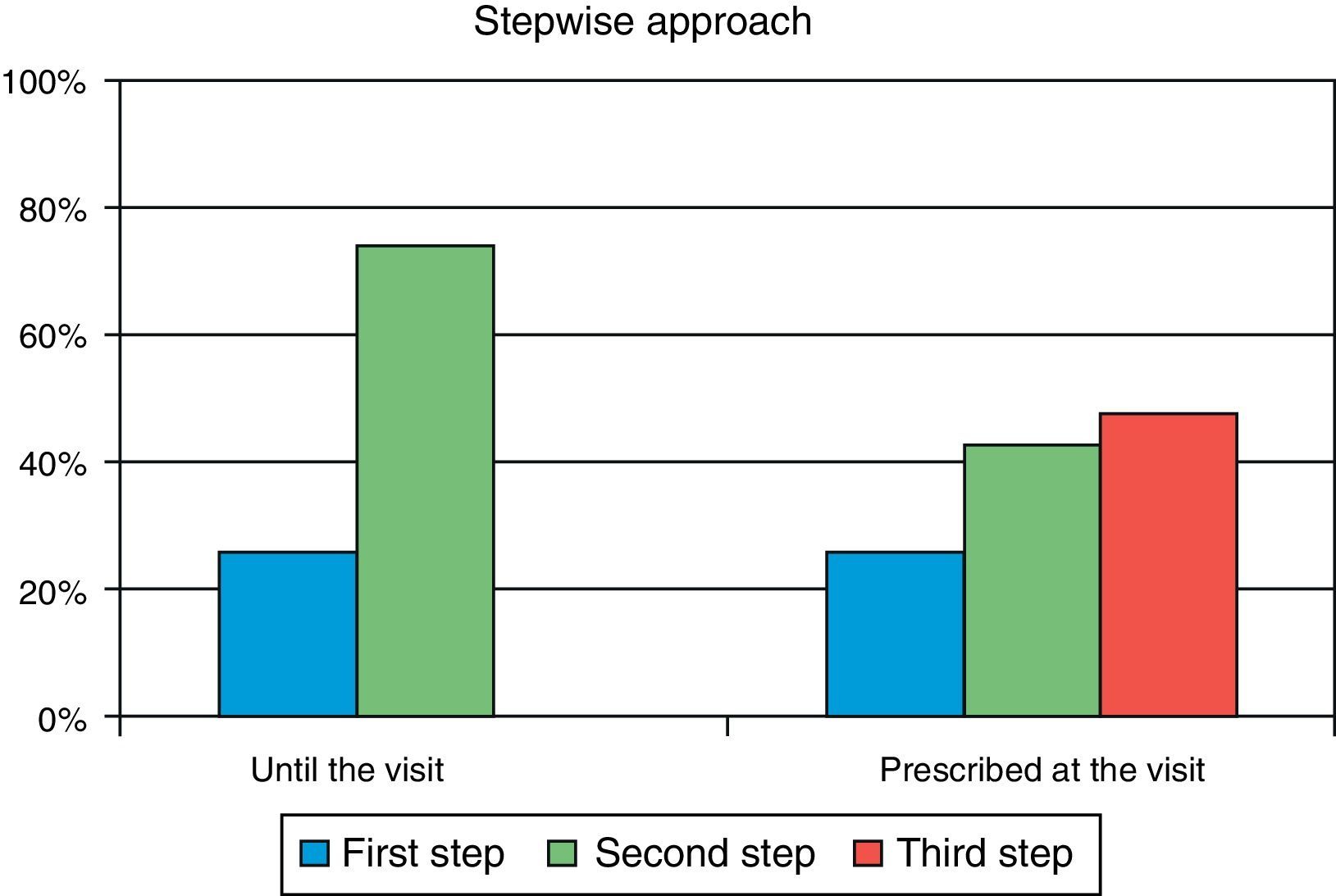
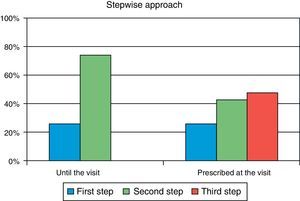
ResultsA total of 285 patients (57.6% males) were assessed. Mean (SD) age was 63.1 (9.7) years, mean HbA1c was 8.5 (1.2) %, mean FPG was 171.7 (43) mg/dL, and mean postprandial plasma glucose was 206.8 (50) mg/dL. In primary care, 26.0% of patients were at S1 and 74.0% were at S2. After referral to the endocrinologist, 9.8% of patients moved onto S1, 42.8% onto S2, and 47.4% onto S3. Oral antidiabetic drugs most commonly prescribed in primary care were metformin (90.2%), DPP-4 inhibitors (34.4%), and sulfonylureas (30.5%), while drugs most commonly used in the specialized endocrinology setting were metformin (86%), insulin (56.8%), and DPP-4 inhibitors (49.8%). The most commonly followed guidelines were those of the American Diabetes Association and the consensus guidelines of the Spanish Society of Diabetes, in 77% and 45% of cases respectively.
