To assess the effect of moderate regular aerobic physical activity not associated to body weight changes on insulin resistance and the associated metabolic changes in general population.
Subjects and methodsA cross-sectional, observational study in an adult population (n=101 subjects aged 30–70 years) with no personal history of disease and with stable weight in the three months prior to the study. The group with regular exercise performed 30–60min of moderate regular physical exercise 5 days per week (7.5–15h MET per week), while a control group performed no regular physical exercise and had a sedentary lifestyle. Subjects were age- and sex-matched. Lipids, lipoproteins, and HOMA index were measured using standard methods.
ResultsThe group with regular physical activity consisted of 48 subjects (21 male/27 female), while the group with no regular physical activity included 53 subjects (31 male/22 female). No significant differences were found between the groups in age, sex, BMI, waist circumference, and blood pressure. Significant differences were found between the groups in fasting serum triglyceride, HDL-C, and apoB levels. Fasting plasma insulin levels (12.1±4.13 vs 14.9±4.8mU/L, p=.004) and HOMA index (2.8±1.1 vs 3.5±4.1, p=.001) were significantly lower in the group with regular physical activity as compared to the sedentary group. Prevalence rates of metabolic syndrome were 20.7% and 45.8% (p=.01) in the regular physical activity and sedentary groups respectively.
ConclusionModerate regular physical activity is associated to higher insulin sensitivity, an improved lipid profile, and a decrease in components of metabolic syndrome with no change in weight or BMI.
Estudiar el efecto del ejercicio físico aeróbico practicado de forma regular y habitual, no acompañado de cambios en el peso corporal, sobre la resistencia a la insulina y las alteraciones metabólicas acompañantes en la población general.
Sujetos y métodosEstudio observacional y transversal en la población adulta, 101 sujetos (30-70 años), sin enfermedad conocida y sin cambios en su peso corporal en los 3 últimos meses. El grupo de ejercicio regular realizaba ejercicio moderado entre 30-60minutos/día 5días/semana (7,5-15h MET/semana) y el grupo control no realizaba ejercicio físico de forma habitual y tenía un estilo de vida sedentario. Los sujetos estaban equiparados en edad y sexo. Se estudiaron parámetros lipídicos, HOMA y síndrome metabólico (SM).
ResultadosEl grupo de ejercicio regular eran 48 sujetos (21 hombres/27 mujeres) y 53 (31 hombres/22 mujeres) el grupo sedentario. No hubo diferencias significativas entre ambos grupos en edad, sexo, IMC, perímetro de cintura y presión arterial. Encontramos diferencias estadísticamente significativas en: TG, cHDL, c-No-HDL y apoB, no así en el CT y cLDL. También hubo diferencias significativas en la insulina plasmática basal (12,1±4,13 y 14,9±4,8mU/l; p=0,004) y en el índice HOMA (2,8±1,1 y 3,5±4,1; p=0,001) en el grupo que realizaba ejercicio frente al grupo sedentario. Los sujetos con SM fueron un 20,7% y un 45,8% (p=0,01) en el grupo con ejercicio y sedentario, respectivamente.
ConclusiónLa realización de una vida activa con el ejercicio físico habitual y moderado conduce a un aumento de la sensibilidad a la insulina, un mejor perfil lipídico y una disminución de los componentes del SM sin modificar necesariamente el peso corporal.
In developed societies, excess calorie intake and sedentary lifestyles have led in recent years to an increase in the prevalence of obesity, dyslipidemia, insulin resistance (IR), and type 2 diabetes.1
A sedentary lifestyle or physical inactivity is an independent factor for IR and its metabolic complications. Physical activity is a significant mechanism for preventing or improving IR and its metabolic complications. This is of paramount importance because IR and its complications are responsible for a high morbidity and mortality and significant social and healthcare expenses in developed countries.2
Physical exercise decreases IR, stimulating the migration of type 4 glucose transporters or GLUT4 to the membranes of the skeletal muscle cells by mechanisms independent of the insulin receptor.3,4 A moderate aerobic physical exercise program increases insulin sensitivity in non-obese and non-diabetic subjects in the absence of body weight changes.5 It is important to assess the effect of exercise on other risk factors (systolic blood pressure [SBP], diastolic blood pressure [DBP], lipid factors, etc.).
