Skin changes associated with thyroid disease include specific lesions such as thyroglossal duct cyst and skin metastases, nonspecific signs such as those secondary to hormonal changes due to hyperfunction and hypofunction, and dermatological changes associated with thyroid diseases, of which we provide two clinical examples.
The prevalence rate of primary autoimmune hypothyroidism (PAIH) is 5%, and up to 8.3% if subclinical hypothyroidism is included.1 Skin manifestations associated with PAIH include a number of skin diseases common to patients with this condition (defined as the presence of autoantibodies even in a euthyroid state) and others directly dependent on thyroid function.
In the former group, the frequency of thyroid dysfunction is variable, occurring in 40–70% of patients with melanin spots of centrofacial location, in 42% of males and 62% of females with vitiligo, in 50% of patients with chronic mucocutaneous candidiasis, in 34% of patients with herpetiform dermatitis, in 8% of delayed hypersensitivity reactions, and in 8% of patients with alopecia areata. Autoimmune thyroid disease is also commonly associated with pemphigus and other bullous diseases, systemic lupus erythematosus, scleroderma, Kaposi's sarcoma, erythema annulare centrifugum, generalized granuloma annulare, multicentric reticulohistiocytosis, elastic pseudoxanthoma, reticular erythematous mucinosis, anemia (pernicious anemia, red blood cell aplasia), herpes gestationis, dermatomyositis, Sjögren's syndrome, polymyositis, other endocrine diseases (acanthosis nigricans, multiple endocrine neoplasia, McCune-Albright syndrome, Sweet's syndrome), CREST syndrome (calcinosis, Raynaud's syndrome, esophageal dysfunction, scleroderma, and telangiectasis), psoriasis, Cowden syndrome with multiple hamartomas, ANOTHER syndrome (alopecia, nail dystrophy, hypohydrosis, and ephelides), acropachies,2 and atopic manifestations such as urticaria, dermatographism and angioedema.3–5
Skin changes directly dependent on thyroid hypofunction include:
- -
Typically dry, pale, and cold skin due to decreased capillary flow, sweating, and thermogenesis; palmoplantar keratoderma, which may become generalized and convert into xeroderma, but dramatically responds to replacement therapy.
- -
Keratosis pilaris of follicles leading to permanent alopecia, thinned hair, and lateral loss of eyebrows. It may be associated with livedo reticularis in the limbs.6
- -
Generalized myxedema or cutaneous mucinosis, due to the accumulation of hyaluronic acid and glycosaminoglycans in the skin. This causes the characteristic hypothyroid facies: thick skin, periorbital edema, and mucosal thickening with dysphonia. There may be periocular hyperpigmentation (Jellinek's sign)7 and hypercarotenemia due to the lack of hepatic metabolism of carotene, which accumulates in the corneal layer, is excreted in sweat, and becomes deposited in areas rich in sebaceous glands.
- -
An uncommon lesion related to primary hypothyroidism and autoimmune polyglandular syndrome type I, erythema annulare centrifugum, consists of a ring-shaped eruption with central clearing occurring in the buttocks, thighs, and proximal part of the arms. Histological examination shows a perivascular lymphocyte infiltrate in the middle and deep dermis.8
- -
Granuloma annulare and oral lichen planus, not well known by most endocrinologists, may also be associated with hypothyroidism. Two cases are reported here, and their relationship to autoimmune thyroid disease is analyzed.