ConclusionApproximately half the patients treated with oral antidiabetic drugs in primary care are prescribed insulin after referral to an endocrinology specialist. The most commonly followed guidelines in specialized care are the American Diabetes Association guidelines.
Evaluar el control glucémico en pacientes con diabetes tipo 2 que son remitidos a Endocrinología desde Asistencia Primaria (AP) por no estar controlados con antidiabéticos orales sin insulinoterapia; y el grado de implementación del consenso nacional de la Sociedad Española de Diabetes, valorando los sucesivos escalones, primero (Pe), segundo (Se) y tercero (Te), del abordaje terapéutico.
Material y métodosEstudio observacional retrospectivo en el que 81 endocrinólogos evaluaron los pacientes mayores de 40 años remitidos por AP entre julio de 2012 y julio de 2013, tratados con 1–2 antidiabéticos orales, no insulinizados, con una hemoglobina glucosilada (HbA1c) ≥ 6,5%, y en los que se disponía en los 3 meses previos de Hb1Ac, glucosa capilar en ayunas y glucosa capilar posprandial.
ResultadosFueron evaluables 285 pacientes (57,6% varones), con una edad media (DE) de 63,1 (9,7) años, HbA1c media de 8,5 (1,2)%, glucosa capilar en ayunas 171,7 (43) mg/dl y glucosa capilar posprandial 206,8 (50) mg/dl. En AP el 26,0% de los pacientes se situaban en Pe terapéutico y el 74,0% en el Se. En atención especializada solo el 9,8% de la cohorte está en el Pe, el 42,8% en el Se y el 47,4% en el Te. Los fármacos más prescritos en AP fueron metformina (90,2%), inhibidores DPP-4 (34,4%) y sulfonilureas (30,5%), mientras que en Endocrinología fueron metformina (86%), insulina (56,8%) e inhibidores DPP-4 (49,8%). Las guías clínicas más seguidas fueron las de la American Diabetes Association y el consenso de la Sociedad Española de Diabetes, en un 77 y 45% respectivamente.
ConclusionesAproximadamente la mitad de los pacientes con diabetes mellitus 2 no insulinizados y tratados con antidiabéticos orales en AP, son tratados con insulina en Endocrinología. La guía clínica más seguida por el especialista es la de la American Diabetes Association.
