Despite the high prevalence of chronic kidney disease in the elderly population, few data are available on the frequency of secondary hyperparathyroidism in the Spanish population affected by this problem. We undertook a study on this issue in patients attending the internal medicine departments in our area.
Design and methodsAn observational, cross-sectional survey performed at internal medicine departments on 415 patients with stage 3 and 4 chronic kidney disease. Clinical history and risk factors were collected using a standardized protocol. Serum creatinine, phosphate, calcium, intact parathormone (PTH) and 25-hydroxy-cholecalciferol (25-OH-vitD) levels were measured in all patients.
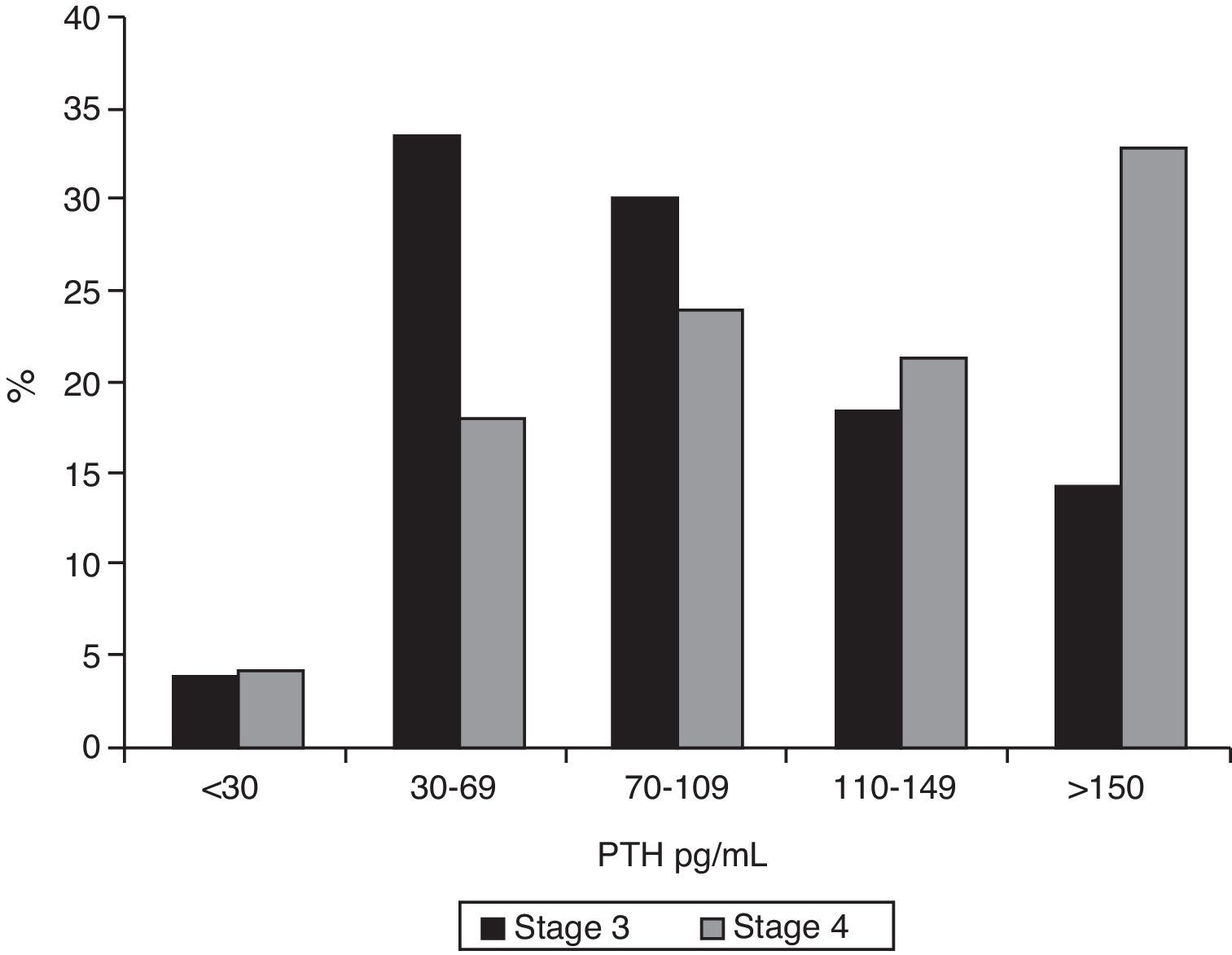
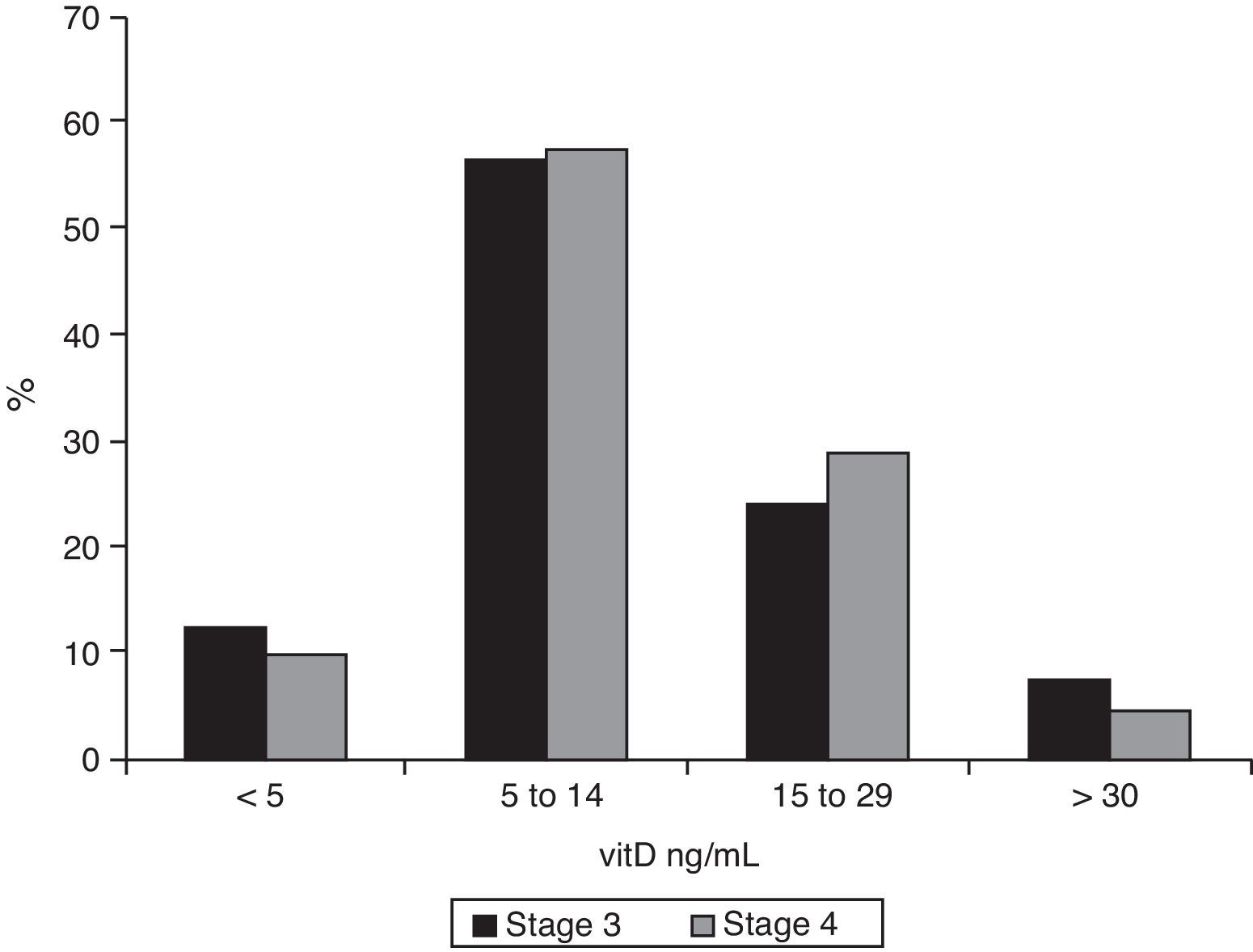
ResultsAmong stage 3 patients, 62.9% had PTH levels ≥70pg/mL and 32.7% levels ≥110pg/mL. Median PTH level in stage 4 patients was 120pg/mL (p<0.001), and 77.9% of these patients had PTH ≥70pg/mL (p<0.001) and 54.1% ≥110pg/mL (p=0.015). Adequate 25-hydroxy-cholecalciferol levels were found in only 7.2% of stage 3 patients and 4.1% of stage 4 patients. Only 7.2% of stage 3 patients had hyperphosphatemia, as compared to 25.4% of stage 4 patients (p<0.001).
ConclusionsHyperparathyroidism is a common complication of stage 3 and 4 chronic kidney disease which is not associated to detectable changes in serum calcium and phosphate levels. It is therefore advisable to measure PTH levels in all patients with decreased glomerular filtration rate.
A pesar de la elevada prevalencia de insuficiencia renal en la población anciana existen escasos datos sobre frecuencia del hiperparatiroidismo secundario en la población española afecta de este trastorno. Nos propusimos estudiar este aspecto a partir de las personas atendidas por los servicios de medicina interna de nuestra área.
