In 2006, the Chicago Consensus1 on the management of intersex disorders proposed the replacement of terms such as intersex, hermaphrodite, and pseudohermaphroditism by the term “sexual development disorders”, defined as congenital diseases where the development of chromosomes, gonads, or anatomical sex is atypical. The birth of a child with any of these characteristics undoubtedly has a great social and psychological impact on his/her relatives. A rapid clinical, hormonal, genetic, molecular, and radiographic evaluation that allows for assessing both the etiology of the case and adequate management is therefore required. Initial work-op of a child with genital ambiguity should include family history, complete physical examination, sex chromosome evaluation, and a study of internal anatomy, as well as gonadal and adrenal steroid secretion levels. Diagnosis of these children with undifferentiated sex is, however, complex, because of a multifactorial and highly variable etiopathogenesis.
We report the case of a 19-year-old woman of Romanian origin, diagnosed with congenital adrenal hyperplasia on drug treatment, who was referred to the clinical genetics unit by the endocrinology department. Her personal history included resection of clitoris hypertrophia at 3 years of age after a laparotomy showed the presence of a uterus and ovaries. She had an unremarkable family history. She reported primary amenorrhea and hirsutism. A complete physical examination revealed a height of 1.50m, a weight of 105kg, truncal obesity, female phenotype and marked virilization signs in the face, arms, chest, and legs, a deep voice, and normal female external genitalia.
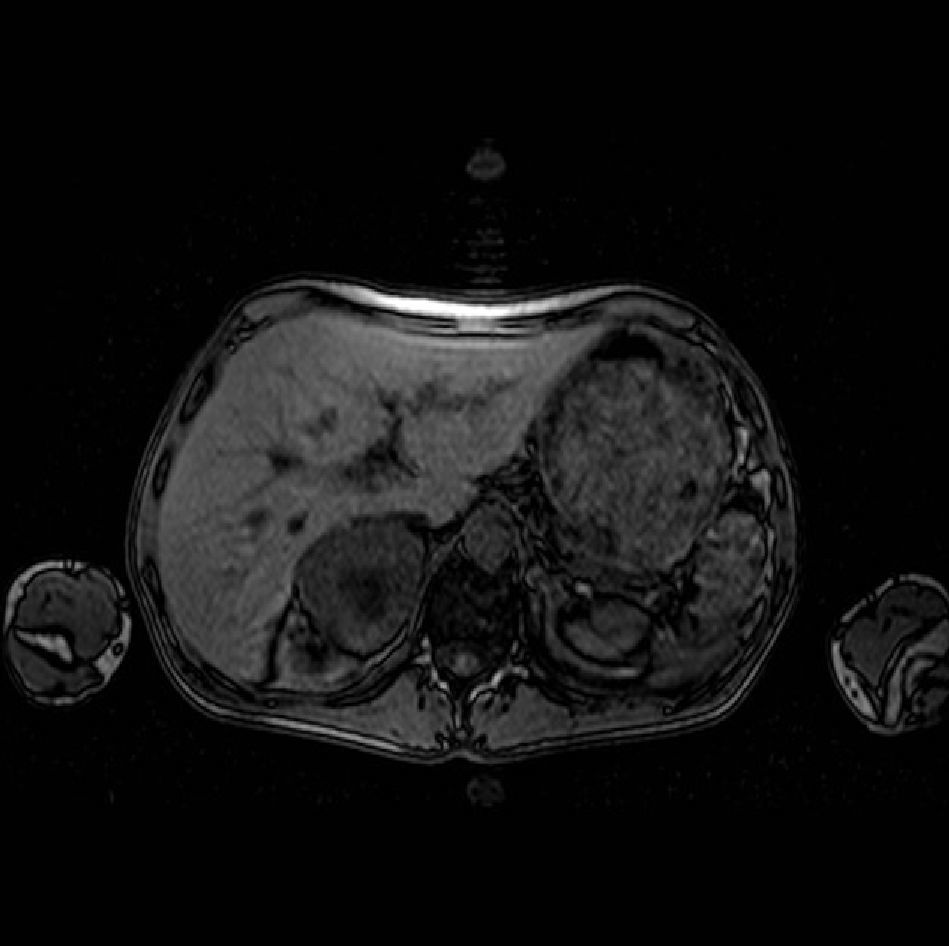
Except for low estradiol levels (<10pg/mL), all chemistry test results were within the normal range, including a 17-hydroxyprogesterone (17OHP) level of 0.91ng/mL. Gynecological ultrasound examination showed an anteverted, regular, and empty uterus measuring approximately 59mm×17mm×30mm, atrophic for the patient's age, and ovoid hyperechogenic images 19mm×7mm and 29mm×16mm in size on the right and left sides, respectively that suggested ovaries also atrophic for the patient's age. Abdominal magnetic resonance imaging (MRI) confirmed the presence of markedly atrophic uterus and ovaries, with no other genitourinary abnormalities. An additional MRI showed normal adrenal glands.
