Thyroid squamous cell carcinoma (SCC) is an uncommon condition, because the normal thyroid gland has no squamous epithelium. The incidence of this tumor is 0.7–3%, and less than 50 cases have been reported. Papillary thyroid carcinoma (PTC) associated with SCC is an exceptional thyroid gland tumor and shows phenotypical and immunological characteristics of both components in the same lesion1. We report the case of a female patient who experienced PTC associated with thyroid SCC.
This was a 62-year-old woman diagnosed with primary hypothyroidism treated with levothyroxine 150μg/day who complained of a mass in the anterior neck area associated with dysphonia, dysphagia, and dyspnea. Chest X-rays showed an intrathoracic mass causing a tracheal deviation to the right. Neck ultrasound revealed an irregular, enlarged thyroid gland at the expense of the left lobe and an 18mm adenopathy with cystic areas in the left laterocervical lymph node chain. Computed tomography (CT) showed a heterogeneous 6.5cm×5cm×6.5cm thyroid mass that had affected the left thyroid lobe and, partially, the right lobe, infiltrated the esophagus and trachea, decreasing their lumen to 9mm, and eroded the cricoid and thyroid cartilages. Multiple left cervical adenopathies up to 20mm in diameter were also detected. Fiberoptic bronchoscopy revealed left vocal cord palsy, paresis in the right vocal cord, and extrinsic fixed stenosis of the airway. FNA of the adenopathy showed tumor cells consistent with carcinoma.
Based on these findings, total thyroidectomy and laryngectomy, a cervical esophagectomy, bilateral functional cervical node excision, and the resection of two tracheal rings and a portion of the left internal jugular vein due to gross infiltration were performed. A tracheostomy was performed at the upper sternal level.
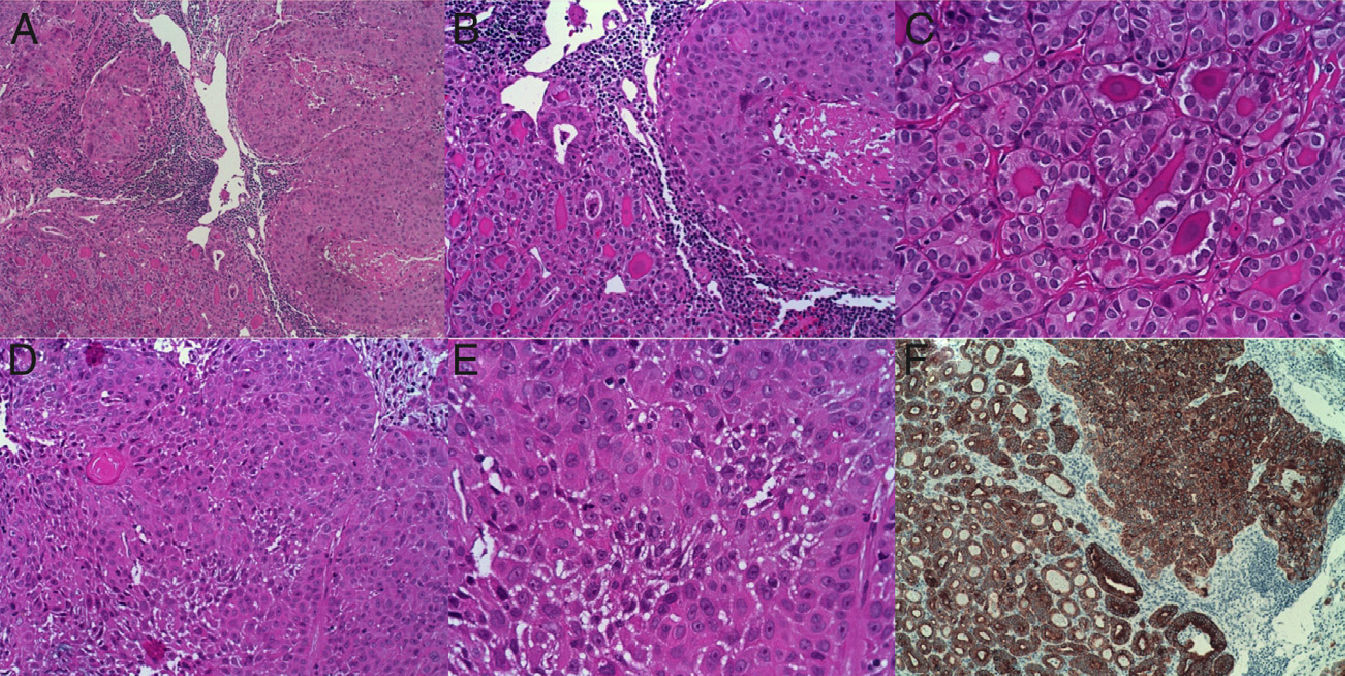
A pathological study found PTC of the tall cell follicular variant with areas of poorly differentiated epidermoid carcinoma that had infiltrated the larynx, esophagus and prelaryngeal muscles, with vascular and perineural invasion and tumor-free surgical margins (stage pT4), associated with chronic thyroiditis. Metastases were also found in six left cervical lymph nodes excised. Immunohistochemistry showed the tumor to be positive for CK19, thyroglobulin, thyroid transcription factor-1 (TTF-1), and MIB-1. The area consistent with epidermoid carcinoma was positive for CK19, CD10, and CK5/6 (Fig. 1).