Diseño y métodosEstudio transversal observacional realizado en servicios de medicina interna. Se incluyeron 415 pacientes con insuficiencia renal crónica estadios 3 y 4. Se recogieron antecedentes y factores de riesgo según un protocolo estandarizado. En todos los casos se determinaron niveles séricos de creatinina, fósforo, calcio, parathormona (PTH) intacta y 25-hidroxi-colecalciferol (25-OH-vitD).
ResultadosDe los pacientes en estadio 3 el 62,9% presentaban una PTH ≥70pg/ml, y en el 32,7% los valores eran ≥110pg/ml. En los enfermos en estadio 4 la mediana de la concentración de PTH era 120pg/ml (p<0,001). De ellos, el 77,9% tenía una PTH ≥70pg/ml (p<0,001) y el 54,1% una PTH ≥110pg/ml (p=0,015). Los valores de 25-OH-vitD eran adecuados en el 7,2% de los pacientes en estadio 3 y en el 4,1% de los de estadio 4. Solamente el 7,2% de los pacientes en estadio 3 tenían hiperfosfatemia, frente al 25,4% en estadio 4 (p<0,001).
ConclusionesEl hiperparatiroidismo es una complicación frecuente de la insuficiencia renal crónica en los estadios 3 y 4, sin que aparezca alteración detectable en los niveles de calcemia o fosfatemia, por lo que es aconsejable determinar PTH en todos los pacientes con filtrado glomerular disminuido.
Chronic kidney failure (CKF) is a clinical syndrome derived from progressive, generalized, and irreversible kidney function impairment secondary to nephron mass destruction. The prevalence of CKF varies depending on both countries and studies. The criterion normally used for CKF is an estimated glomerular filtration rate (eGFR) less than 60mL/min, according to the recommendations of The National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NFK-KDOQI), which stratify CKF into five stages based on eGFR calculated by different formulas, of which the most commonly used in the abbreviated MDRD-4 version.1–3 Using these criteria, the prevalence of CKF is relatively high, particularly in elderly populations. The EPIRCE study, conducted all over Spain, reported a prevalence close to 6% in the general population,4 while the HERMEX study, conducted in Extremadura, found a 4% prevalence.5 Over the age of 65 years, the prevalence of kidney disease exceeded 21% in the EPIRCE study and was three times higher in the HERMEX study.
Decreased kidney function causes metabolic changes that lead to increased parathormone (PTH) levels. Decreased functional kidney mass results in calcitriol deficiency, which “uninhibits” the production of PTH mRNA. In addition to hypocalcemia and decreased calcitriol, phosphorus retention resulting from decreased glomerular filtration is an additional factor which, by different mechanisms, promotes the development of secondary hyperparathyroidism.6 Increased circulating PTH is initially detected at the early stages of kidney function, and kidney function impairment is associated with a progressive increase in PTH levels.7
Despite the high prevalence of CKF in the elderly population, very few data are available on the frequency of occurrence of hyperparathyroidism secondary to kidney disease in the Spanish population with this disorder. The purpose of our study was to analyze this issue based on people with CKF attending the internal medicine departments in our region.
Design and methodsThis was an observational, cross-sectional study conducted at the internal medicine departments of 14 hospitals in Extremadura and Andalusia. Information was collected from patients seen consecutively at internal medicine clinics or hospital wards. A total of 415 patients with stage 3 and 4 CKF based on K/DOQI criteria were enrolled into the study.3 Of these patients, 293 were at stage 3 (70.6%; mean eGFR 43.3±8.7mL/min) and 122 at stage 4 (29.4%; eGFR 24.7±6.2mL/min). Male patients accounted for 47.7% of the sample, and the mean patient age was 78.5±8.8 years, with a median Charlson comorbidity index=2 (mean 2.7). Sixty-one percent of the patients enrolled were hospitalized (median Charlson index=3, mean 3.14). Calcium supplements were being taken by 10.6% of patients (males 7.8%, females 13.5%), 11.2% were being administered vitamin D (98.8% calciferol), and 4.8% were being treated with bisphosphonates. Corticosteroid treatment was being administered to 7.5% of patients.
Clinical histories and risk factors, as well as treatments received, were collected from all participants according to a standardized protocol. Fasting serum creatinine, phosphorus, and calcium levels were measured in all patients. The glomerular filtration rate was estimated using the abbreviated MDRD-4 formula. Intact parathormone (PTH) and 25-hydroxy-cholecalciferol (25-OH-vitD) levels were also measured by the laboratory method used in each participating hospital. Based on the recommendations of the Spanish Society of Nephrology for the management of bone and mineral metabolism disorders in patients with chronic kidney disease,8 secondary hyperparathyroidism was defined as PTH ≥70pg/mL, and 25-OH-vitD deficiency as a value <30ng/mL. Hypocalcemia was defined as plasma calcium levels <8.5mg/dL.