The incidence and prevalence of type 2 diabetes mellitus (T2DM) have linearly increased in recent decades due to changes occurring in Western and emergent societies, closely related to the pandemic of obesity.1 Prevalence has also increased in Spain to approximately 14% of the population.2 DM is a significant public health problem because of both its prevalence and associated comorbidities and the huge direct and indirect health costs derived from its care. In Spain, the costs caused by a diabetic patient are 1.5-fold greater as compared to a non-diabetic patient.3
Scientific evidence from controlled trials has shown that intensive glucose-lowering treatment significantly decreases the risk of developing long-term microvascular complications.4,5 The benefits of intensive treatment for macrovascular complications are however not so evident.6 A number of recently reported interventional studies have not shown any reduction in total or cardiovascular mortality (CV) when the blood glucose goal was very stringent (HbA1c≤6.0%7,8 or 6.5%)9. Clinical practice guidelines and recent consensus documents, such as the national consensus document of the Spanish Diabetes Society (SED)10 and the joint consensus document of the American Diabetes Association–European Association for the Study of Diabetes (ADA/EASD),11 include recent evidence-based information. They therefore propose and emphasize that therapeutic management should be performed in the context of the needs, preferences, and tolerability of each patient, stressing that individualization of treatment is essential for success. Moreover, the national SED consensus document10 represents one of the first guidelines for the treatment of T2DM to include insulin therapy as a second step after the failure of metformin associated with another oral antidiabetic (OAD) drug, and even initially when OADs are contraindicated if the HbA1c level ranges from 6.5% to 8.5%, or is >8.5% in the presence of clinical signs of hyperglycemia. However, although prior guidelines issued by scientific societies other than the SED,10 such as those of ADA (2009)12 and the Canadian Diabetes Association (2008),13 address goal values, stepwise management, and patient characteristics, there are some differences between them. This is partly due to the lack of controlled studies with an adequate sample size that would enable us to compare the differences in the goals to be achieved in accordance with the steps proposed in the different guidelines. Therefore, the national consensus document of the SED10 has not only considered treatment individualization based on patient characteristics, but has adapted it to the available Spanish evidence.
The purpose of this study was to assess blood glucose control in insulin-naïve patients with T2DM not controlled with OADs referred from primary care (PC) to the endocrinology department, and the degree of implementation of the national SED consensus document on the drug treatment of hyperglycemia, assessing the sequential steps one (S1), two (S2), and three (S3) in the therapeutic approach.
Patients and methodsThis was an observational, retrospective, multicenter study including patients with T2DM treated with OADs. The study was conducted by endocrinologists at outpatient clinics from Spanish hospitals or other centers representative of all the autonomous communities, weighted by population density. Patients who met the selection criteria were consecutively enrolled.
Selection criteria and objectivesSelection criteria included: (a) age older than 40 years, (b) current treatment with one or more hypoglycemic drugs other than insulin, (c) HbA1c≥6.5% in the three months prior to the first visit to the endocrinologist, (d) the availability of blood glucose records for at least 3 months, and (e) informed consent. Patients with other etiologies of DM were excluded.
The primary study objective was to assess glycemic control parameters in patients with T2DM previously attending PC with suboptimal control on a regimen consisting of 1–2 oral hypoglycemic drugs and who first attended the endocrinology department. The secondary objective was to assess the degree of implementation of the national consensus for drug treatment of T2DM by analyzing the stepwise approach to treatment used by the endocrinologist and recording the clinical practice guidelines followed by endocrinologists.
Study conductEach endocrinologist had to record clinical information from the first four patients who met the selection criteria. Researchers collected at a single visit retrospective information through a complete clinical history taken in standard clinical practice conditions during the second visit of the patient to the endocrinologist after referral from PC. Information was collected from the clinical records, as they included the variables to be taken into consideration in the study objectives. Demographic data, associated cardiovascular risk factors (HBP, dyslipidemia, overweight-obesity, smoking, physical exercise), associated CV conditions, and data related to T2DM (duration and organ impact: nephropathy, retinopathy, diabetic foot, and neuropathy), anthropometric data (body mass index, abdominal circumference), information from physical examination (blood pressure), and laboratory parameters (total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and serum creatinine) were also collected. Metabolic control assessed in the primary objective consisted of plasma levels of HbA1c (NGSP), measurements of fasting capillary blood glucose (FCG), measurements of postprandial capillary blood glucose (PCG), and the number of episodes of symptomatic or severe hypoglycemia. For FBG and PBG, information in endocrinologist records on prior measurements made in PC and mean measurements in the previous three months and in the previous 30 days was collected. For the secondary objective, treatments prescribed to patients by PC physicians, those prescribed by endocrinologists after clinical evaluation of the patients, and the clinical practice guidelines used were collected. Treatments prescribed at PC for DM and data to assess the degree of implementation of the national consensus for drug treatment of T2DM were taken from clinical records verbally reported by patients at the visit to endocrinology.
