To assess lipid profile changes in post-menopausal women treated with testosterone gel.
MethodsThirty-six oophorectomized women on estradiol treatment who received transdermal testosterone gel (5mg daily) were enrolled into our study. Cholesterol, triglycerides, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), very low density-lipoprotein cholesterol (VLDL-C), and lipoprotein (a) were tested before and after 6 months of treatment.
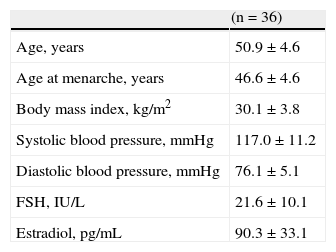
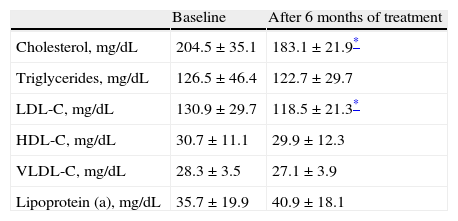
ResultsSelected participants had a mean age of 50.9±4.6 years and a body mass index of 30.1±3.8kg/m2. Significantly decreased cholesterol levels were found after 6 months of treatment (204.5±35.1mg/dL before treatment as compared to 183.1±21.9mg/dL after treatment; p<0.05). A significant reduction was also seen in LDL-C levels after 6 months of treatment with testosterone gel as compared to baseline (130.9±29.7mg/dL versus 118.5±21.3mg/dL; p<0.05). No differences were found in triglyceride, HDL-C, VLDL-C, and lipoprotein (a) levels (p=NS).
ConclusionTestosterone gel, associated to estrogen treatment in oophorectomized women, decreased cholesterol and LDL-C levels after 6 months of treatment, without affecting serum triglyceride, HDL-C, VLDL-C, and lipoprotein (a) levels.
Determinar las modificaciones del perfil lipídico en menopáusicas tratadas con gel de testosterona.
MétodosSe incluyeron 36 mujeres ooforectomizadas, tratadas con estradiol, que recibieron tratamiento con gel de testosterona (5mg transdérmicos diarios). Se analizaron la presencia de obesidad, concentraciones séricas de colesterol, triglicéridos, colesterol unido a proteínas de alta densidad (HDL-C) y colesterol unido a proteínas de baja densidad (LDL-C), colesterol unido a proteínas de muy baja densidad (VLDL-C) y lipoproteína (a) antes y después de 6 meses de tratamiento.
ResultadosLa edad promedio de las participantes seleccionadas fue de 50,9±4,6 años, con un índice de masa corporal de 30,1±3,8Kg/m2. Se observó una disminución significativa de las concentraciones de colesterol (204,5±35,1mg/dL antes del tratamiento comparado con 183,1±21,9mg/dL después del tratamiento; p<0,05). También se observó una disminución significativa en las concentraciones de LDL-C entre las concentraciones iniciales y luego de 6 meses de tratamiento con gel de testosterona (130,9±29,7mg/dL comparado con 118,5±21,3mg/dL; p<0,05). No se encontraron diferencias en las concentraciones de triglicéridos, HDL-C, VLDL-C y lipoproteína (a) (p=ns).
ConclusiónEl gel de testosterona, asociado a tratamiento estrogénico en mujeres ooforectomizadas, produce disminución de las concentraciones de colesterol y LDL-C posterior a 6 meses de tratamiento, sin afectar las concentraciones de triglicéridos, HDL-C, VLDL-C y lipoproteína (a).