Quantitative variables are given as the mean and standard deviation (SD), or as median and interquartile range (IQR) depending on their distribution. Qualitative variables are given as absolute and relative frequencies with their corresponding confidence intervals. The difference between means was analyzed using a Mann–Whitney test. A Chi-square test was used for differences between prevalence rates. SPSS 21.0 statistical software (IBM, Armonk, New York, USA) was used for statistical analysis. Sample size was estimated by considering as the primary variable the proportion of iPTH, estimated at 37%, a 95% confidence level, and an absolute precision of 5%. Based on the above assumptions, the estimated sample size was 359 patients.
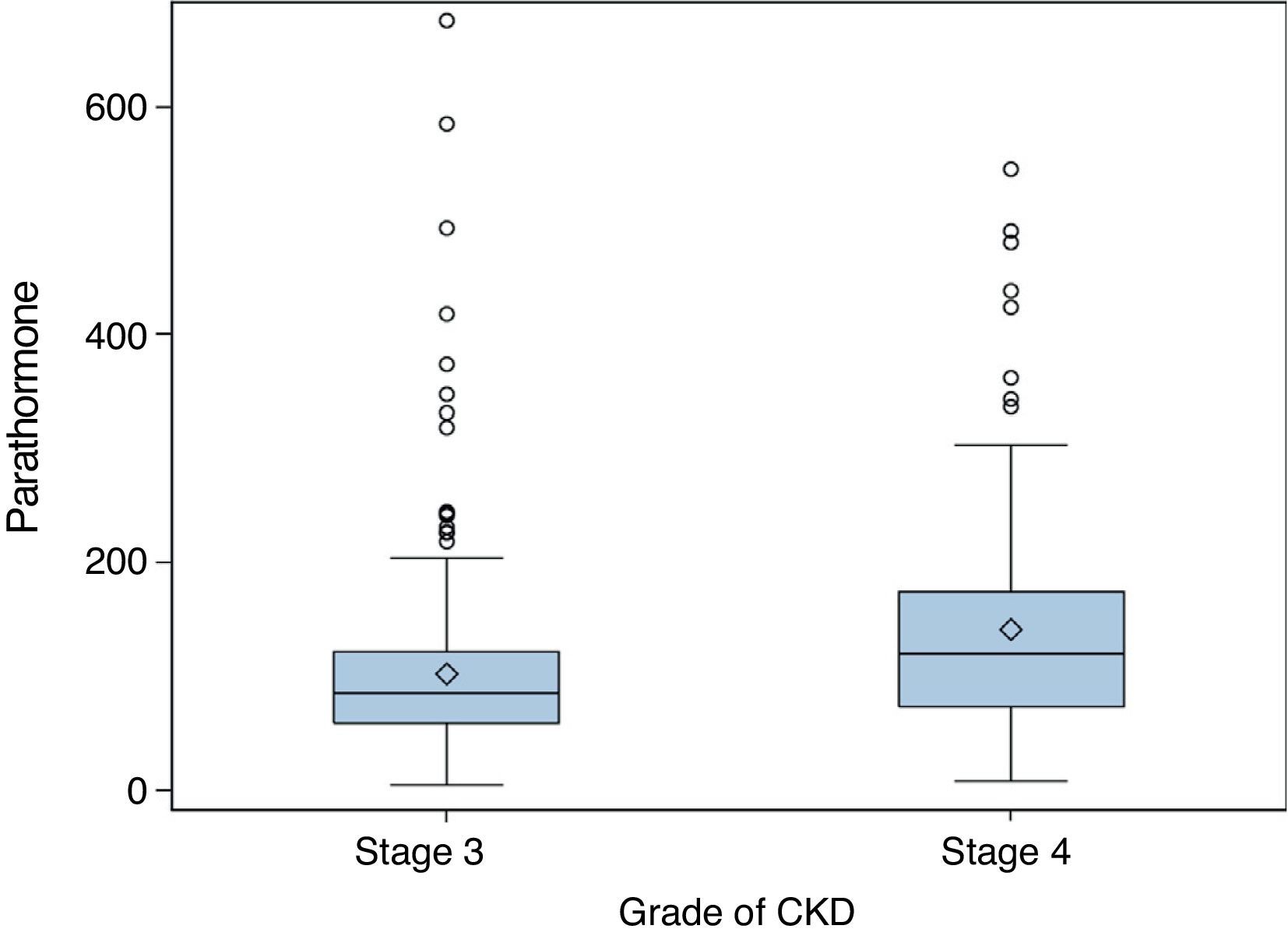
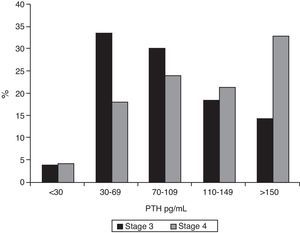
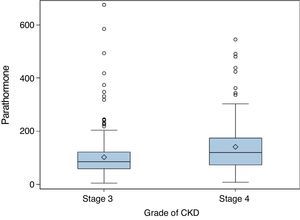
ResultsMedian PTH in stage 3 patients was 86pg/mL (IQR 60–122); 62.9% of these patients (95% CI 0.57–0.68) had PTH levels ≥70pg/mL, and 32.7% values ≥110pg/mL (95% CI 0.28–0.38). In stage 4 patients, median PTH level was 120pg/mL (IQR 74–175, p<0.001). Of these, 77.9% (95% CI 0.70–0.84) had PTH ≥70pg/mL (p<0.001), and 54.1% (95% CI 0.45–0.63) PTH ≥110pg/mL (p=0.015). Figs. 1 and 2 break the data down.
