Diabetes and frailty are two conditions that frequently occur concurrently and are increasingly prevalent in the older patient. We review the concept, epidemiology and consequences of frailty, and the implications of the presence of frailty in the management of diabetes. Frailty is associated with decreased quality of life, a risk of falls, new or increased disability, hospitalization, and increased mortality. All of these factors affect the management of diabetes in older patients. It is important to rule out frailty in all diabetic patients aged >70 years; if frailty is suspected, a comprehensive and multidisciplinary medical and functional assessment of the patient should be conducted to develop an individualized treatment plan. This plan should include nutritional measures, physical activity, and education on self-care and diabetes; drugs should not be used without a clear indication. Antihyperglycemic drugs that may cause excessive weight loss and/or are associated with a high risk of hypoglycemia should be avoided.
La diabetes y la fragilidad son 2 procesos que se producen a menudo simultáneamente y son cada vez más prevalentes en los pacientes mayores. Revisamos aquí el concepto, la epidemiología y las consecuencias de la fragilidad, y las implicaciones de la presencia de fragilidad en el tratamiento de la diabetes. La fragilidad se asocia con un empeoramiento de la calidad de vida, riesgo de caídas, aparición o aumento de la discapacidad, hospitalización y aumento de la mortalidad. Todos estos factores afectan al tratamiento de la diabetes en los pacientes de mayor edad. Es importante descartar la existencia de fragilidad en todos los pacientes diabéticos de más de 70 años de edad; si se sospecha fragilidad, debe efectuarse una valoración médica y funcional, exhaustiva y multidisciplinaria, del paciente para idear un plan de tratamiento individualizado. Este plan debe incluir medidas nutricionales, actividad física y educación sobre los cuidados personales y la diabetes; no deben utilizarse fármacos si no están claramente indicados. Deben evitarse los hipoglucemiantes que puedan causar una pérdida de peso excesiva o que se asocien con un riesgo elevado de hipoglucemia.
Increasing life expectancies and declining birth rates mean that the segment of the population comprising people aged >60 years is growing at a rapid rate in most countries. Worldwide, it is estimated that this population will increase from 600 million to 2 billion between 2000 and 2050.1 In Spain, according to estimates from the National Statistics Institute (2014), the population of people aged >65 years, currently 18.2%, will increase up to 24.9% in 2029, and 38.7% in 2064.2
This demographic change can be perceived as an achievement of public health policies and socioeconomic development; however, this change also poses a major challenge to society, which must adapt to improve the health and functional capacity of older people.1 In this context, frailty, which is considered to be the most characteristic clinical condition of an aging population, is particularly important.3
This article provides an overview of the concept, epidemiology and consequences of frailty, as well as the implications of the presence of frailty in the management of diabetes.
FrailtyDefinition and diagnostic criteriaFrailty can be defined as a situation of extreme vulnerability to the effects of low-intensity stressors. It results from difficulty maintaining homeostasis due to loss of functional reserve.3 Two conceptual frameworks have been used to explain frailty.
- (1)
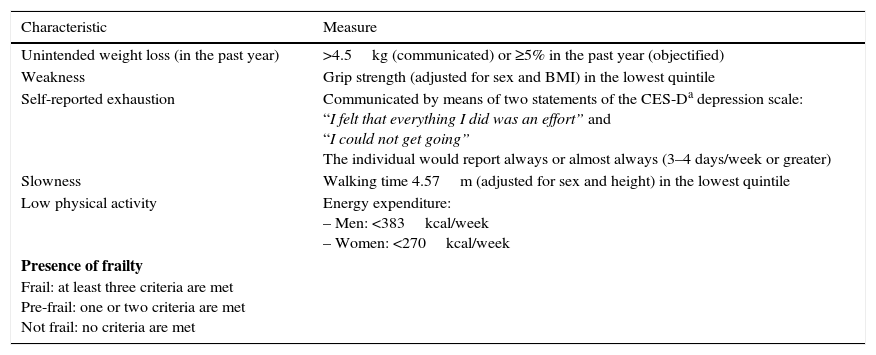
The ‘frailty phenotype’ was described by Fried et al.4 in 2001 using data obtained from the Cardiovascular Health Study. According to this phenotype, frailty represents an alteration in different physiological systems that causes the aforementioned drop in functional reserve and is expressed clinically through the five criteria shown in Table 1.4 According to this definition, a person is considered frail if they meet at least three of the five criteria and pre-frail if they meet one or two of these criteria. Those who do not meet any of the criteria are classified as robust.4
Table 1.Operational criteria of the frailty phenotype.
Characteristic Measure Unintended weight loss (in the past year) >4.5kg (communicated) or ≥5% in the past year (objectified) Weakness Grip strength (adjusted for sex and BMI) in the lowest quintile Self-reported exhaustion Communicated by means of two statements of the CES-Da depression scale:
“I felt that everything I did was an effort” and
“I could not get going”
The individual would report always or almost always (3–4 days/week or greater)Slowness Walking time 4.57m (adjusted for sex and height) in the lowest quintile Low physical activity Energy expenditure:
– Men: <383kcal/week
– Women: <270kcal/weekPresence of frailty
Frail: at least three criteria are met
Pre-frail: one or two criteria are met
Not frail: no criteria are metAdapted from Fried et al.,4 2001. - (2)
In the ‘cumulative deficit model’ created by Rockwood et al.,5 frailty is defined by the presence of deficits that accumulate over time and progress to a level of vulnerability that becomes incompatible with independent living and survival. The operational translation of this conceptual framework is embodied in the Frailty Index, which quantitatively describes the health status of the patient and the progression of their frailty over time and evaluates potential deficits the patient may have in the following categories: disease, cognitive status, health conditions, nutritional status, ability to communicate, independence for activities of daily living, motivation, perception of health status, strength, balance, mobility, sleep, social aspects, geriatric syndromes, and disability.5–7 The value of this index for each individual is calculated as the ratio of observed deficits to the total number of possible deficits.