Cardiovascular diseases are the leading cause of death worldwide.1 Their increased prevalence in men may be attributed to low/high density lipoprotein levels as compared to premenopausal women.2 The reduction of total and low density lipoprotein cholesterol (LDL-C) levels decreases the incidence of coronary heart disease. An inverse relationship exists between high density lipoprotein cholesterol (HDL-C) and coronary heart disease in the developed world. When HDL-C levels are less than 50mg/dL, postmenopausal women have a three times greater chance of dying than women with normal levels.3 An 11% decrease in LDL-C and a 12% increase in HDL-C have been related to a 34% decrease in coronary heart disease.4 Different sex hormone levels in men and women are thought to be significant factors that contribute to sex differences in lipoprotein levels. This observation shows that the use of hormone replacement therapy decreases cardiovascular risk.5
In healthy women with normal menses, ovaries and adrenal glands contribute to the production of approximately 300μg of testosterone daily.6 However, the physiological role of testosterone in women is unclear.7 From the age of 30, women experience a significant reduction in serum androgen levels as a consequence of age-related decrease.8 This may lead to a clinical female androgen deficiency syndrome characterized by fatigue, decreased well-being and libido, body composition changes, and bone loss. If associated with low free testosterone levels, these signs and symptoms may be relieved by androgen administration, currently considered as a treatment option.7
It has been suggested that the restoration of physiological testosterone levels may protect the cardiovascular system in postmenopausal women,9 but hyperandrogenemia is associated with decreased LDL levels, hyperinsulinemia, insulin resistance, and increased abdominal fat accumulation, all of which contribute to increasing the risk of cardiovascular disease.10 It has also been suggested that increased lipolytic activity of visceral fat induced by androgens causes the excess release of fatty acids into the portal vein, which decreases insulin clearance in the liver and increases the production of glucose and very low density lipoproteins.11
In men with hypogonadism, the administration of testosterone gel has various effects on lipids. Transdermal 5 alpha-dihydrotestosterone decreases cholesterol and low density lipoproteins without causing changes in triglycerides or high density lipoproteins.12 Transdermal testosterone decreases high density lipoprotein levels and increases the total cholesterol/HDL-C ratio.13 Subsequent studies have shown no untoward effects on the lipid profile.14 Controversy still exists about the potential effects of androgen gel in women with hypogonadism induced by oophorectomy treated with estrogens.
The purpose of this research was to determine lipid profile changes in postmenopausal women treated with testosterone gel.
Patients and methodsThe research was conducted from March 2009 to December 2010 on 36 women with hypogonadism due to oophorectomy attending the outpatient clinics of internal medicine, endocrinology, and gynecology of Hospital Central Dr. Urquinaona and treated with testosterone gel. The ethics committee of the hospital approved the study, and written consent was obtained from all participants.
The women were between 41 and 59 years of age, had undergone hysterectomy and bilateral salpingo-oophorectomy with climacteric symptoms, smoked less than five cigarettes daily, and had normal blood pressure values (less than 140/90mmHg). All the women had to have been previously using hormone replacement therapy with estradiol 1mg for at least 12 months and had not used drugs altering lipid metabolism, antihypertensives, vitamins, or antioxidants within 12 months of the start or during the study. Exclusion criteria were the use of alcohol, a history of testosterone use in the previous six months, the use of antidepressants, and prior cardiac, neoplastic, cerebrovascular, thromboembolic, hepatic, or renal disease. Surgery had been performed at least 12 months before the women were enrolled into the study. No woman had transaminase or alkaline phosphatase values more than two times the normal laboratory values. Participants with a history of hyperandrogenism such as hirsutism and polycystic ovary syndrome were also excluded. An electrocardiogram was performed on all participants, and those with abnormal ECG were excluded from the study.
The participants were treated for six months with oral estradiol 1mg and a daily dose of testosterone 5mg (one application) provided by a gel containing 1% testosterone, ethyl alcohol, and distilled water. The gel was packaged in a 40mL bottle with a dispenser supplying each time 0.5g of gel containing 5mg of testosterone. The gel was applied onto the medial lateral surface of the thigh at night (10 PM). The participants were informed that they should wash their hands immediately after application and could not bathe for 6h. Venous blood samples were taken in the morning, after fasting for at least 12h, at study start and after six months of treatment.