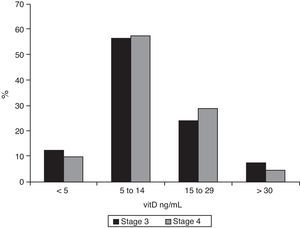
As regards 25-OH-vitD levels, only 7.2% (95% CI 0.05–0.11) of stage 3 patients had adequate values, and this proportion was even lower among stage 4 patients (4.1%, 95% CI 0.02–0.09), but the difference was not significant. The data are broken down in Fig. 3. Median 25-OH-vitD levels were 10.9ng/mL (IQR 6.9–17.3) in stage 3 patients and 11.0ng/mL (IQR 7.1–17.0) in stage 4 patients (not statistically significant).
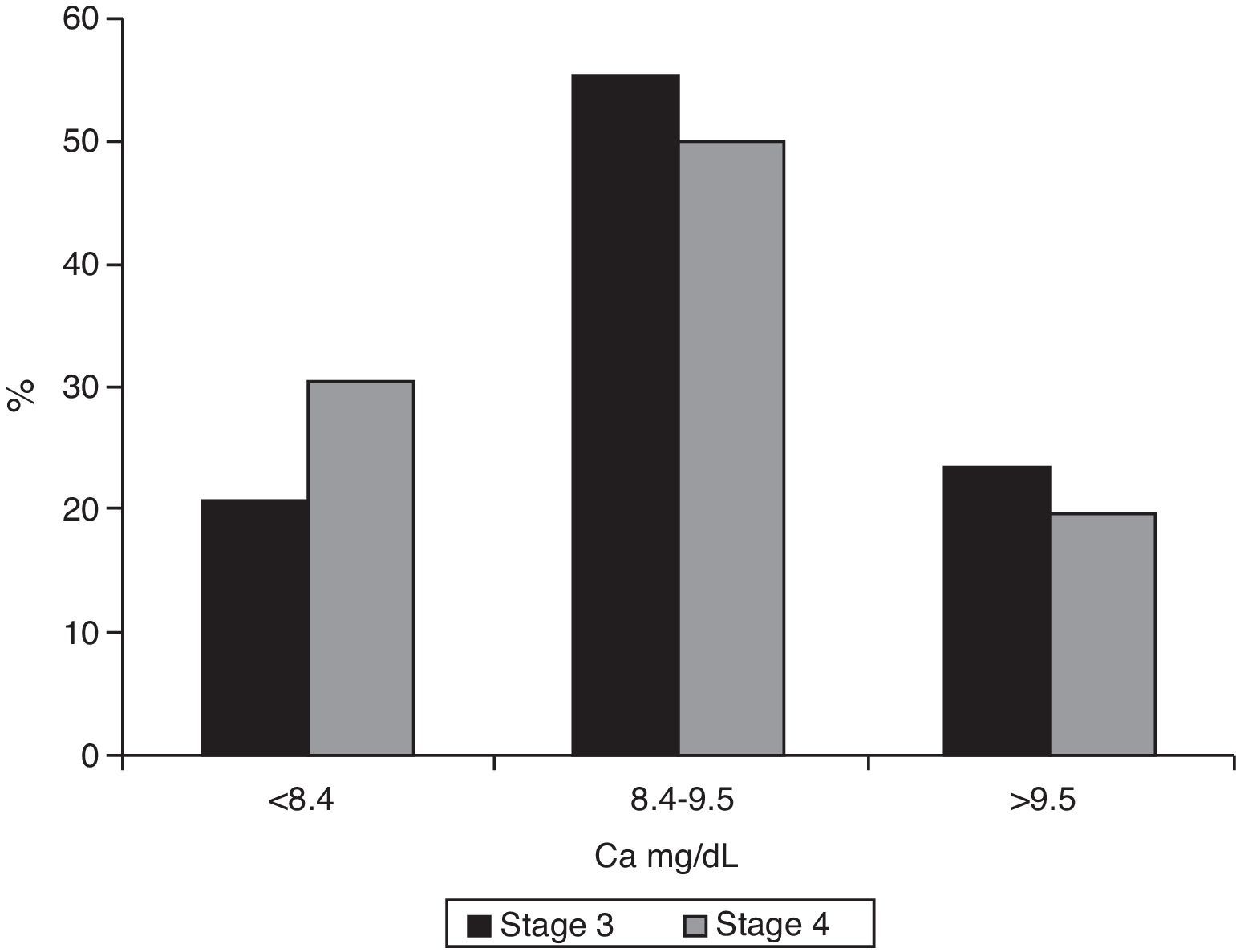
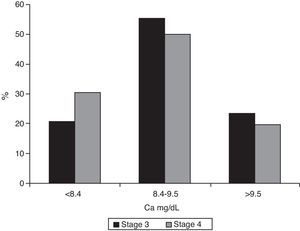
Median calcium levels were 9.0mg/dL (IQR 8–9.4) in stage 3 patients and 8.7 (IQR 8.2–9.3) in the stage 4 groups (p=0.007). Hypocalcemia was found in 20.9% (95% CI 0.17–0.26) of patients with stage 3 CKF and 30.3% (95% CI 0.23–0.39) of stage 4 patients (p=0.039, Chi-square test) (Fig. 4).
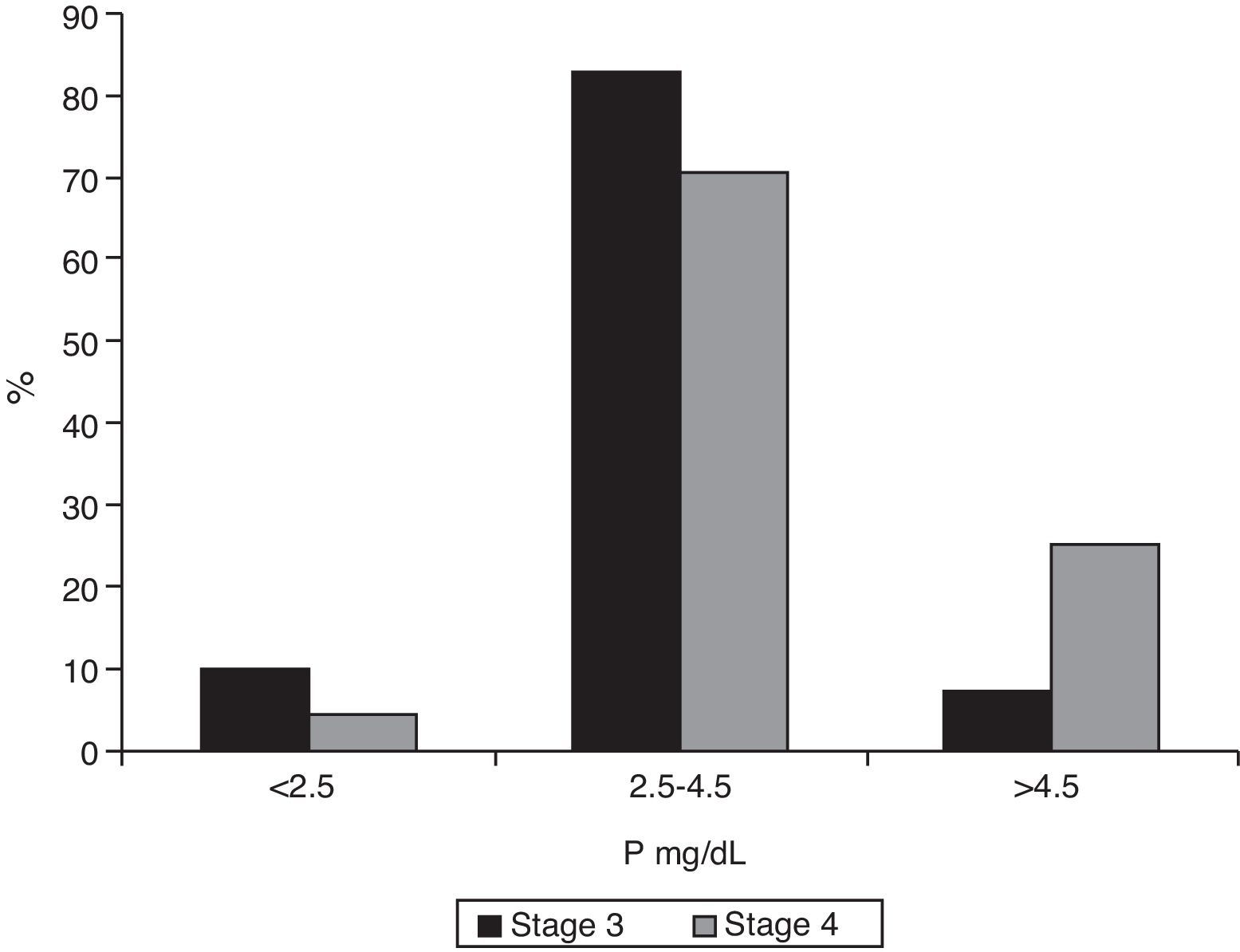
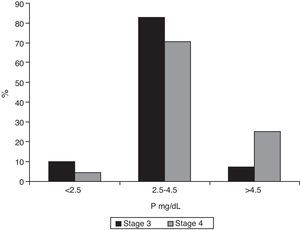
Median phosphorus levels were 3.3mg/dL (IQR 2.9–3.8) in patients with stage 3 CKF and 3.9mg/dL (IQR 3.5–4.9) in stage 4 patients (p<0.001). Only 7.2% (95% CI 0.05–0.11) of stage 3 patients had phosphate levels ≥4.5mg/dL (95% CI 0.19–0.34), as compared to 25.4% of stage 4 patients (p<0.001). Fig. 5 shows the distribution of phosphorus levels.
The median of the calcium-phosphorus product was 30.0 (IQR 25.2–34.8) in stage 3 and 34.3 (IQR 29.9–39.0) in stage 4 patients. The number of patients with a calcium-phosphorus product >55 was very low: 0.68% (95% CI 0.002–0.02) in stage 3 and 2.5% (95% CI 0.01–0.07) in stage 4 (not statistically significant).