As noted by other authors, the Fried phenotype and Frailty Index have significant differences that may influence their use; these are discussed in detail elsewhere.3,8 The Frailty phenotype can be used at the patient's first visit; in contrast, the Frailty Index requires a thorough initial geriatric assessment.8 It should be highlighted that the modified Frailty Index has a potentially superior ability to discriminate between patients with moderate frailty and those with severe frailty3 and is more sensitive to change.8 Therefore, it is theoretically more useful for assessing the outcome of interventions or describing the evolution of the health status of the patient. However, it should be noted that both models have demonstrated convergent validity with respect to health outcomes and are therefore helpful in identifying frailty.3,8
Attempts to arrive at an operative and universal definition of frailty have not yet been successful. Experts from different disciplines collaborating on the “Frailty Operative definition – Consensus Conference” project (2012) concluded that frailty is a multidimensional syndrome that affects physical function (i.e., gait and mobility), nutritional status, and mental health and cognition, and is the result of a decrease in physiological reserve and resistance to stressors.9 In a similar sense, representatives from six international societies (the International Association of Gerontology and Geriatrics; Society on Sarcopenia, Cachexia and Wasting Diseases; the International Academy of Nutrition and Aging; the European Union Geriatric Medicine Society; the American Medical Directors Association; and the American Federation for Aging Research) have defined frailty as “a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function that increases an individual's vulnerability for developing increased dependency and/or death.”10
EpidemiologyA systematic review showed that, if psychosocial components are included in the definition of frailty, the prevalence of frailty in the population aged ≥65 years is 13.6%; if the definition of frailty is limited to a physical condition, the prevalence is only 9.9%.11 Overall, the average prevalence of frailty is 10.7%; this prevalence increases with age and is higher in women than in men.11 In another systematic review that included 24 studies conducted in individuals aged ≥65 years, the prevalence of frailty was 14% when the Fried phenotype was used and 24% when the cumulative deficit model was used.12 These authors also found that the prevalence of frailty increases with age and is more prevalent in women. It is also noteworthy that Black individuals are four times more likely to develop frailty than are White individuals.13
Previous studies conducted in Spain indicated that frailty has a significant impact on the older population. In these studies, the prevalence of frailty ranges from 8.4% to 16.9%, and the prevalence of pre-frailty ranges from 41.8% to 48.5% when the Fried Frailty Phenotype is applied.14–18 Although the variables analyzed in different studies varied, the factors most commonly associated with frailty were age, comorbidity, depression, and cognitive disorders. Variability in these prevalences may be due to differences in the age of the studied populations, the use of modified Fried criteria in certain studies, and the use of different cut-off values for some of the items.
Pathophysiology and outcomesFrailty is the manifestation of disorders in different physiological systems of the body, including the central nervous, endocrine, immune, musculo-skeletal, cardiovascular, respiratory, and renal systems. Frailty becomes apparent when a (currently not accurately defined) level of decline in physiological reserves occurs.19,20
Several longitudinal studies conducted in older populations have demonstrated an association between frailty and a deterioration in quality of life, a risk of falls, new or increased disability, hospitalizations, nursing home entry, and mortality.4,21–27 Subjects who are moderately or severely frail have a relative risk (RR) for institutionalization of 8.6 (95% confidence interval [CI], 4.9–15.2) when compared with subjects who are physically active.21 Frail subjects are more likely to have an impaired quality of life and/or to have been hospitalized.26 As compared to non-frail patients, frail patients have a greater likelihood of losing basic activities of daily life (hazard ratio [HR] 2.5, 95% CI 1.3–4.8), mobility (HR 2.7, 95% CI 1.5–5.0), and instrumental activities of daily life (HR 1.9, 95% CI 1.1–3.3).27 It has also been reported that frail women have a higher age-adjusted likelihood of presenting with recurrent falls (odds ratio [OR], 2.4), disability (OR 2.2–2.8), and death (HR 2.4–2.7).23 In another study, both women and men showed an increased likelihood of death with almost identical effect sizes compared with the previous study: a HR of 2.8 (95% CI 1.5–5.3) for women, and a HR of 2.4 (95% CI 1.0–5.9) for men.24 The effect of frailty on survival has also been studied in a systematic review of studies conducted in randomly sampled older individuals.12 In this systematic review, the existence of a gradient between frailty and mortality was found, with reduced survival associated with increasing numbers of frailty criteria.12