Physical exercise has significant benefits in subjects with IR and metabolic syndrome (MS) by inducing a favorable change in the lipid profile with a reduction in LDL and triglyceride (TG) levels and an increase in HDL. In subjects with abnormal glucose tolerance, moderate physical exercise, alone or combined with a low-calorie diet, significantly prevents the onset of type 2 diabetes.6
Physical activity or exercise also contributes to the prevention of many other diseases, including coronary disease, some types of cancer, and mental diseases such as dementia and depression.7
The amount of physical activity required to prevent metabolic complications is not well known. In 2008, the guidelines of the American Heart Association and the Department of Health and Social Services of the United States recommended at least 150min/week (7.5 metabolic equivalents [MET]-h/week) of physical activity of moderate intensity to maintain a good state of health.8,9
Few studies have analyzed the effect of regular physical exercise, not associated with a low-calorie diet or body weight changes, on insulin sensitivity and lipid metabolism in the general population.
Our objective was to assess in the general population the effect on IR and its associated metabolic changes of regular, routine aerobic physical exercise not associated with restrictive diets or body weight changes. The design of our study would allow us to establish associations between exercise and metabolic parameters. However, the cross-sectional nature of the study, would not allow us to state that such relationships were causal.
Subjects and methodsDesignA cross-sectional, observational study in an adult population (aged 30–70 years) of both sexes attending a health center (in the Valencia metropolitan area) for different reasons during a period of one year. Opportunity sampling was used. This study was approved by and complied with the rules of the research and ethics committee of the center.
Inclusion criteriaVoluntary participation happened. The following are considered as inclusion criteria: No known disease, no current smoking or smoking cessation more than one year before, no or moderate (<20g/day) alcohol consumption, free diet, and no use of any programmed diet. Normal laboratory test results (liver and kidney function, complete blood count, thyroid hormones, and urine analysis) were taken note of.
Exclusion criteriaThe following are considered as inclusion criteria: Age outside the described range; current use of a low-calorie diet or weight gain or loss greater than 10% in the prior three months; hypothyroidism, including subclinical hypothyroidism (TSH<5); hepatic, renal, or cardiac failure; neoplasms; obesity (BMI≥30); diabetes (blood glucose≥126mg/dL or prior diagnosis); alcohol consumption (≥20g/day); smoking; and diagnosed or treated hypertension.
Subjects who met the inclusion criteria and none of the exclusion criteria and who had performed moderate exercise for 30–60min/day at least five days a week (between 7.5 and 15MET-h/week) during the past year were included in the regular exercise arm. Moderate regular exercise was defined as walking fast, jogging, running, riding a bicycle (including a static bicycle), the use of machines to do aerobic exercise, dancing, tennis, squash, and swimming. Exercise performance was assessed based on the individualized survey.
The control arm consisted of age and sex-matched subjects who met the inclusion criteria and none of the exclusion criteria but who did not perform regular physical exercise, either leading a sedentary lifestyle or performing exercise for less than 150min/week (less than 7.5MET-h/week). These subjects comprised the sedentary arm.
Study protocolThe clinical history of every participant was recorded including age, sex, personal history of drug use, toxic habits, ischemic heart disease, stroke or peripheral vascular disease, high blood pressure, diabetes, smoking, alcohol consumption, and physical exercise. Any family history of high blood pressure, diabetes, cardiovascular disease, and dyslipidemia was collected. Every subject's weight three months before (taken from the clinical history or provided by the subjects themselves) was recorded. A qualitative dietary survey was performed. Blood pressure was measured as follows: after resting supine for 10min, two measurements were taken five minutes apart. Current weight and height were measured to calculate body mass index (BMI), and waist circumference was measured.