DiscussionOur data show a very high prevalence of hyperparathyroidism secondary to kidney disease in the population with stage 3 and 4 kidney failure seen at internal medicine departments both when diagnostic levels and treatment goals for such stages are considered. Similar results were seen in the MERENA study,9 which compared morbidity and mortality data from 1129 patients with stage 3 and 4 CKF according to KDOQI criteria attending nephrology clinics who were distributed into two cohorts, diabetic (n=461) and non-diabetic patients (n=668). Mean age was similar to that of our sample (70 years), and the proportion of patients with PTH outside the therapeutic range was 71.3% in stage 3 (PTH ranging from 35 to 70pg/mL) and 79.3% in stage 4 (range, 70–110pg/mL). In both cases, the proportions were much higher than those found in our study, which may be explained by the different origin of the samples. While the poor control of hyperparathyroidism seen at nephrology clinics may appear surprising, the OSERCE study, conducted on 634 patients from 32 Spanish nephrology units, reported similar results.10 The same occurs when a comparison is made with other studies which also reported a very high prevalence of hyperparathyroidism secondary to kidney failure.11–14
Taking into account the relatively high prevalence of CKF in the Spanish population,4,5,15 both the MERENA study and our results should be a cause of concern and show the need for screening all of these patients for hyperparathyroidism, despite the resultant costs. This is not a minor issue, because apart from