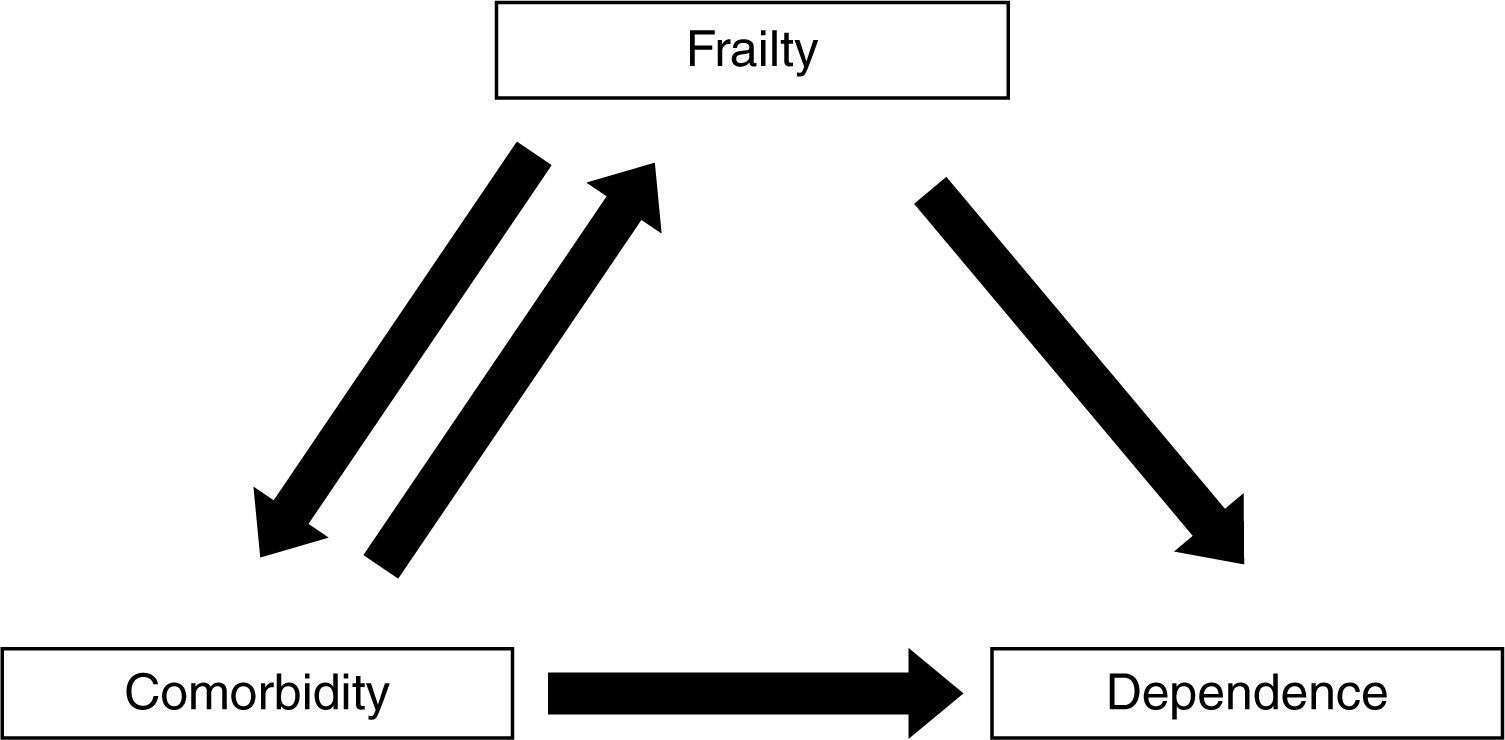
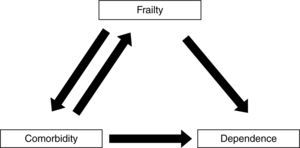
Frailty, comorbidity, and disabilityFrailty is not synonymous with comorbidity or disability, even though these concepts have overlapping definitions. Instead, comorbidity is one of the etiological factors of frailty, and disability is a result of frailty (Fig. 1).4
Interactions between frailty and various chronic diseases, such as anemia, cardiovascular diseases, chronic renal disease, cancer, HIV infection, cognitive disorder, Parkinson's disease, depression, and diabetes mellitus have been identified.19 However, five chronic diseases have been shown to be most significantly associated with the development of frailty and its progression toward disability: heart failure, cancer, renal failure, HIV infection and diabetes mellitus. All of these diseases are associated with an increased risk of frailty and other adverse outcomes.10
The diabetes–frailty association is of particular importance for the following reasons: (1) both disease states are commonly encountered in the older patient; (2) both entities share several common pathophysiological mechanisms,28–30 which could explain their frequent coexistence; (3) diabetes accelerates the aging process and therefore places the individual at greater risk of becoming frail;30 and (4) the presence of frailty in diabetic patients increases the likelihood of complications,31 functional deterioration,32,33 and mortality,34 and therefore impacts the management of these patients.35
From diabetes to frailtyEpidemiologyAn estimated 382 million people have diabetes worldwide (46% undiagnosed), and this figure is expected to grow by 55% to 592 million in 2035.36 As is the case with frailty, the incidence and prevalence of diabetes increases with age, and more than 25% of the people aged ≥65 years has diabetes.37 The International Diabetes Federation (IDF) estimates that 18.6% of people aged between 60 and 79 years have diabetes, and more than 35% of all diabetes cases are in this age group.36 In Spain, the Di@betes study showed that at least 30% of people aged between 61 and 75 years and more than 35% of people aged >76 years have diabetes.38
The prevalence of diabetes increases with the presence of frailty. The Cardiovascular Health Study showed that the prevalence of diabetes was 18.8% in individuals without frailty, 24.5% in individuals with pre-frailty, and 32.4% in individuals with frailty.39 Likewise, the presence of frailty is higher in patients with diabetes. Data from this study indicate that frailty is present in 25% of individuals with diabetes and pre-frailty is present in 18.2% compared with a prevalence of frailty of 6.9% in the whole sample who were aged ≥65 years.4 However, in Spain, the Toledo study showed that frailty is present in only 10.2% of individuals with diabetes and in 7.8% of individuals without diabetes; moreover, this study found no association between these two factors in a multivariate analysis.15 A study that evaluated the progression of frailty and pre-frailty in an older cohort living in the community found that the presence of diabetes in women with pre-frailty reduced by 50% the likelihood of their frailty improving.40
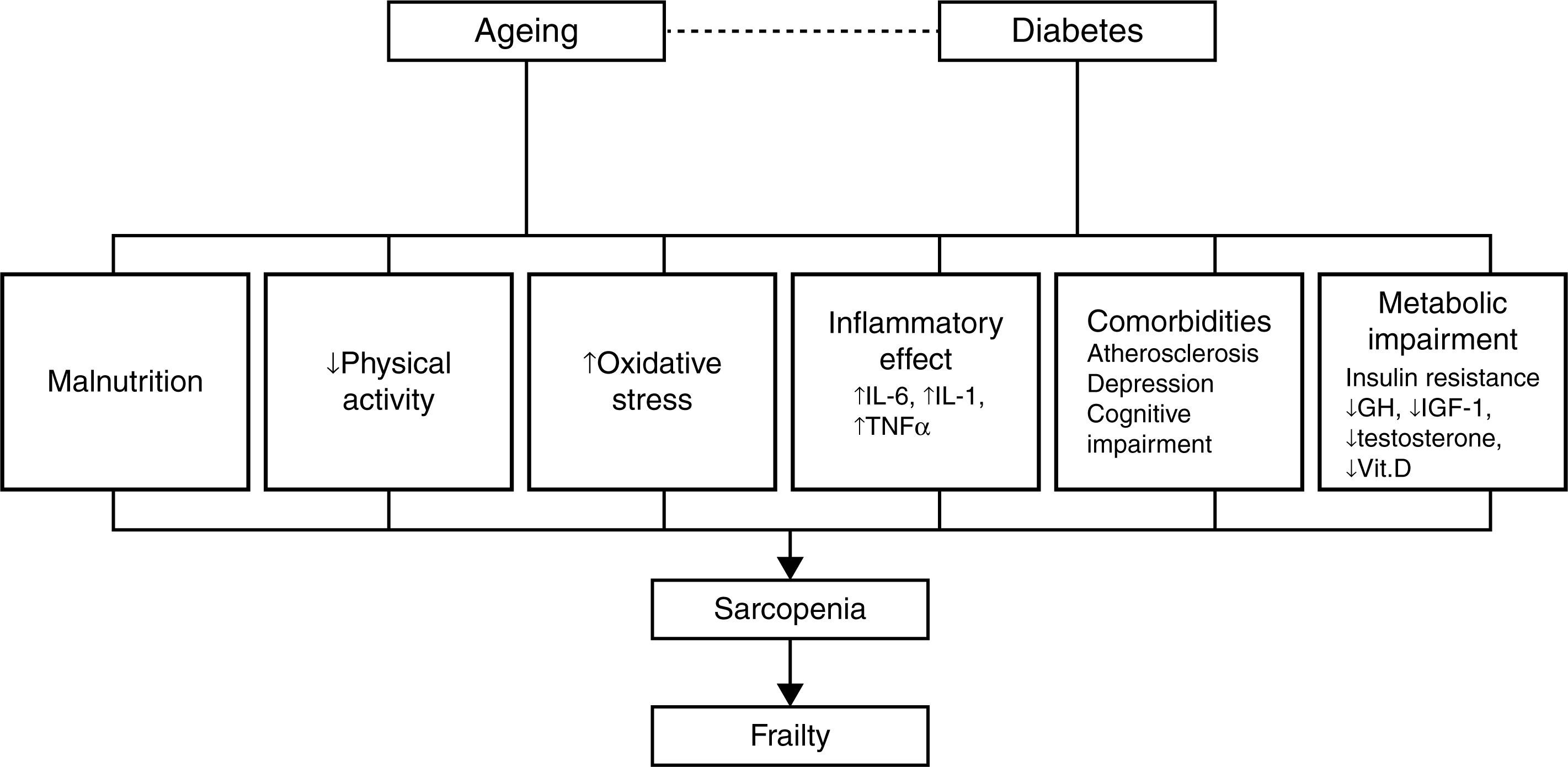
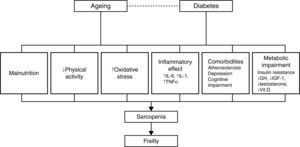
PathophysiologySarcopenia, defined as a reduction in muscular mass and function (strength or performance),41 is considered a key component of frailty and may represent the pathophysiological link between diabetes and frailty.28 Data from several studies reviewed by other authors28,30,42,43 show a close relationship between diabetes, insulin resistance, the chronic low-grade inflammation characteristic of diabetes and sarcopenia, and/or muscle deterioration. Many studies have shown that muscle strength and quality decrease in patients with diabetes, and this decline becomes more pronounced the longer the patient is affected with diabetes and the poorer their glycemic control. Insulin resistance is associated with a decrease in muscle strength, most likely due to a decrease in protein synthesis, increased degradation, and a resultant loss of muscle mass. At the same time, insulin resistance in aging patients can lead to mitochondrial alterations that result in a decrease in production of the energy required for muscle contraction and an increase in oxidative stress.