MethodsA blood sample was drawn after a 12-hour overnight fast. Plasma was separated immediately by refrigerated centrifugation at 2500–3000rpm for 10min. Samples were processed immediately or within one week after storage at −20°C.
Total cholesterol (TC) and TG were measured using enzymatic methods10,11 in a Technicon RATM-1000 analyzer. Low density lipoprotein (LDL) cholesterol was tested after separation by precipitation with phosphotungstic acid-magnesium chloride.12 LDL cholesterol (LDL-C) was calculated using the Friedewald formula, and non-HDL cholesterol (non-HDL-C) by the difference between TC and HDL-C. Apoprotein B (apoB) was tested by immunoturbidimetry.13 Glucose was tested using an enzymatic method,14 and insulin by an immunoenzymatic assay.15 The homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated using the formula reported by Matthews et al.16: insulin (μU/mL)x[glucose (mmol/L)/22.5]. The definition of IR with HOMA was ≥3.2.17
The presence of MS was defined based on the recommendations of the American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. A diagnosis of MS was therefore based on the presence of three or more of the following characteristics: abdominal waist circumference ≥94cm in males and ≥80cm in females, fasting plasma TG levels ≥150mg/dL or current treatment for hypertriglyceridemia, HDL cholesterol ≤40mg/dL in male or ≤50mg/dL in females or current treatment for low HDL-C levels, high blood pressure (SBP≥130mmHg or DCBP≥85mmHg) or current antihypertensive treatment, fasting blood glucose≥100mg/dL or current glucose lowering treatment.18
Statistical analysisFor the descriptive analysis, after verifying the normality of variables using a Kolmogorov–Smirnov test, the standard measures of central tendency and dispersion were used: mean and standard deviation (SD). For the bivariate analysis, a difference of means test (Student's t-test) was used for parametric variables, while a Mann–Whitney U test was used for nonparametric variables. A Chi-square test was used to compare proportions. All analyses were performed using SPSS statistical software version 15.
ResultsOne hundred and one randomly selected subjects of both sexes, aged 30–70 years, were studied. Forty-eight subjects (21 males/27 females) were enrolled into the regular exercise arm and 53 subjects (31 males/22 females) were enrolled into the sedentary arm, with no significant differences between the groups (p=0.139).
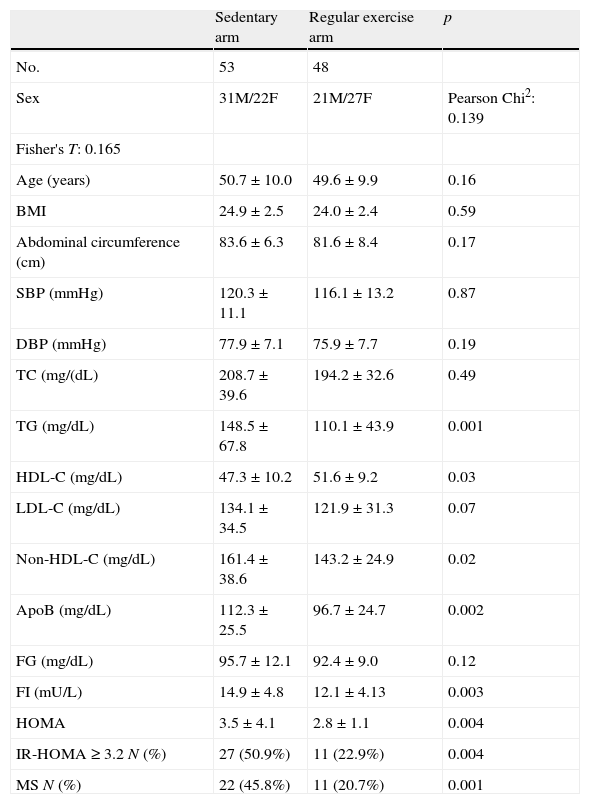
Table 1 shows the general characteristics of the study arms (regular exercise and sedentary).