DefinitionsUncontrolled DM was defined as fasting blood glucose≥126mg/dL, postprandial blood glucose≥180mg/dL, and HbA1c≥6.5%.14 HBP was defined, using the values proposed for DM patients, as values higher than 130/85mmHg or the use of antihypertensive drug treatment.15 Obesity was defined as a body mass index (weight in kg/height in m2) of 30kg/m2 or higher according to the Spanish Society for the Study of Obesity,16 and central obesity as a waist circumference greater than 102cm in men and 88cm in women.17 Dyslipidemia was defined as LDL cholesterol levels of 100mg/dL or higher and/or triglyceride levels of 150mg/dL or higher and/or HDL cholesterol levels less than 40mg/dL in men and 50 in women,14 or the current administration of drug treatment for dyslipidemia. Left ventricular hypertrophy was determined based on echocardiographic or ECG criteria, according to the clinical records. Stratification of the three treatment steps of T2DM was based on the sequential phases suggested by the SED.10 The first step essentially consists of introducing metformin, or a different OAD if metformin is not tolerated, if HbA1 ranges from 6.5–8.5%, and metformin with another OAD if HbA1 is >8.5%. The second step is a combination of two drugs, one of which may be insulin, and in the third step, insulinization, if not previously given, with or without other OADs, or triple oral therapy is started. The change to a higher step depends on whether or not metabolic control is achieved three months after the prior evaluation.
Statistical analysisThe descriptive statistics of all variables assessed included measures of central tendency and dispersion for quantitative variables and absolute and relative frequencies for qualitative variables, with 95% confidence intervals in both cases. Sample size calculation assumed a two-sided 95% confidence interval to estimate the mean with an error ±0.1, a standard deviation of 0.9, and the confidence interval was based on the statistical Z value for large samples, so that the calculated sample was 320 patients.
ResultsBaseline sample dataEighty-one endocrinologists participating in the study enrolled 320 patients with T2DM from July 2012 to July 2013; 35 of these patients were excluded from the study. The reasons for exclusion included treatment with more than two non-insulin agents at the first visit to the endocrinologist (28 patients), HbA1c<6.5% in the previous three months (7 patients), and age under 40 years (two patients). Two patients excluded did not meet more than one criterion. A total of 285 patients with a mean age of 63.1 years were evaluable for the study. Fifty-one per cent of patients were obese. One third of the patients had trunk adiposity, with an abdominal circumference greater than recommended. LDL cholesterol levels were higher than 100mg/dL in 61% of patients.
ObjectivesPrimary objectiveData are also given on the proportion of patients with uncontrolled HbA1c, FPG and PPG levels according to ADA criteria.14 The low proportion of patients with a self-measured capillary glucose profile within the objectives should be noted: only 20% and 25% of them had controlled FCG and PCG respectively. Approximately 80% had HbA1c values ≥7.5%. Daily, weekly, and monthly blood glucose self-monitoring was performed by 25.9%, 50.4%, and 23.7% of patients respectively. No hypoglycemia was reported by 90.6% of patients, while 4.7% of patients reported one episode of hypoglycemia and 4.7% two episodes of hypoglycemia in the month prior to the visit to endocrinology.