Insulin-like growth factor type 1 plays an important role in protein synthesis, and levels of this molecule decrease with age and in patients with diabetes.44,45 This decrease is related to the development of frailty, functional decline, and disability in the older patient. Research on markers related to inflammation in frail and non-frail individuals indicates that the frailest subjects have elevated levels of high sensitivity C-reactive protein, a soluble biomarker of inflammation,46,47 and other procoagulant factors. Patients with diabetes also have elevated levels of cytokines, such as tumor necrosis factor alpha and interleukin-6, that stimulate proteolysis and apoptosis in muscle cells. Additionally, different studies describe a dysregulation of the levels of various hormones, such as testosterone and cortisol, and nutrients, such as vitamin D, in patients with insulin resistance, sarcopenia, and functional decline.28–30,42
These mechanisms provide a direct association between diabetes and functional sarcopenia/impairment, and explain most of the attributable risk of disability in older patients with diabetes. However, other factors can explain the relationship between these two entities, including atherosclerosis, depressive illness, and cognitive decline.29,48 Patients with diabetes develop atherosclerosis at an accelerated rate.29 Atherosclerosis can be related to sarcopenia and frailty through processes that affect muscle performance (i.e., peripheral vascular disease and peripheral neuropathy) or chronic kidney disease, which can result in inactivity, loss of muscle mass, and a decline in physical and cognitive function.28,29 Diabetes and depression are interrelated, and depression has been linked to a progressive decline in strength. It has also been shown that diabetes and impaired glucose tolerance are associated with a worsening of cognitive function.29 Patients with type 2 diabetes are at an increased risk of developing mild cognitive impairment, vascular dementia, and Alzheimer's disease. Additionally, elevated glucose levels have been associated with an increased risk of developing dementia in patients without diabetes.48 The inter-relations among these factors are summarized in Fig. 2.
Frailty as a complicating factor in the management of patients with diabetesScreening and diagnosis of frailtyAccording to the international consensus on frailty (Frailty Consensus: A Call to Action) and the Consensus Document on Prevention of Frailty and Falls in the Older Person, which was approved by the Interterritorial Council of the Spanish National Health System and is scheduled to be implemented between 2015 and 2025 all persons aged 70 years or older, should be screened for frailty.10 Given the significant repercussions that frailty has on older individuals (especially patients with diabetes), and the implications for the management of diabetes, the IDF also recommends screening patients with diabetes who are aged ≥70 years for frailty.
Functional status is an important prognostic factor in older patients. Consequently, a comprehensive functional assessment that quantitatively encompasses the physical, cognitive, and emotional status of the patient should be a critical part of the clinical evaluation of older patients with diabetes.37
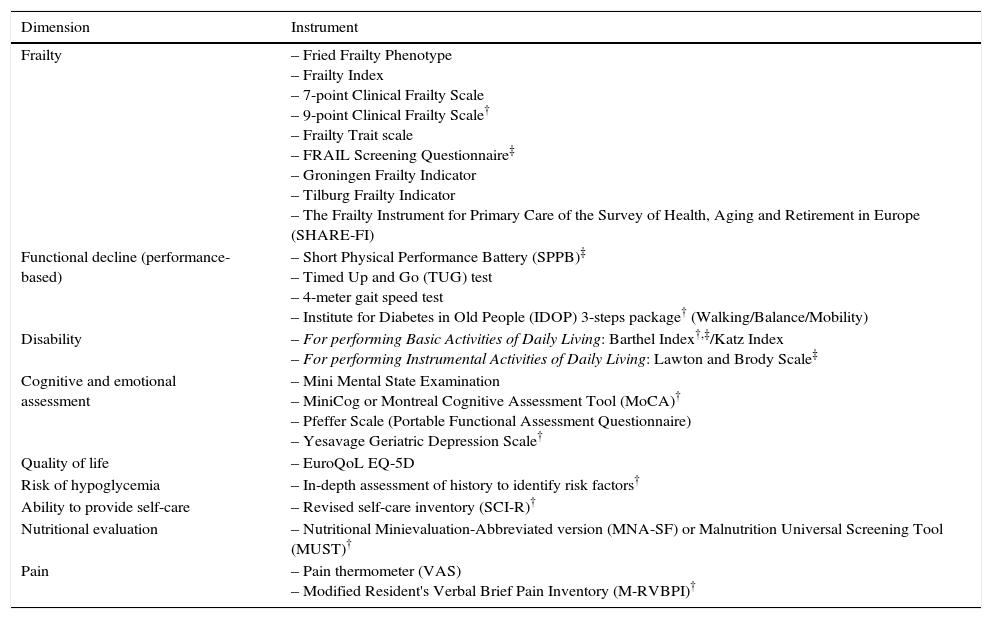
The international consensus on frailty endorses validated instruments to assess the presence of frailty, including the FRAIL questionnaire, the CHS Frailty Screening Measure (corresponding to the Fried Phenotype), the Clinical Frailty Scale (a tool recommended by the IDF), and the Gérontopôle Frailty Screening Tool.10 These tools can identify people with frailty who need further evaluation by specialists. In Table 2, we list a number of instruments that can be used to assess frailty.