the immediate problem of renal bone disease, PTH has traditionally been considered a uremic toxin and has been associated with various systemic effects, in particular with the occurrence of vascular calcifications.16 Left ventricular hypertrophy, a known vascular risk factor highly prevalent in kidney failure, has also been related to the occurrence of hyperparathyroidism.17 Its relationship to overall mortality18,19 and cardiovascular morbidity and mortality has also been suggested.20 Since cardiovascular mortality is the leading cause of death in patients with renal failure,21 the measurement of PTH levels is particularly necessary in any patient with eGFR <60mL/min.
Our data show that changes in phosphate and calcium levels rarely occur, even when PTH levels are higher than 110pg/mL. Phosphorus retention has traditionally been related to the occurrence of hyperparathyroidism, which is the main factor responsible for bone disease associated with kidney failure.22 Epidemiological evidence has also accumulated relating hyperphosphatemia both to cardiovascular and all-cause mortality of renal patients and to the progression of kidney disease.23 Based on our results, the kidney appears to maintain its capacity to adjust phosphorus balance in stage 3 of kidney disease. However, this involves the stimulation of PTH production and the occurrence of hyperparathyroidism, which can only be detected by specific PTH measurement even in the absence of hyperphosphatemia. Hyperphosphatemia, even if mild, occurs in a significant number of patients with stage 4 CKF.