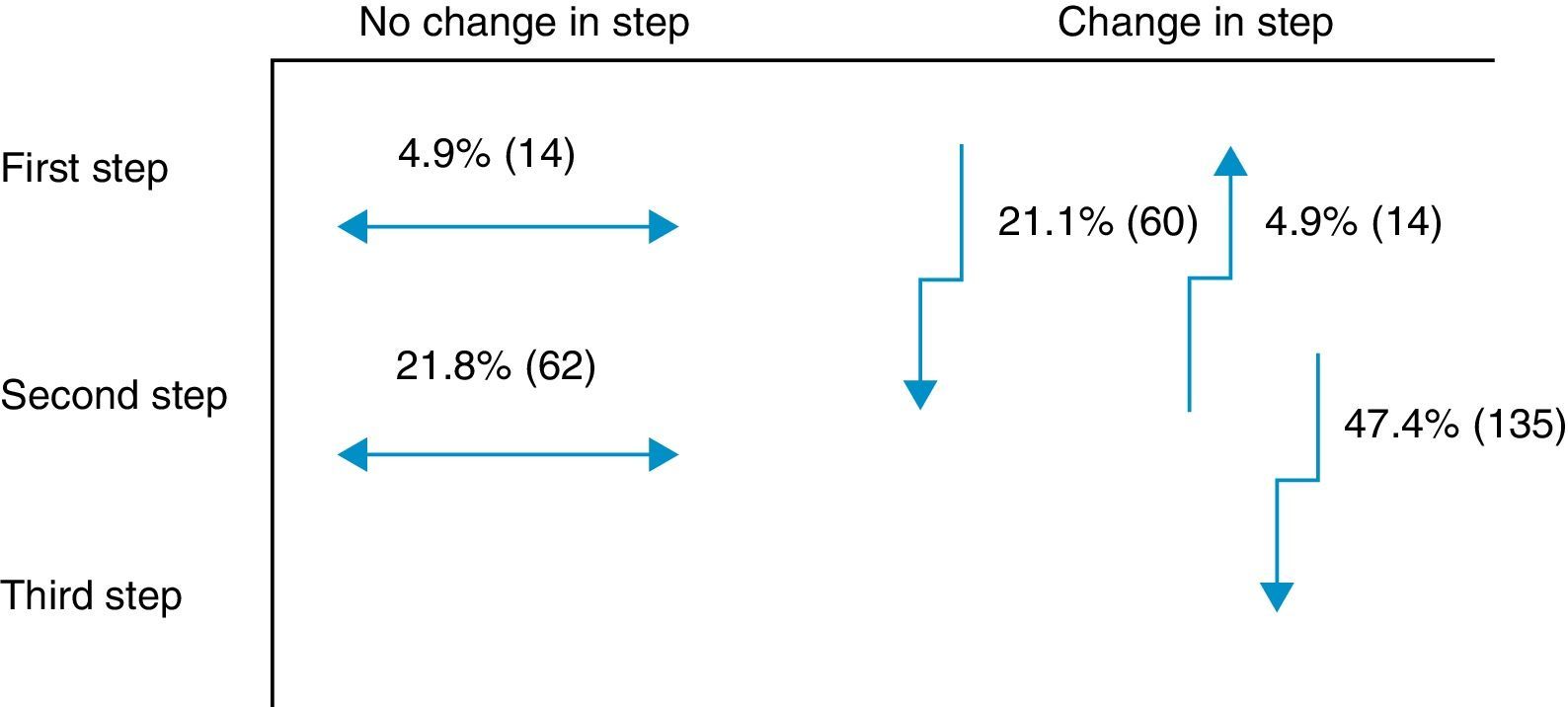
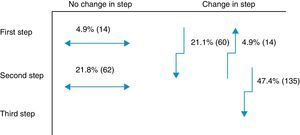
Secondary objectiveAt the time of intervention by endocrinologists, 26% of patients were receiving one drug (first step) and 74% were receiving two drugs (second step). With the regimen prescribed by the endocrinologist, only 9.8% of patients were in the first step, 42.8% in the second step, and 47.4% in the third step. Fig. 1 shows the change in step at the visit to the endocrinologist. It can be seen that the most common treatments in PC were metformin, DPP-4 inhibitors, and sulfonylureas, received by 90%, 34%, and 31% of patients respectively, while endocrinologists most commonly prescribed metformin (86%), insulin (57%), and DPP-4 inhibitors (50%). At endocrinology, insulin therapy was started in 56.8% of patients, all of them given a basal insulin scheme and from all treatment steps: 5.3% from the first step, 17.2% from the second step, and 34.4% from the third step. The clinical practice guidelines most commonly used in endocrinology were the ADA guidelines (76.6%), followed by those of the SED (45.3%), EASD (44.9%), and the Canadian Diabetes Association (1.1%) (Fig. 2).