Instruments for evaluating frailty and its components and/or associated areas.
| Dimension | Instrument |
|---|---|
| Frailty | – Fried Frailty Phenotype – Frailty Index – 7-point Clinical Frailty Scale – 9-point Clinical Frailty Scale† – Frailty Trait scale – FRAIL Screening Questionnaire‡ – Groningen Frailty Indicator – Tilburg Frailty Indicator – The Frailty Instrument for Primary Care of the Survey of Health, Aging and Retirement in Europe (SHARE-FI) |
| Functional decline (performance-based) | – Short Physical Performance Battery (SPPB)‡ – Timed Up and Go (TUG) test – 4-meter gait speed test – Institute for Diabetes in Old People (IDOP) 3-steps package† (Walking/Balance/Mobility) |
| Disability | – For performing Basic Activities of Daily Living: Barthel Index†,‡/Katz Index – For performing Instrumental Activities of Daily Living: Lawton and Brody Scale‡ |
| Cognitive and emotional assessment | – Mini Mental State Examination – MiniCog or Montreal Cognitive Assessment Tool (MoCA)† – Pfeffer Scale (Portable Functional Assessment Questionnaire) – Yesavage Geriatric Depression Scale† |
| Quality of life | – EuroQoL EQ-5D |
| Risk of hypoglycemia | – In-depth assessment of history to identify risk factors† |
| Ability to provide self-care | – Revised self-care inventory (SCI-R)† |
| Nutritional evaluation | – Nutritional Minievaluation-Abbreviated version (MNA-SF) or Malnutrition Universal Screening Tool (MUST)† |
| Pain | – Pain thermometer (VAS) – Modified Resident's Verbal Brief Pain Inventory (M-RVBPI)† |
As recommended by the IDF and treatment guides for older diabetics, multidimensional and, whenever possible, multidisciplinary assessments of older patients with diabetes should be performed to collect information about medical, functional, cognitive, emotional, and social functioning of the patient.50,51 A comprehensive geriatric assessment is likely the most complete way to assess a patient with frailty.49 This assessment is a dynamic and structured diagnostic process that detects and quantifies the problems, needs, and abilities of the older individual in four key areas: clinical, functional, mental, and social. This assessment can then be used to develop an interdisciplinary plan for intervention, treatment, and long-term monitoring, thereby enabling the patient to maintain a high degree of independence and an acceptable quality of life. During this evaluation, emphasis should be placed on managing these complex diseases and evaluating the patient's quality of life.49,50,52 At a minimum, the evaluation should assess the patient's functional capacity, cognitive function, and mental health.50Table 2 lists evaluations and instruments proposed by the IDF that can be used with minimal training in daily clinical practice.
Screening and diagnosis of diabetesGiven the elevated prevalence of diabetes in older patients and because approximately 40% of cases of diabetes remain undiagnosed,38 all older patients should be periodically evaluated to detect diabetes. These evaluations are especially warranted in certain groups, including in all patients admitted to a nursing home.50 The IDF recommends using the same diagnostic criteria for diabetes that are used for the general population; however, only the simplest possible tests should be used in frail patients.
Therapeutic management of diabetes in older frail patientsClinical trials evaluating treatments for diabetes typically exclude frail patients and patients with multiple comorbidities or functional disabilities. Therefore, no evidence-based recommendations for the treatment of these patients are available. Additionally, until recently, clinical practice guidelines did not contain specific recommendations for the management of these patients.35,53 Current guidelines for managing diabetes in the older patient recognize the need to consider frailty, comorbidities, and functionality when making decisions.37,50,51,54–56 Other factors should also be considered, including cardiovascular disease, advanced microvascular disease, undetected hypoglycemias, and other individual aspects of the patient (resources and support systems).57,58
Glycemic control objectivesThere is widespread consensus that the objectives of glycemic control in the frail older patient should be individualized and that the strict control of glycemia (for example, glycated hemoglobin [HbA1c] <7%) is associated with a risk of hypoglycemia and functional decline.35,50,51,53,54 Because the estimated time to achieve benefits from intensive glycemic control is at least 8 years (United Kingdom Prospective Diabetes Study), the available guidelines currently recommend less stringent control objectives (HbA1c <8.0–8.5%) in frail patients or patients with a limited life expectancy (<5 years). Table 3 describes the main recommendations of the American Diabetes Association (ADA), the IDF and other consensuses for achieving glycemic control and managing diabetes in the frail older patient.
Objectives for the management of complications and comorbidities associated with diabetes in the frail older patient with diabetes mellitus.
| IDF recommendations, 201350 | ADA recommendations, 201537,70,71 | Other consensuses* | |||
|---|---|---|---|---|---|
| *Functionally dependent patient/frail patient | *Patient with a complex or intermediate health status | *Patient with a very complex health status | *Frail patient (EDWPOP)54 | *Frail patient (Spanish consensus, type 2 diabetes in the older patient)48 | |
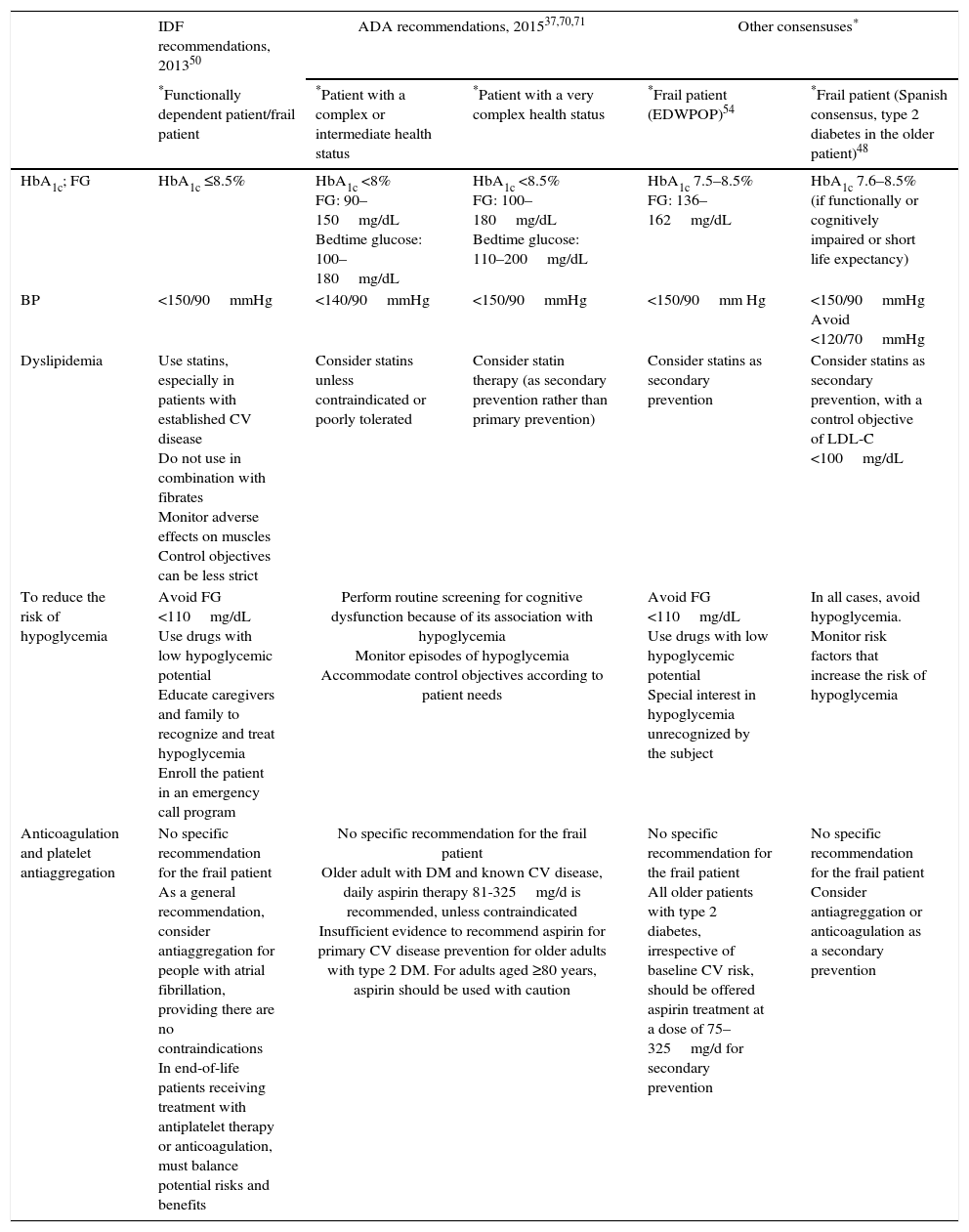
| HbA1c; FG | HbA1c ≤8.5% | HbA1c <8% FG: 90–150mg/dL Bedtime glucose: 100–180mg/dL | HbA1c <8.5% FG: 100–180mg/dL Bedtime glucose: 110–200mg/dL | HbA1c 7.5–8.5% FG: 136–162mg/dL | HbA1c 7.6–8.5% (if functionally or cognitively impaired or short life expectancy) |