General characteristics of the study arms.
| Sedentary arm | Regular exercise arm | p | |
| No. | 53 | 48 | |
| Sex | 31M/22F | 21M/27F | Pearson Chi2: 0.139 |
| Fisher's T: 0.165 | |||
| Age (years) | 50.7±10.0 | 49.6±9.9 | 0.16 |
| BMI | 24.9±2.5 | 24.0±2.4 | 0.59 |
| Abdominal circumference (cm) | 83.6±6.3 | 81.6±8.4 | 0.17 |
| SBP (mmHg) | 120.3±11.1 | 116.1±13.2 | 0.87 |
| DBP (mmHg) | 77.9±7.1 | 75.9±7.7 | 0.19 |
| TC (mg/(dL) | 208.7±39.6 | 194.2±32.6 | 0.49 |
| TG (mg/dL) | 148.5±67.8 | 110.1±43.9 | 0.001 |
| HDL-C (mg/dL) | 47.3±10.2 | 51.6±9.2 | 0.03 |
| LDL-C (mg/dL) | 134.1±34.5 | 121.9±31.3 | 0.07 |
| Non-HDL-C (mg/dL) | 161.4±38.6 | 143.2±24.9 | 0.02 |
| ApoB (mg/dL) | 112.3±25.5 | 96.7±24.7 | 0.002 |
| FG (mg/dL) | 95.7±12.1 | 92.4±9.0 | 0.12 |
| FI (mU/L) | 14.9±4.8 | 12.1±4.13 | 0.003 |
| HOMA | 3.5±4.1 | 2.8±1.1 | 0.004 |
| IR-HOMA≥3.2 N (%) | 27 (50.9%) | 11 (22.9%) | 0.004 |
| MS N (%) | 22 (45.8%) | 11 (20.7%) | 0.001 |
Apo B: apolipoprotein B; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; TC: total cholesterol; non-HDL-C: total cholesterol-HDL-C; FG: fasting glucose; M/F: male/female; HOMA: homeostatic model assessment; FI: fasting insulin; BMI: body mass index; IR-HOMA≥3.2: insulin resistance; N: number of subjects; DBP: diastolic blood pressure; SBP: systolic blood pressure: MS: metabolic syndrome; TG: plasma triglycerides.
No differences were found between the regular exercise and sedentary arms in terms of age, BMI, waist circumference, SBP, or DBP.
As regards lipid parameters, significant differences were found between the regular exercise and sedentary arms in TG (110.1±43.9 vs 148.5±67.8mg/dL; p=0.001), HDL-C (51.6±9.2 vs 47.3±10.2mg/dL; p=0.03), non-HDL-C (143.2±24.9 vs 161.4±38.6mg/dL; p=0.02), and apoB (96.7±24.7 vs 112.3±25.5mg/dL; p=0.002), while no statistically significant differences were seen in TC and LDL-C levels (Table 1).
No differences were found in fasting plasma glucose levels, which were 92.4±9.0mg/dL in the exercise arm and 95.7±12.1mg/dL in the sedentary arm (p=0.12). By contrast, significant differences were seen in basal plasma insulin (12.1±4.13 vs 14.9±4.8mU/L) and the HOMA index (2.8±1.1 vs 3.5±4.1) between the group performing exercise and the sedentary group (p=0.004 for insulinemia and p=0.001 for the HOMA index) (Table 1).