Data from this study suggest that 41% of patients with T2DM not given insulin therapy seen at PC and not controlled with one or two OADs have HbA1c levels ≥8.5%, while the remaining 59% have values ranging from 6.5% to 8.5%. HbA1c level was ≥7.5% in up to 82% of the cohort. Referral to endocrinology implies a basal insulinization of 56.8% of patients in all steps, in accordance with the recommendations of the SED consensus document,10 although the clinical guidelines for the treatment of T2DM followed by the greatest proportion of specialists (77%) were those of the ADA. The characteristics of the patients assessed in this study were similar to those of recent series reported in Spain in primary care (the LAURA study),18 in the specialized setting (the INSTIGATE study),19 and in both settings together (the DIABES study),20 except for the prevalence of obesity, which was higher in our study (51%). One of the selection criteria for patients assessed by endocrinologists was an HbA1c value ≥6.5%. The mean value of this parameter at the last measurement recorded by the PC physician was 8.5%, similar to that reported in the INSTIGATE study19 before insulinization by specialists, and they were also previously treated with two OADs. The evaluated sample may be considered representative of patients referred to specialists from PC.
It is well known that many patients have a lower than recommended control before basal insulin is started. This was confirmed by the finding of HbA1c values >8.5% in 41% of patients when they were referred to the endocrinologist on one or two OADs. DM control was the poorer, the longer the duration of the disease,20 which was 10 years on average in our series. The SED10 considers basal insulin therapy associated with metformin to be an adequate option after the failure of metformin monotherapy. Delay in insulinization is common both in our environment and outside it.21,22 The importance of early insulin therapy had already been stressed in the 2009 ADA guidelines, which recommended therapy in patients not achieving the goal of HbA1c<7% after 2–3 months with metformin together with lifestyle changes.12 This objective was qualified in the 2010 SED consensus document.10 The 2009 ADA12 and SED10 guidelines were effective at the time this study was conducted, but the new ADA/EASD guidelines were issued at the same time as the study was being performed, leading to a joint ADA/EASD document11 in 2012. After publication of the studies Action to Control Cardiovascular Risk in Diabetes,8Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation10 and the Veterans Affairs Diabetes Trial,9 showing that intensive, indiscriminate treatment of diabetic patients was not associated with benefits in CV mortality and, in the Action to Control Cardiovascular Risk in Diabetes study,8 showing that it increased CV and total mortality, the therapeutic recommendations were changed, and treatment individualization based on patient characteristics was emphasized.