| BP | <150/90mmHg | <140/90mmHg | <150/90mmHg | <150/90mm Hg | <150/90mmHg Avoid <120/70mmHg |
| Dyslipidemia | Use statins, especially in patients with established CV disease Do not use in combination with fibrates Monitor adverse effects on muscles Control objectives can be less strict | Consider statins unless contraindicated or poorly tolerated | Consider statin therapy (as secondary prevention rather than primary prevention) | Consider statins as secondary prevention | Consider statins as secondary prevention, with a control objective of LDL-C <100mg/dL |
| To reduce the risk of hypoglycemia | Avoid FG <110mg/dL Use drugs with low hypoglycemic potential Educate caregivers and family to recognize and treat hypoglycemia Enroll the patient in an emergency call program | Perform routine screening for cognitive dysfunction because of its association with hypoglycemia Monitor episodes of hypoglycemia Accommodate control objectives according to patient needs | Avoid FG <110mg/dL Use drugs with low hypoglycemic potential Special interest in hypoglycemia unrecognized by the subject | In all cases, avoid hypoglycemia. Monitor risk factors that increase the risk of hypoglycemia | |
| Anticoagulation and platelet antiaggregation | No specific recommendation for the frail patient As a general recommendation, consider antiaggregation for people with atrial fibrillation, providing there are no contraindications In end-of-life patients receiving treatment with antiplatelet therapy or anticoagulation, must balance potential risks and benefits | No specific recommendation for the frail patient Older adult with DM and known CV disease, daily aspirin therapy 81-325mg/d is recommended, unless contraindicated Insufficient evidence to recommend aspirin for primary CV disease prevention for older adults with type 2 DM. For adults aged ≥80 years, aspirin should be used with caution | No specific recommendation for the frail patient All older patients with type 2 diabetes, irrespective of baseline CV risk, should be offered aspirin treatment at a dose of 75–325mg/d for secondary prevention | No specific recommendation for the frail patient Consider antiagreggation or anticoagulation as a secondary prevention | |
Functionally dependent and frail patients (IDF)50: they are characterized by a combination of significant fatigue, recent weight loss, severe restriction of mobility and strength, and a greater risk of falls and hospitalization. Individualize glycemic control objectives, taking into account the patient's functional status, comorbidity, CV disease, history, and risk of hypoglycemia and microvascular complications.
Patients with a complex or intermediate health status (ADA):37,70,71 multiple chronic diseases coexist or a limitation exists for performing more than two IADL or mild or moderate cognitive impairment. Patients with intermediate life expectancy, high risk of hypoglycemia, vulnerability, and risk of falls.
Patients with a very complex health status (ADA)37,70,71: presence of advanced-stage chronic disease or severe cognitive impairment or lack of independence in more than two IADL. Patients with limited life expectancy in whom the benefit of treatment is uncertain.
European Consensus (EDWPOP).54 Frail patient: dependent patients with multisystemic disease who have been hospitalized, including those with dementia in whom there is a high risk of hypoglycemia and in whom it is key to avoid metabolic decompensation.
Spanish Consensus (Treatment of type 2 diabetes in the older patient)51: Frail patient: patient with multiple comorbidities, a high risk of hypoglycemia, functional disability or life expectancy less than 5 years (less likely to benefit from reduced risk of vascular complications and more likely to suffer serious adverse effects such as hypoglycemia). Individualize therapy by performing a risk/benefit analysis of antidiabetic treatment based on the functional and cognitive status of the patient, comorbidities, risk of hypoglycemia, ability to take care of oneself, life expectancy, and quality of life.
ADA, American Diabetes Association; BP, blood pressure; CV, cardiovascular; EDWPOP, European Diabetes Working Party for Older People; FG, fasting glucose; IADL, instrumental activities of daily living; IDF, International Diabetes Federation; LDL-C, low-density lipoprotein cholesterol.
Below, we summarize the limited information available for treating older patients with diabetes that considers the frailty of the patient.
Nonpharmacological interventionsNutritionA nutritional evaluation that can detect malnutrition or weight loss and provides an appropriate and individualized nutritional plan should be performed. Food with high protein and energy content may be necessary to improve the nutritional and functional status of the patient.50,58
Performing physical activity and exerciseThis is an important component of the treatment plan for diabetic patients. Light resistance and balance training can be performed to improve physical performance, strengthen the lower body, and prevent the deterioration of the patient's functional status.49,50 Interventions focused on physical activity have been shown to be effective in delaying and even reversing frailty and disability,3,59 and improving cognitive status and emotional wellbeing.60 According to a systematic review of studies on frail older patients,61 the best strategy for improving frailty and preventing falls involves implementing interventions designed to address strength, endurance, and balance.