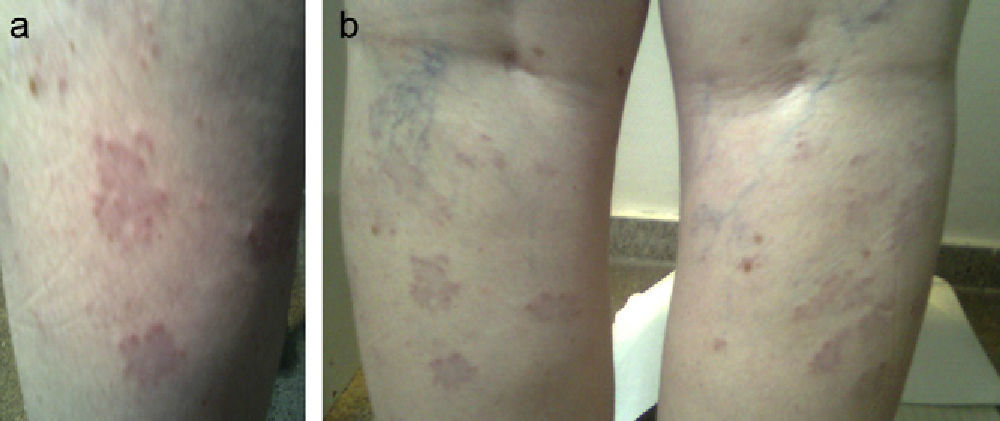
A female patient was referred at 41 years of age for hypothyroidism. Her history included a daughter with vitiligo and PAIH, and the patient herself had undergone surgery for melanoma in situ two years before and was in remission. Laboratory tests requested by the dermatology department revealed subclinical PAIH (TSH [thyroid-stimulating hormone]: 8.7mcU/mL, FT4 [free thyroxine]: 0.92ng/dL, ATA-TPO [antiperoxidase antibodies]: >1300U/mL) which was monitored at the clinic without treatment. Nine years later, the patient reported aphonia, dry skin, and asthenia with postmenopausal menstrual changes (TSH: 8.34mcU/mL, FT4: 0.98ng/dL). She had grade I goiter with an irregular surface and a hypoechoic, pseudonodular ultrasound image 8.7mm in size in the upper pole of the left lobe. Subsequently, she was treated with levothyroxine at doses of 1.11mcg/kg (TSH: 2.18mcU/mL). One year later, confluent papular lesions appeared in the posterior aspect of both legs below the popliteal fossa. The lesions were biopsied and were diagnosed as granuloma annulare (Fig. 1a and b). The patient was treated with topical corticosteroids with no lesion improvement, and is currently receiving PUVA therapy (psoralens and ultraviolet A radiation).
Granuloma annulare is an indurated, non-scaling lesion usually occurring in the limbs as ring-shaped plaques and papules of the color of the skin. It is considered to be a chronic, benign self-limiting dermatosis of unknown etiology and characterized by granulomatous inflammation due to a mechanism of type IV hypersensitivity of the skin. A genetic predisposition has been suggested because it has been related to haplotypes HLA-BW35 and HLA-A29. Granuloma annulare has been related to autoimmune thyroiditis (in 5.7–12% of cases),9,10 diabetes mellitus, and some neoplasms such as Hodgkin's lymphoma. Because of this, patients with atypical granuloma or elderly patients should be tested to rule out solid and hematological tumors, as well as immunosuppression states.11 Some authors postulate that its association with autoimmune thyroid disease may be due to chance because of its high prevalence, while other authors report a statistically more frequent association which they explain by genetic predisposition, an autoimmune pathophysiological mechanism that induces apoptosis or triggering factors, common to both conditions.10 Keratinocytes, Langerhans cells, and melanocytes are thought to release cytokines that stimulate inflammatory cells. Of the four existing forms (localized, disseminated, linear perforating, and subcutaneous), generalized granuloma annulare, consisting of 10 or more papular lesions tending to annular confluence, accounts for less than 10% of cases. Most patients are diagnosed between the fourth and seventh decades of life. Lesions are usually symmetrically located in the trunk and limbs. Pathological examination reveals palisading granulomata with perivascular lymphocyte infiltration and predominant T-helper lymphocytes. In more than half the patients, granuloma resolves spontaneously without treatment within two months to two years, but may recur in up to 40% of cases, particularly in children, and may sometimes persist for up to 10 years. No treatment is usually required for the localized, self-limiting form. In patients who demand treatment or in generalized granuloma,9 intralesional corticosteroid injections (triamcinolone 2.5–5mg/mL), cryotherapy, and electrodessication may be attempted, after warning patients of the potential occurrence of atrophy or scars.12 Other treatments used include interferon beta-1, PUVA, retinoic acid, tacrolimus, laser and oral retinoid treatment, dapsone, or cyclosporin.13
Case 2This was a 50-year-old female patient with an unremarkable family and personal history who had been monitored at the endocrinology clinic since 1998 for subclinical AIPH diagnosed at 38 years of age for which no treatment had been given until 2005. Laboratory test results at menopause included: TSH, 22mcU/mL; FT4, 0.81ng/dL; and ATA-TPO, >1300U/mL. The patient had grade I small, elastic goiter with no clinical evidence of hormone dysfunction or neck compression. Replacement therapy was started at a dose of 1.77mcg/dL. The patient reported erosive and ulcerative lesions in oral mucosa since 2000. Physical examination revealed whitish reticular lesions in both cheeks with superficial erosions. Laboratory tests, including serology for hepatitis C, were negative. Based on the diagnosis of oral lichen planus, treatment was started with systemic and topical corticosteroids, synthetic antimalarial drugs and, finally, after recurrence upon treatment discontinuation, oral cyclosporine. At the last visit, oral mucosal lesions persisted in the inner cheek, and also in the tongue with a geographic pattern.