In the past, PTH increase and the development of parathyroid gland hypertrophy in CKF was attributed to low calcium levels. It was subsequently shown that calcitriol levels gradually decreased as kidney function decreased24 and that vitamin D receptors, on which calcitriol acts, regulated the proliferation of parathyroid cells and PTH synthesis.25,26 As occurred with serum phosphorus levels, changes in calcium levels do not appear to be a frequent finding in patients with CKF, even at stage 4, and changes in the calcium-phosphorus product are not usually found either. Measurements of both parameters have therefore a poor diagnostic yield in these patients.
Vitamin D is a lipid-soluble vitamin which, in addition to being an essential micronutrient, should be regarded as a hormone involved in a complex endocrine system that regulates mineral homeostasis, protects skeletal integrity, and modulates cell growth and differentiation in a wide variety of tissues.27 Several published studies suggest that a considerable part of the population has vitamin D deficiency. Thus, in a Spanish study of an outpatient population over 64 years of age with no known risk factors for hypovitaminosis, the prevalence of hypovitaminosis D (defined as 25-OH-vitD levels <25ng/mL) was 87%.28 In another study, 61% of healthy, young university undergraduates in the Canary Islands had vitamin D deficiency or insufficiency (25-OH-vitD levels <30ng/mL), and 32% had levels less than 20ng/mL.29 The prevalence of vitamin D deficiency obviously depends on the cut-off plasma level used. The value proposed by the WHO is 20ng/dL, but there is currently a trend toward defining vitamin D insufficiency as levels <30ng/mL, which increases the prevalence.30 Our study results show a similar situation, in this case in patients with stage 3 and 4 renal failure. It should be noted that only 10% of these patients were receiving vitamin D supplements. This disorder is not inherent to kidney disease per se, but is a common finding in the general population. Vitamin D deficiency is associated with low sun exposure, combined with a low intake of foods containing the vitamin or with conditions that cause fat malabsorption. This is very surprising in the Spanish population, considering the high number of hours of sunshine in our country. Although individual variability in vitamin D production through the action of sunlight is very high, this can hardly explain the homogeneity of the results.31 It is a worrying finding, however, because vitamin D deficiency appears to be associated with multiple conditions, including cardiovascular diseases,32 and supplementation has not been shown to have therapeutic benefits.33
The main strength of this study lies in its sample size and the fact that the sample came from a circumscribed area with little immigration from either other regions of Spain or from abroad, thus giving the study population a considerable homogeneity. However, this in turn raises some doubts regarding the reproducibility of our data in other regions. The main weakness of the study is that the sample consisted of patients, rather than subjects from the general population, with a rather old mean age, which increases the prevalence of asymptomatic secondary hyperparathyroidism. In addition, parathormone was not measured at a central laboratory, and the variability in the methods used at the different laboratories may have influenced the results, although the differences between the various reference values were small.
In conclusion, hyperparathyroidism is an extremely frequent complication of stage 3 and 4 CKF. This complication usually occurs before any detectable change in calcium and phosphate levels. It is, therefore, advisable to measure parathormone levels in all patients with a glomerular filtration rate decreased to less than 60mL/min. Vitamin D deficiency is also very common in this patient group, but does not appear to be related to kidney failure.
AuthorshipList of participating physicians:García Sánchez, Francisco (Hospital Virgen del Puerto, Plasencia); Luque, Maria José (Hospital de la Siberia, Talarrubias); Morales, Rocío (Hospital Infanta Cristina, Badajoz): Megías, Ana (Hospital de la Siberia, Talarrubias); Muñoz Díaz, Fernando (Hospital de Llerena); Ramiro, Jose Manuel (Hospital de Coria); Romero, Manuel (Hospital Infanta Elena, Huelva); Gutiérrez Díaz, María Dolores (Hospital de Llerena); Gómez Casero, Lourdes (Hospital Tierra de Barros, Almendralejo); Nevado, Leticia (Hospital Perpetuo Socorro, Badajoz); Bureo, Nicolás (Hospital Perpetuo Socorro, Badajoz); Buenavida, Jose Ramón (Hospital de Llerena); Fernández Auzmendi, Verónica (Hospital de la Siberia, Talarrubias); Maciá, Enrique (Hospital Perpetuo Socorro, Badajoz); Masero, Antonio (Hospital de Don Benito-Villanueva de la Serena); Pijierro, Agustin (Hospital Infanta Cristina, Badajoz).
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Bureo JC, Arévalo JC, Antón J, Adrados G, Jiménez Morales JL, Robles NR. Prevalencia del hiperparatiroidismo secundario en pacientes con enfermedad renal crónica estadios 3 y 4 atendidos en medicina interna. Endocrinol Nutr. 2015;62:300–305.