Diabetes and education on self-careEducation on self-care should take into account mental and physical functional disorders, comorbidities, impaired vision or hearing, manual dexterity, and social setting. Education should be provided to both health professionals and caregivers.50 Recommendations from the Diabetes Care Program of Nova Scotia indicate that it may not be necessary to routinely measure plasma glucose in patients who have remained stable with oral antidiabetics or well-established doses of insulin alone.35
Pharmacological measuresAntidiabetic drugsBecause renal disease is prevalent among older patients, renal function should be evaluated before starting a treatment regimen for diabetes. If the patient has adequate renal function, metformin is the drug of choice. Most recent consensuses,37,50,58 recommend that the dose of metformin should be reduced if the estimated glomerular filtration rate (eGFR) is between 30 and 60mL/min, and it should not be used in patients with eGFR values <30mL/min or in the presence of any comorbidities that could interfere with its use (e.g., heart failure). According to the summary of product characteristics, metformin should not be used in patients with renal clearance values <60mL/min.62 One of the most common problems with metformin encountered in the older patient is a high rate of gastrointestinal intolerance (which can occur in up to 30% of patients) and resultant anorexia and weight loss. This can lead to sarcopenia and declines in function, which would necessitate the discontinuation of this drug or a dose reduction. In this case, dipeptidyl peptidase 4 (DPP-4) inhibitors or sulfonylureas, with lower risks of inducing hypoglycemia, may be the drugs of choice.56 If monotherapy with metformin is not sufficient to achieve the desired glycemic control, another drug must be added, preferably a DPP-4 inhibitor50,51,54,56, in patients at a high risk of experiencing hypoglycemia (i.e., frail patients; patients recently discharged from the hospital; patients with cognitive impairment, disability, or erratic intake; and patients in a residential care setting). In patients who cannot tolerate metformin, the combination of a DPP-4 inhibitor and a sulfonylurea with low risk of hypoglycemia is recommended. Similarly, according to the treatment algorithm for hyperglycemia in patients with type 2 diabetes published by the Network of Study Groups of Diabetes in Primary Health Care (Red de Grupos de Estudio de la Diabetes en Atención Primaria de la Salud[RedGDPS]), patients aged >75 years or frail patients should receive a DPP-4 inhibitor instead of a sulfonylurea to reduce the risk of hypoglycemia.61 Furthermore, the RedGDPS algorithm emphasizes the need to consider potential renal dysfunction. Thus, in patients with GFRs <30mL/min, the drug of choice would be a DPP-4 inhibitor (with dose adjustment, if required).63
Several studies64,65 indicate that DPP-4 inhibitors (linagliptin and vildagliptin) are a safe option for diabetic patients aged >70 years, and most guidelines list DPP-4 inhibitors as the drugs of choice for older patients in whom metformin is contraindicated,50,51,63,66 being an alternative to sulfonylureas, which in most cases are associated with an increased risk of hypoglycemia.
If a patient requires insulin treatment, the safest option is to add a long-acting insulin analog along with oral agents and provide educational information tailored to the patients and/or their caregivers.50 Use of basal insulin alone is recommended if possible, to avoid hypoglycemia associated with the use of rapid-acting insulins. If these are required because of, for instance, an erratic eating pattern, they should be administered only after a caloric intake to prevent hypoglycemia.67 In all cases, it will be necessary to evaluate factors such as the cognitive function of the patient, the presence of caregivers, the ability and degree of independence of the patient, and accessibility of healthcare.51
Table 4 summarizes the main characteristics, precautions and warnings of each group of diabetes medications when used in older and frail patients.
Recommendations on the use of hypoglycemic drugs in patients with comorbidities associated with frailty.
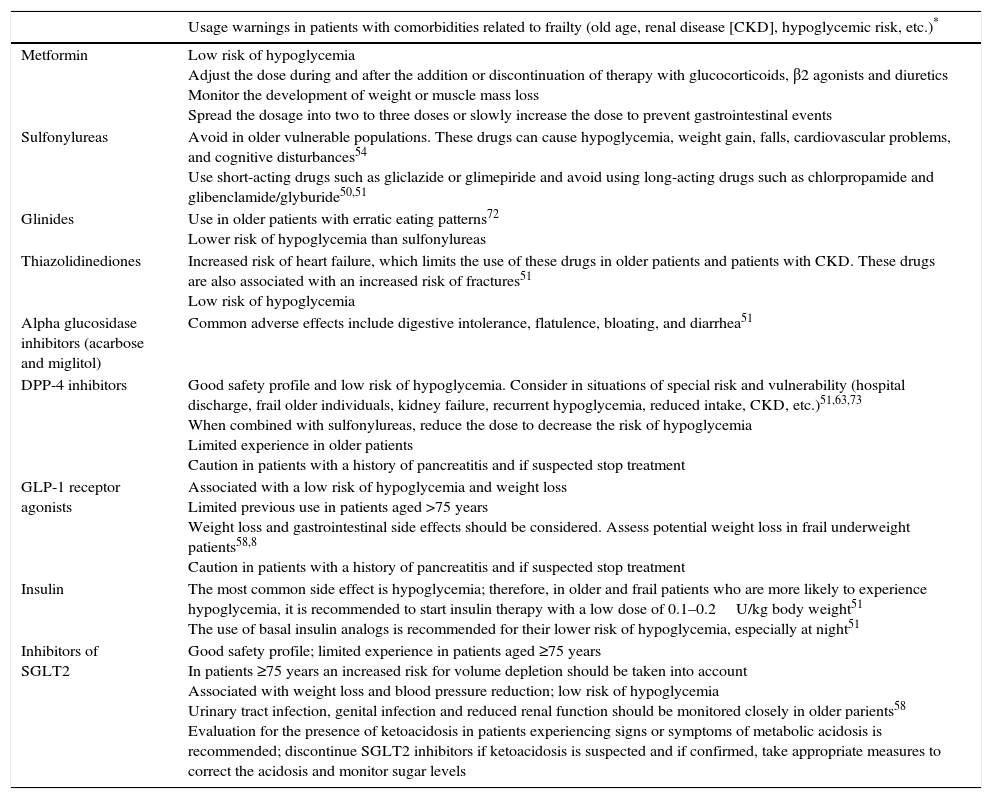
| Usage warnings in patients with comorbidities related to frailty (old age, renal disease [CKD], hypoglycemic risk, etc.)* | |
|---|---|
| Metformin | Low risk of hypoglycemia Adjust the dose during and after the addition or discontinuation of therapy with glucocorticoids, β2 agonists and diuretics Monitor the development of weight or muscle mass loss Spread the dosage into two to three doses or slowly increase the dose to prevent gastrointestinal events |
| Sulfonylureas | Avoid in older vulnerable populations. These drugs can cause hypoglycemia, weight gain, falls, cardiovascular problems, and cognitive disturbances54 Use short-acting drugs such as gliclazide or glimepiride and avoid using long-acting drugs such as chlorpropamide and glibenclamide/glyburide50,51 |
| Glinides | Use in older patients with erratic eating patterns72 Lower risk of hypoglycemia than sulfonylureas |
| Thiazolidinediones | Increased risk of heart failure, which limits the use of these drugs in older patients and patients with CKD. These drugs are also associated with an increased risk of fractures51 Low risk of hypoglycemia |
| Alpha glucosidase inhibitors (acarbose and miglitol) | Common adverse effects include digestive intolerance, flatulence, bloating, and diarrhea51 |
| DPP-4 inhibitors | Good safety profile and low risk of hypoglycemia. Consider in situations of special risk and vulnerability (hospital discharge, frail older individuals, kidney failure, recurrent hypoglycemia, reduced intake, CKD, etc.)51,63,73 When combined with sulfonylureas, reduce the dose to decrease the risk of hypoglycemia Limited experience in older patients Caution in patients with a history of pancreatitis and if suspected stop treatment |
| GLP-1 receptor agonists | Associated with a low risk of hypoglycemia and weight loss Limited previous use in patients aged >75 years Weight loss and gastrointestinal side effects should be considered. Assess potential weight loss in frail underweight patients58,8 Caution in patients with a history of pancreatitis and if suspected stop treatment |
| Insulin | The most common side effect is hypoglycemia; therefore, in older and frail patients who are more likely to experience hypoglycemia, it is recommended to start insulin therapy with a low dose of 0.1–0.2U/kg body weight51 The use of basal insulin analogs is recommended for their lower risk of hypoglycemia, especially at night51 |
| Inhibitors of SGLT2 | Good safety profile; limited experience in patients aged ≥75 years In patients ≥75 years an increased risk for volume depletion should be taken into account Associated with weight loss and blood pressure reduction; low risk of hypoglycemia Urinary tract infection, genital infection and reduced renal function should be monitored closely in older parients58 Evaluation for the presence of ketoacidosis in patients experiencing signs or symptoms of metabolic acidosis is recommended; discontinue SGLT2 inhibitors if ketoacidosis is suspected and if confirmed, take appropriate measures to correct the acidosis and monitor sugar levels |
Information on warnings can be checked in the corresponding Summary of Product Drug characteristics and are as reported at http://www.aemps.gob.es/cima/ and at https://sinaem.agemed.es/CartasFarmacovigilanciaDoc/2015/DHPC_definitiva_glifozinas_09_07_2015.pdf for the inhibitors of SGLT2, unless otherwise indicated.