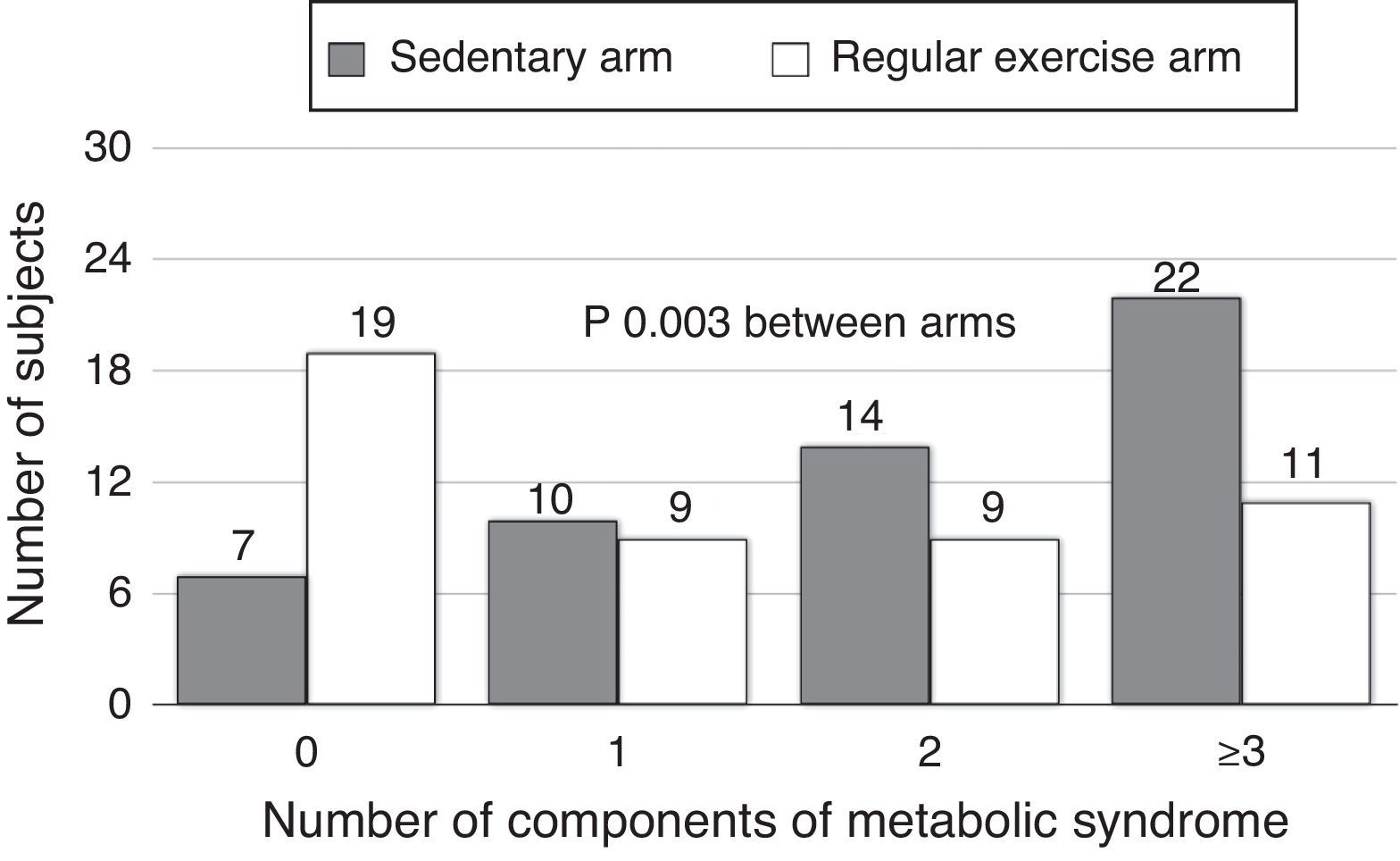
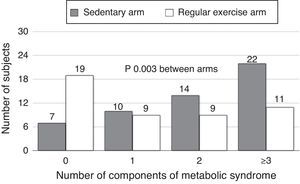
The proportions of subjects with IR (HOMA≥3.2) were 50.9% in the sedentary arm and 22.9% in the exercise arm, with a statistically significant difference (p=0.004). Subjects with MS were 45.8% and 20.7% in the sedentary and exercise arms respectively (p<0.001) (Table 1). A greater number of subjects in the exercise arm were found to have no or only one component of MS, while a greater number of subjects in the sedentary arm had 2, 3, or 4 MS factors (p=0.003) (Fig. 1).