(A) Thyroid tumor with biphasic pattern of papillary carcinoma (PC) and squamous carcinoma (SC). A follicular pattern of PC is shown on the left, and a solid pattern of SC on the right (10×). (B) The same image as in (A) at higher magnification (20×). (C) Follicular pattern of papillary carcinoma. (D and E) Areas of atypical squamous differentiation. (F) Immunohistochemistry strongly positive for cytokeratin 19 in both PC and SC areas.
Eight weeks after surgery, 104mCi of 131I was administered. A subsequent scan showed accumulation of the tracer in the left thoracic region. Suppressive therapy with levothyroxine 200μg/day was then started.
A control CT scan showed disease progression with the growth of a neck mass, which extended from the tracheostomy to the hyoid invading the tongue, with jugular vein thrombosis, adenopathic conglomerate in the submental and submandibular areas and in the posterior cervical triangle, and bilateral millimetric lung nodules probably related to metastases. At this time, laboratory test results included TSH 0.02μIU/mL (0.465–4.68), FT4 1.68ng/dL (0.78–2.19), thyroglobulin <0.5ng/mL, and negative thyroglobulin and microsomal antibodies.
After verifying disease progression, chemotherapy was started with cisplatin 75mg/m2 and 5-fluorouracil 1000mg/m2 every 21 days. Four cycles were administered with no response. The patient showed absolute dysphagia, and a jejunostomy was performed for feeding. The patient died eight months after diagnosis.
When SCC is found in the thyroid gland, the possibility that the tumor is a metastatic disease or an invasion of a local structure should be ruled out. For this, chest X-rays, laryngoscopy, bronchoscopy, and esophagoscopy should be performed. The presence of embryological remnants of the thyroglossal duct, the last branchial arch, or the thymic epithelium may explain the presence of squamous cells in the thyroid gland.2 It has also been suggested that squamous metaplasia occurring in the follicular epithelium due to chronic thyroid gland inflammation promotes the development of SCC in the affected epithelium.2
SCC in the thyroid gland has been reported to be associated with other thyroid diseases such as Hashimoto's thyroiditis,3 PTC,4 follicular carcinoma,5 and anaplastic carcinoma.6
The mean age at diagnosis of patients with SCC ranges from the fifth to the sixth decades, but the condition may occur at any age. Most cases have been reported in women.7 Patients usually have a rapidly growing neck mass, obstructive symptoms, and dysphonia. Nodal metastases and the invasion of local structures such as the trachea and esophagus are common at diagnosis. The clinical differentiation of SCC from thyroid anaplastic carcinoma is impossible. FNA is important for diagnosis, but surgical specimens should be studied. The final diagnosis of SCC is crucial because treatment and prognosis differ from those of other types of thyroid tumors. According to the classification of the World Health Organization, SCC of the thyroid gland is a malignant tumor consisting completely of cells with squamous differentiation. To diagnose SCC, a metastasis or direct invasion from an adjacent organ should be ruled out.8 In immunohistochemistry, SCC is positive for cytokeratin, but not for thyroglobulin.
The treatment options are limited. Surgery alone, chemotherapy, or radiotherapy alone is ineffective7 because there is a high probability of recurrence and local invasion. The only option for survival is extensive surgery to remove all tumor tissue, leaving tumor-free surgical margins. This is possible if the tumor is diagnosed in the early stages. In advanced stages, SCC infiltration makes total resection of tumor tissue virtually impossible.7
As regards adjuvant treatment, the administration of 131I is not helpful because squamous cells have no follicular differentiation and do not take up iodine. Combined treatment with surgery and postoperative radiotherapy decreases local recurrence, and some patients treated with this scheme have survived longer.9 Other treatment options include the combination of surgery with postoperative radiotherapy and chemotherapy. This option is recommended because it decreases local disease recurrence. Several clinical trials conducted using adriamycin, bleomycin, cisplatin, vincristine, doxorubicin, and cyclophosphamide found no advantages in any of them.7 Thyroid squamous cells may show EGFR gene polymorphisms and increased EGFR protein expression, which may represent a therapeutic target.10 SCC of the thyroid gland is a tumor with a poor prognosis with an aggressive course similar to anaplastic thyroid carcinoma. Mean survival is less than one year, and death is usually secondary to direct invasion or compression of the trachea.2,7
Please cite this article as: Manrique Franco K, Cedeño Díaz Oderay M, Aragón Valera C, Sánchez-Vilar Burdiel O, Rovira Loscos A. Carcinoma papilar tiroideo asociado al carcinoma de células escamosas. Endocrinol Nutr. 2014;61:226–228.