CKD, chronic kidney disease; DPP-4, dipeptidyl-peptidase-4; GLP-1, glucagon-like peptide-1; SGLT2, sodium-glucose cotransporter 2.
It is important to periodically review other medications that the patient receives and, if possible, avoid polypharmacy because of its link with adverse events in the older patient. An evaluation of the indication of each drug should be performed. The STOPP-START (Screening Tool of Older Person's Prescriptions and Screening Tool to Alert doctors to Right Treatment)49 criteria are a useful tool that can be used to determine when to discontinue drugs that negatively affect older patients who are at increased risk of adverse effects (falls, functional impairment, urinary incontinence, sleep disturbances, weight loss, etc.).
To choose the most appropriate drug, the ADA and the European Association for the Study of Diabetes also recommend following a patient-centered approach, taking into account the efficacy, cost, and adverse effects of the drug, its effects on the weight of the patient, associated comorbidities, hypoglycemia, resource availability, and preferences of patients or their caregivers.66
Overall, we summarize our key recommendations on the treatment of diabetes in the frail patient in the following: (1) do not try to reduce HBA1c below 7%; (2) avoid hypoglycemia; (3) when selecting a drug, consider with the same weight the balance between efficacy and safety, and bear in mind potential drug–drug interactions; (4) as part of the treatment plan include a resistance training program; (5) look for short-term treatment goals; and (6) simplify treatment regimens, including the use of insulin whenever possible.
Prevention and management of complications associated with diabetes in the frail patientNot all patients clearly fit into one category. Therefore, the preferences and characteristics of the patient and their caregivers are important factors to consider when developing individualized treatment plans. Treatment goals for the management of complications depend on the functional status of the patient, including their frailty, cognitive status, risk of hypoglycemia, and life expectancy.58
The recommendations of the IDF and ADA for the management of certain complications and comorbidities associated with diabetes are summarized in Table 3.
Interventions to address patients’ cardiovascular risks are necessary to prevent or delay cardiovascular disease in people with diabetes. In this context, the ADA recommends preventive measures in all patients aged ≥60 years to address some of the main risk factors for cardiovascular disease (tobacco use, blood pressure, dyslipidemia, renal function, glycemic control, obesity, periodontitis, sleep apnea, and peripheral vascular disease). However, the role of cardiovascular disease as a prognostic marker of death and functional impairment in older patients with diabetes is controversial, especially in patients aged >75 years.68
Hypoglycemia is the main side effect of diabetes treatment in general older patients, and hypoglycemia can have serious consequences, including cognitive impairment, falls, fractures, and cardiovascular events, that lead to frailty and disability, creating a vicious circle of hypoglycemia and frailty.67 Avoiding hypoglycemia should be one of the main goals of treatment. Thus, glycemic targets should be individualized, focusing on the short term to avoid hypoglycemia instead of having a long term strategy.67 In addition to drug treatment, under-nutrition plays an important role in the occurrence of hypoglycemia in frail patients. Therefore, the improvement of the energy intake is central to the management of these patients.67 Recommendations on the use of insulins have been made above. It is recommended that family members and caregivers be educated to recognize and treat hypoglycemia50 and that frail patients living in the community should be enrolled in an ‘SOS-call’ program.
The IDF also makes general recommendations for handling hyperglycemic emergencies.50 Glycemic goals should minimize severe hyperglycemia and reduce the risk of diabetic ketoacidosis or a hyperglycemic hyperosmolar state. Both medical and non-medical teams should be taught to recognize risk factors and manage hyperglycemic emergencies in older patients. Additional research on the consequences of frailty in diabetic patients and the development of intervention programs specifically designed for these patients could help improve their clinical care. In this context, the MID-FRAIL study, which was funded under the 7th Framework Programme of the European Union and led by Spain, provides an opportunity to explore the usefulness of a multidimensional intervention for frail and pre-frail patients aged >70 years with type 2 diabetes.69
ConclusionsFrailty can be associated with various chronic diseases, especially diabetes. Both conditions are highly prevalent in older individuals, share pathophysiological characteristics, and act synergistically to cause functional impairment in older patients. Moreover, frailty affects the management of diabetes in older patients, an issue that is increasingly being recognized in the therapeutic guidelines of major national and international scientific societies. Therefore, it is important to rule out frailty in all diabetic patients aged >70 years. With this aim, current guidelines for the management of diabetes in older patients recommend several validated evaluation tools which can be administered during routine clinical practice. If frailty is suspected, a comprehensive and multidisciplinary evaluation that assesses medical and functional aspects of the patient should be conducted to establish an individualized intervention plan that requires less strict glycemic targets, a less stringent control of associated comorbidities, and a consideration of the life expectancy of the patient. The intervention plan should contain specific measures for addressing nutrition, physical activity, education on diabetes and self-care recommendations, and avoidance of polypharmacy in older patients. It is important to take precautions when using drugs to treat hyperglycemia that can cause excessive weight loss and/or an increased risk of hypoglycemia. It is necessary to train health professionals to detect, diagnose, and manage frailty and its potential consequences.
Conflict of interestsAmelia Cobo, Luis Alberto Vázquez and Jesús Reviriego are employees of Eli Lilly and Company. Leocadio Rodríguez-Ma¿nas has given lectures on diabetes in the elderly funded by Eli Lilly, Boehringer, Novartis, Servier, and Sanofi.
The authors wish to thank Fernando Rico-Villademoros (COCIENTE S.L., Madrid, Spain) and Rx Communications (Mold, United Kingdom) for their help in the medical writing and preparation of the article, funded by Eli Lilly and Company.