DiscussionOverweight and obesity represent significant health problems in developed countries. In our community, subjects with BMI≥25 currently account for 63% of the adult population. Obesity has a genetic basis and a significant relationship with environmental factors, particularly excess calorie intake and a sedentary lifestyle or physical inactivity.
Physical activity has a significant impact on the metabolic changes related to obesity. Physical activity guidelines have been established based on a systematic review of studies conducted in Western countries.19 In 2008, the performance of physical activity of moderate intensity for longer than 150min/week (7.5MET-h/week)8,9 was recommended as adequate to maintain a good state of health. This was the cut-off point used in our study to differentiate the subjects doing regular physical activity from sedentary subjects.
Regular physical activity is associated with a better metabolic profile. Atherogenic dyslipidemia, characteristic of subjects with MS, insulin resistance, and diabetes is characterized by increased TG levels, decreased HDL-C levels, and increased non-HDL-C and apoB levels, and is related to high cardiovascular risk.20,21 Our study found significant differences in lipid parameters (atherogenic dyslipidemia) between sedentary subjects and those who performed routine physical exercise, in agreement with data reported by other authors who found that routine exercise and low to moderate physical activity had considerable benefits on the lipid profile, with significant changes in plasma TG and HDL-C.22,23
Our study had some limitations which should be taken into account when interpreting its results. Firstly, 56% of subjects in the exercise arm were female, as compared to only 41.5% in the control arm. This difference is not statistically significant due to the sample size, but should be noted because gender has a well known impact on the tested parameters. Menopausal status, which could have modified the results obtained, was not included among the data collected from the women enrolled. It should finally be noted that differences in BMI and age were 0.9kg/m2 and 1.1 years respectively. The statistical differences between these parameters are obviously not significant, and p values (0.59 and 0.16 respectively) do not even approach statistical significance. Some parameters with statistical significance may have appeared if a larger sample had been used, but the metabolic changes would also have been greater. For the design used, we think that the absence of statistical significance in these parameters does not detract any value from our findings.
Although the design of our study only allowed us to establish associations and not causal relationships, significant differences were found in the lower apoB levels in the group doing exercise with no differences in LDL-C. Similar data have been reported by other authors in healthy subjects who performed exercise three times a week and had a decrease in apoB and the apoB/apoA1 ratio as compared to sedentary subjects.24 Other authors25 found beneficial effects on cardiovascular risk markers in healthy young adults after short training with moderate exercise. These lipid changes were more marked in the group doing intense exercise.
The definition of insulin resistance based on the HOMA index has been widely used in clinical and epidemiological studies and validated with the hyperinsulinemic euglycemic clamp. Insulin resistance is defined as HOMA index values ≥3.2.17,26 In healthy, non-obese, and non-diabetic people, mild to moderate aerobic exercise increases insulin sensitivity, leading to the conclusion that a moderate physical exercise program is beneficial for improving insulin sensitivity and preventing the consequences of insulin resistance: MS and type 2 diabetes.27
Many studies have shown that moderate physical exercise decreases the risk of MS.28,29 Increases in physical activity levels in leisure time, in terms of duration or intensity, are linearly associated with a reduction in MS risk.30
We conclude that an active life including routine moderate physical exercise, with no intervention regarding diet or body weight, is related to an increase in insulin sensitivity, a better lipid profile, and a decrease in MS components. In addition, it is also probably related to a decreased incidence of diabetes and cardiovascular disease.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please, cite this article as: Caro J, et al. Efecto metabólico del ejercicio físico regular en la población sana. Endocrinol Nutr. 2013;60:167–72.