FSH and estradiol levels were measured by radioimmunoassay using commercial kits (Immulite 2000, Diagnostic Product Corp., USA). Intra- and inter-assay coefficients of variation were 4% and 7% for FSH and 5% and 9% for testosterone. Total cholesterol and triglycerides were measured using automated enzymatic methods (COBASs Integra Cholesterol and COBASs Integra triglycerides) in a Roche/Hitachi 74 analyzer. HDL-C was measured after selective precipitation using heparin–manganese and subsequent enzymatic cholesterol measurement. Low density lipoproteins were calculated using the Friedewald formula [LDL-C=(total cholesterol−HDL-C)−(triglycerides/5)]. Serum levels of lipoprotein (a) were measured using an immunoturbidimetric test (Polymedco, USA). The detection limit was 10mg/dL. Intra-assay coefficients of variation were <3%.
A Student's t-test for related samples was used to assess changes in serum lipid and lipoprotein levels caused by the testosterone gel. A value of p<0.05 was considered statistically significant.
ResultsTable 1 shows the general characteristics of postmenopausal women. Selected participants had a mean age of 50.9±4.6 years, a body mass index of 30.1±3.8kg/m2, and FSH and estradiol levels of 21.6±10.1IU/L and 90.3±33.1pg/mL, respectively.
Table 2 shows mean lipid and lipoprotein levels before and after treatment with testosterone gel. Cholesterol levels decreased more than 10% at six months of treatment as compared to baseline (204.5±35.1mg/dL before treatment as compared to 183.1±21.9mg/dL after treatment; p<0.05). As regards plasma triglyceride values, mean baseline level was 126.5±46.4mg/dL, with a non-significant decrease greater than 3%, to 122.7±29.7mg/dL, after six months of treatment (p=NS).
Lipid-lipoprotein levels before and after treatment (n=36).
| Baseline | After 6 months of treatment | |
| Cholesterol, mg/dL | 204.5±35.1 | 183.1±21.9* |
| Triglycerides, mg/dL | 126.5±46.4 | 122.7±29.7 |
| LDL-C, mg/dL | 130.9±29.7 | 118.5±21.3* |
| HDL-C, mg/dL | 30.7±11.1 | 29.9±12.3 |
| VLDL-C, mg/dL | 28.3±3.5 | 27.1±3.9 |
| Lipoprotein (a), mg/dL | 35.7±19.9 | 40.9±18.1 |
HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; VLDL-C: very low density lipoprotein cholesterol.
With regard to lipoproteins (Table 2), a significant decrease (greater than 9%) was found in LDL-C levels after six months of treatment with testosterone gel as compared to baseline (130.9±29.7mg/dL versus 118.5±21.3mg/dL, p<0.05). HDL-C levels decreased from 30.7±11.1mg/dL at baseline to 29.9±12.3mg/dL (−2.6%) at six months of treatment (p=NS). VLDL-C levels were 28.3±3.5mg/dL at baseline and decreased more than 4% to 27.1±3.9mg/dL (p=NS). Lipoprotein (a) levels were 35.7±19.9mg/dL at baseline and 40.9±18.1mg/dL after treatment, a more than 12% increase with no statistical significance (p=NS).
DiscussionThe results of this research showed that the testosterone gel induced significant changes in total cholesterol and LDL-C levels. It was also shown to cause no significant changes in the levels of triglycerides, HDL-C, VLDL-C, and lipoprotein (a).
Among the different known risk factors for coronary heart disease, arterial hypertension, diabetes mellitus, smoking, hyperlipidemia, and obesity are most commonly cited. Sex disparity in the incidence of cardiovascular disease has been considered to reflect estrogen-mediated protection against atherogenesis, in contrast to the promotion of atherosclerosis by androgens. Randomized clinical studies have shown that the use of an estrogen/progestogen combination provides no cardiovascular benefits in post-menopausal women.15 The greater susceptibility of men to cardiovascular disease may be due to genetic, hormonal, or lifestyle factors or to a combined mechanism.