One innovative aspect of the SED consensus document10 is insulinization associated with metformin in the second treatment step, which has been included in the latest joint ADA/EASD guidelines.11 The importance of adding basal insulin and being able to improve effectiveness was demonstrated in the TULIP study,23 where patients with HbA1c levels ranging from 7.0% to 8.0% treated with maximum doses of metformin and sulfonylurea were randomized for an intensification of measures to modify lifestyle or insulin glargine, and a better control was seen in the insulin therapy arm. Insulin was prescribed to 57% of this study cohort in the different steps. Of the 47% of patients changed to the third step at the visit to the endocrinologist, 27% were prescribed a third OAD and the remaining 73%, insulin. In this third step, most endocrinologists appear to have followed the recommendations of the SED10 and the 2012 ADA/EASD,11 which placed at the same level triple therapy with three OADs and triple therapy with insulin. An argument to reinforce this latter option is that triple therapy with three OADs has not shown an additive effect24 and is associated with a greater chance of adverse effects as compared to insulin addition.25 Moreover, treatment adherence is lower26 due to multiple drug administration. Combination with insulin is most often recommended according to the SED guidelines.27 Insulin therapy, however, has had to overcome, and sometimes still meets, a certain resistance on the part of physicians and patients themselves, particularly due to the perception of a risk of hypoglycemia28 and a potential impairment in quality of life.29 However, the introduction of long-acting analogs in a basal regimen administered once daily partly overcomes these disadvantages, especially as regards the rate of hypoglycemia, particularly at night.30 Thus, the LAUREL study31 demonstrated a better blood glucose control after switching to a long-acting insulin; in PC, the RESCUE study32 stressed the decreased risk of hypoglycemia after switching to a combined regimen of OAD and insulin glargine. In the LAURA study,18 conducted in PC, a 6.6% rate of symptomatic or severe hypoglycemia was found in patients prescribed insulin glargine in the month prior to the visit. In our study, a 9.4% rate of symptomatic and severe hypoglycemia was found in patients with 1–2 OADs before insulinization.
It should also be noted that the prescription of DPP-4 inhibitors by endocrinologists also increased in this study because they are a drug class with a neutral effect on weight and a low hypoglycemic profile, similar to metformin.11 By contrast, a decrease was seen in the prescription of sulfonylureas, which are associated with increased weight and a high risk of hypoglycemia.11 The combination of metformin and DPP-4 inhibitors is the association most commonly prescribed by endocrinologists. This implies a greater rationalization of the treatment scheme with OADs and greater safety by its being associated with insulin if necessary.
The ADA guidelines for the treatment of T2DM have had the greatest clinical impact in the field of endocrinology. Since the ADA/EASD consensus11 was published during the conduct of this study, it is difficult to know which recommendations were actually followed by endocrinologists. However, the SED consensus document10 and the 2012 ADA/EASD guidelines11 report similar objectives, and it may therefore be assumed that the vast majority of Spanish endocrinologists currently follow similar treatment patterns.
This study had some design limitations which should be taken into account when interpreting the results. The study retrospectively collected clinical variables with no control group and no assessment of the efficacy of the treatment prescribed by the specialists. Moreover, the publication of the ADA/EASD guidelines (2012) during the study conduct did not allow for a clear interpretation of which ADA guidelines the endocrinologist referred to. However, the fact that the study was conducted in the setting of standard clinical practice is a positive aspect to be taken into consideration. In conclusion, in patients with T2DM with suboptimal blood glucose control on 1–2 OADs seen at endocrinology clinics according to standard clinical practice and referred from PC, basal insulin therapy is started in all three treatment steps in more than one half of the cases. On the other hand, the clinical guidelines for the treatment of hyperglycemia most commonly followed by Spanish endocrinologists are the ADA guidelines.
Funding of the studyThe study was funded by Sanofi Aventis Spain.
Conflict of interestDr. Azriel has received funding for papers and clinical trials from Sanofi-Aventis, Novo-Nordisk, Astra-Zenneca, and Lilly-Boehringer.
To all the physicians participating in the basal plus study, without whose cooperation this study could not have been conducted. This manuscript was prepared with the help of Dr. José Mora Maciá, a medical writer funded by Sanofi Aventis Spain.
Please cite this article as: Azriel S, Casal F, Dalama B, Varillas F, Villarroel Á, Soto A, et al. Parámetros de control glucémico en pacientes con diabetes tipo 2 no insulinizados derivados a consulta de Endocrinología, y grado de implementación del consenso nacional sobre el tratamiento de la hiperglucemia. Endocrinol Nutr. 2014;61:541–547.