Lichen planus is a papular, inflammatory, and pruritic eruption with a chronic course affecting the skin and mucosal membranes. Its prevalence rate in the adult European population ranges from 1% to 3%, and is more common in middle-aged women (50–59 years).14,15 It represents an autoimmune reaction mediated by T cells and directed against basal keratinocytes which express autoantigens on their surfaces and have been modified by different causes. The specific antigens triggering the immune response are not clear.14,16 Potential triggers reported include hepatitis C, drugs, contact allergens, and neoplasms. Several pro-inflammatory cytokines such as interleukin-2, 4, 6 and 10, tumor necrosis factor-alpha, interferon alpha, and transforming growth factor-B1 have been implicated. There are various clinical forms in which oral lichen planus most commonly affects the jugal mucosa, but may also affect the tongue, gums, palate, and other mucosal and conjunctival tissues. The reticular form with whitish intertwined lesions forming a network symmetrically affecting both sides of the jugal mucosa is the most common and is usually asymptomatic. An erosive form with superficial ulcers that usually affects the lateral aspects of the tongue also exists. The longer the follow-up time, the more frequent the evolution from a simple to a combined form.16 Skin lesions are found in 15–20% of patients with oral lichen planus, and this condition has been associated with the development of oral tumors in 1–5% of cases.17
A recent study reviewed a database from a healthcare department including 1477 patients with lichen planus and 2856 controls. Several clinical and demographic characteristics were analyzed, and a statistically significant association was found with hypothyroidism and dyslipidemia.18
In a Finnish study published in September 2010, after reviewing the clinical histories of 222 patients with lichen planus and 222 controls, a 15% prevalence of thyroid disease was found in those with thyroid disease regardless of levothyroxine treatment. Up to one third of cases had lichen planus in the oral mucosa and tongue, as occurred in the reported patient. Prior thyroid disease is associated with a two-fold greater risk of having oral lichen planus.6 Complete spontaneous resolution is rare.16 Spontaneous remissions in the first year have been reported, but subsequent recurrence occurs in 20% of cases. Treatment has been attempted with oral corticosteroids, PUVA, retinoids, enoxaparin sodium, sulfasalazine, metronidazole, and biologic therapies such as alefacept, efalizumab, and basiliximab, with variable results. Efficacy studies with a greater number of patients are still needed. Therefore, since spontaneous regression occurs in some cases and treatments have many side effects, the risk-benefit ratio should always be assessed before treatment is started.19
To sum up, we report the clinical cases of two patients with PSIH and paucisymptomatic skin lesions which should be considered during clinical history and physical examination as they may be treated and require monitoring due to their potential risk of malignization.
Please cite this article as: Alcázar Lázaro V, Aguilar Martínez A. Alteraciones dermatológicas asociadas a hipotiroidismo. Endocrinol Nutr. 2013;60:345–7.