The net effect of testosterone on risk factors for cardiovascular disease is difficult to establish for different reasons.16 The first reason is that associations between endogenous testosterone levels and cardiovascular risk factors are confounded by multiple interactions of endogenous androgens, body fat distribution, and insulin sensitivity. Second, exogenous testosterone has marked effects, some of them beneficial, upon several risk factors such as decreased fibrinogen, plasminogen, and insulin levels, while other effects may be considered as negative (decreased HDL-C levels). Third, a causal relationship between risk factors and atherosclerosis has not been fully shown. Fourth, testosterone exerts its metabolic effects both directly and through its metabolites, estradiol and 5 alpha-dihydrotestosterone. Testosterone and estradiol may have additive or counterregulatory effects. Fifth, polymorphisms in androgen, sex hormone binding globulin, and 5 alpha-reductase receptor genes regulate the biological effects and bioavailability of testosterone and 5 alpha-dihydrotestosterone, respectively.
The effects of unopposed oral and transdermal estrogens upon lipid-lipoprotein metabolism are clearly defined. The use of estrogens is known to change the lipid profile and lipoprotein levels.17 Estrogen therapy has been shown to dose-dependently reduce serum LDL-C levels and to increase HDL-C levels.18 In recent years, there has been an increasing interest in androgen treatment for women with hypogonadism after oophorectomy. Although the addition of low testosterone doses to estrogen therapy has been shown to improve body composition19 and bone mineral density,20 little is known about the effects of these substances on cardiovascular risk factors. Prior studies have shown that the main indication for the administration of testosterone as a transdermal gel in postmenopausal women is sex dysfunction, because it improves its symptoms.6,21
Different research studies have suggested that the co-administration of estrogens attenuates the cardioprotective effect of estrogens on serum HDL-C levels, decreases total cholesterol and triglycerides,22,23 and has a neutral effect on blood flow.24 By contrast, parenteral testosterone causes no changes in lipoprotein levels but improves both endothelium-dependent and independent vasodilation associated with hormone replacement therapy in menopause.25
This study showed that testosterone gel (added to estrogen therapy) decreases cholesterol and LDL-C levels. Unlike in prior studies, a neutral effect was also seen on HDL-C levels.23 Studies in men have shown a greater than 30% increase in HDL-C levels after the use of high androgen levels.26 Similar results have been seen with the use of non-aromatic androgens and have been associated with increased hepatic triglyceride lipase activity, which increases the catabolism of HDL-C.12
The clinical significance of the changes reported here and in previous studies for cardiovascular risk factors is unknown, but it has been suggested that changes in HDL-C levels induced by androgens should not be considered as pro-atherogenic, as they may reflect an acceleration of reverse cholesterol transport.27 Clinical and epidemiological studies provide evidence of the cardioprotective effects of HDL-C levels, attributable not only to their role in reverse cholesterol transport, but also to their effect upon endothelial cells and antioxidant activity.
In contrast to the results found in this study (non-significant effects on triglyceride levels), oophorectomized women treated with conjugated estrogens and androgens have been reported to experience a significant reduction in plasma triglyceride levels as compared to those treated with conjugated estrogens alone.28 This finding should be taken into account because lipoproteins containing triglycerides are closely related to plasma viscosity, an established risk factor for cardiovascular disease.29 There is some evidence to suggest that triglycerides are risk factors in some patient subgroups, including postmenopausal women. It is also well known that the effects upon triglyceride levels depend on the hormone administration route.4
The effect of a decrease in LDL-C levels seen in this study may protect against atherosclerosis, as previously suggested.16 Endocytosis of LDL-C molecules allows for cholesterol esters to be released in the different tissues. This uptake is regulated by free cholesterol levels, which modulate LDL-C receptor expression in the cell membrane. Acetylated LDL-C and LDL-C oxidase interact with the recovery receptor located on the macrophage cell surface to allow for intercellular accumulation of LDL-C. This type of process, which forms foam cells, appears to promote the development of atherosclerosis.
In conclusion, testosterone gel, associated with estrogen therapy in oophorectomized women, decreases total cholesterol and LDL-C levels after six months of use.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Fernández-Carvajal J, et al. Modificaciones del perfil lipídico en menopáusicas tratadas con gel de testosterona. Endocrinol Nutr. 2012;59:44-9.