Molecular study of congenital adrenal hyperplasia found no mutation responsible for the development of the condition. Karyotype study in peripheral blood showed, in 14 metaphases tested, 46 chromosomes with the sex formula XY, and in 6 metaphases studied, 45 chromosomes with the sex formula X. The molecular cytogenetic technique of fluorescent in situ hybridization (FISH) with centromeric probes for chromosomes X and Y [Vysis CEPX (DXZ1)/Y(DYZ1)] was performed. The DXZ1 probe showed a signal for the centromeric region of the X chromosome in 30% of the nuclei, while the DYZ1 probe showed two signals for the centromeric region of Y chromosome in 70% of the nuclei (Fig. 1). The resulting conventional chromosome formula was: mos 45, X[30] ish (cepX[DXZ1]+)/46, X, dic(Y) [70] ish (cepX[DXZ1]+/Y[DYZ1]++). A familial study could not be performed because her relatives were not living in Spain (Fig. 1).
FISH performed with centromeric probe for chromosome X (red) and chromosome Y (green). A and B: metaphases, C and D: nuclei. A: metaphase with two green signals (two Y chromosome centromeres) and one red signal (an X chromosome centromere), B: metaphase with a red signal (an X chromosome centromere), C: metaphase with two green signals (two Y chromosome centromeres) and one red signal (an X chromosome centromere), D: metaphase with a red signal (an X chromosome centromere).
A study of the sex-determining region (SRY), the azoospermia factor region (AZF), and the Deleted in AZoospermia gene (DAZ) by polymerase chain reaction (PCR), using probes of 20 loci along the Y chromosome (SRY, DYS271, DYS148, DYS273, KALY, DYS212, SMCY, DYS215, DYS218, DYS219, DYS221, DYS223, DYS224, DYF51S1, DYS236, DAZ, DYS240), detected the presence of all tested regions of the chromosome in the peripheral blood cells of the patient.
Final diagnosis was Turner syndrome, 45,X/46,X, dic (Y) mosaicism.
Congenital adrenal hyperplasia is due to a deficiency in one of the enzymes involved in cortisol biosynthesis. In approximately 95% of cases it is due to a deficiency in 21-hydroxylase, which is altered in the fascicular area of the adrenal cortex so that 17OHP is not converted into 11-deoxycortisol. Because of defective cortisol synthesis, ACTH levels are increased, which results in the overproduction and accumulation of cortisol precursors, particularly 17OHP. This causes excess androgen production resulting in virilization.2 The reported patient was being treated for congenital adrenal hyperplasia and had a history of surgery for hypertrophic clitoris. The molecular study performed ruled out a mutation being responsible for the sexual development disorder.
Mosaicism of the sex chromosomes, consisting of a 45,X associated with another cell line that contains a structurally normal or abnormal Y chromosome, represents one of the main causes of ambiguous genitalia.3 Most cases of 45,X/46,XY mosaicism are attributed to the loss of the non-disjunction of the Y chromosome after disomic normal fertilization.4 The frequency of the Y chromosome or its fragments in Turner syndrome is estimated at 7%.5 The proportion of mosaicism and the involved tissues determine the clinical expression of the defect. In a high proportion of patients where the karyotype shows the line 45,X, Turnerian stigmata of highly variable intensity appear, as occurred in our patient. After puberty, increased gonadotropin levels and highly variable androgen production that determines an also highly variable virilization, usually occur.
The sex-determining region gene (SRY of chromosome Y) is a transcription factor in the short arm of chromosome Y that is critical for testis development. Mutations in the SRY gene in XY individuals lead to female phenotypical development, while patients with karyotype 45,X carrying the SRY gene in an autosome have a male phenotype.6 The early detection of Y chromosome sequences is important because of the high risk of the development of gonadal tumors, particularly gonadoblastoma, a rare gonadal neoplasm. Most such tumors occur in women with an abnormal karyotype in which at least a part of the centromeric region of the short arm of the Y chromosome, a region often called locus GBY, is present.7 The risk of gonadoblastoma or dysgerminoma is estimated at approximately 10%5 to 35%.8 In the event of mosaicism of chromosomes X and Y, detection of specific Y chromosome sequences is required in patients with mosaicism 45X/46XY in order to detect the risk of gonadoblastoma and to recommend gonadectomy.
Patients diagnosed with sexual development disorders may have psychosocial disturbances. Our patient considered herself a woman, and knowledge of the cell line containing the Y chromosome did not cause her any psychological conflict. Gender identity is a complex and sensitive subject, and physicians should have an open, frank discussion with patients and, if necessary, should consider referral to psychiatrists or psychologists experienced in the management of sexual identity disorders.
Despite adequate genetic counseling, our patient refused preventive gonadectomy and the resection of Wolffian structures to prevent the risk of tumors and potential virilization.
To sum up, the reported patient underwent at 3 years of age a laparoscopy that confirmed the presence of a uterus and ovaries, followed by surgery for hypertrophic clitoris. She was diagnosed with congenital adrenal hyperplasia and treated accordingly. A peripheral blood karyotype performed at 19 years showed two line cells, 45,X/46,XY. The Y chromosome was dicentric, that is, it consisted of two segments coming from two chromatids of the same chromosome.9 Since a karyotype does not always identify the Y chromosome or its fragments, molecular studies or FISH using a Y centromeric probe should be performed in any patient with Turner syndrome having a marker chromosome or a chromosomic sex fragment of unknown origin, and they should be complemented if needed.10 The development of molecular and cytogenetic techniques in recent years made it possible to confirm and guide diagnosis and treatment in the reported case.
Please cite this article as: García Benítez Y, et al. Trastorno en el desarrollo sexual en un paciente 45,X/46,X,DIC(Y). Endocrinol Nutr. 2012;59:268–70.