To assess in standard clinical practice the feasibility, efficacy, and safety of switching patients with long-standing type 2 diabetes (T2DM) and poor or unstable blood glucose control to basal-bolus insulin therapy.
Material and methodsThis was a prospective, single center study including 37 patients with T2DM (age 65±8 years, 62.2% men, body mass index 28.8±6.2kg/m2, diabetes duration 18±8 years) with poor or unstable glycemic control, who were switched to a basal-bolus insulin regimen with glargine and rapid-acting insulin analogue at the discretion of their physicians. After a group-structured outpatient diabetes training program, patients were followed in a clinical practice setting for 6 months. Clinical and biochemical variables were collected before switching and at 3 and 6 months.
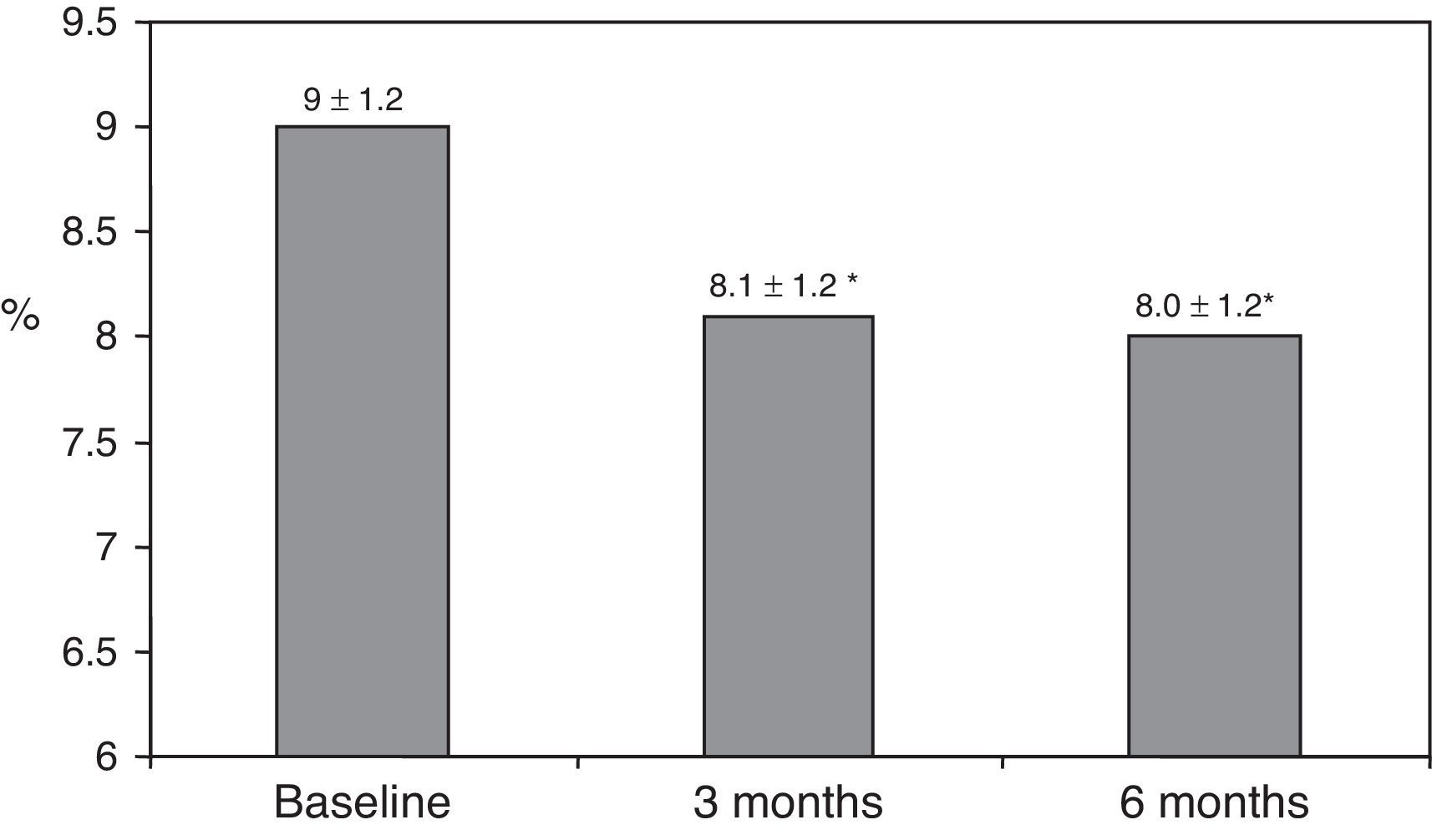
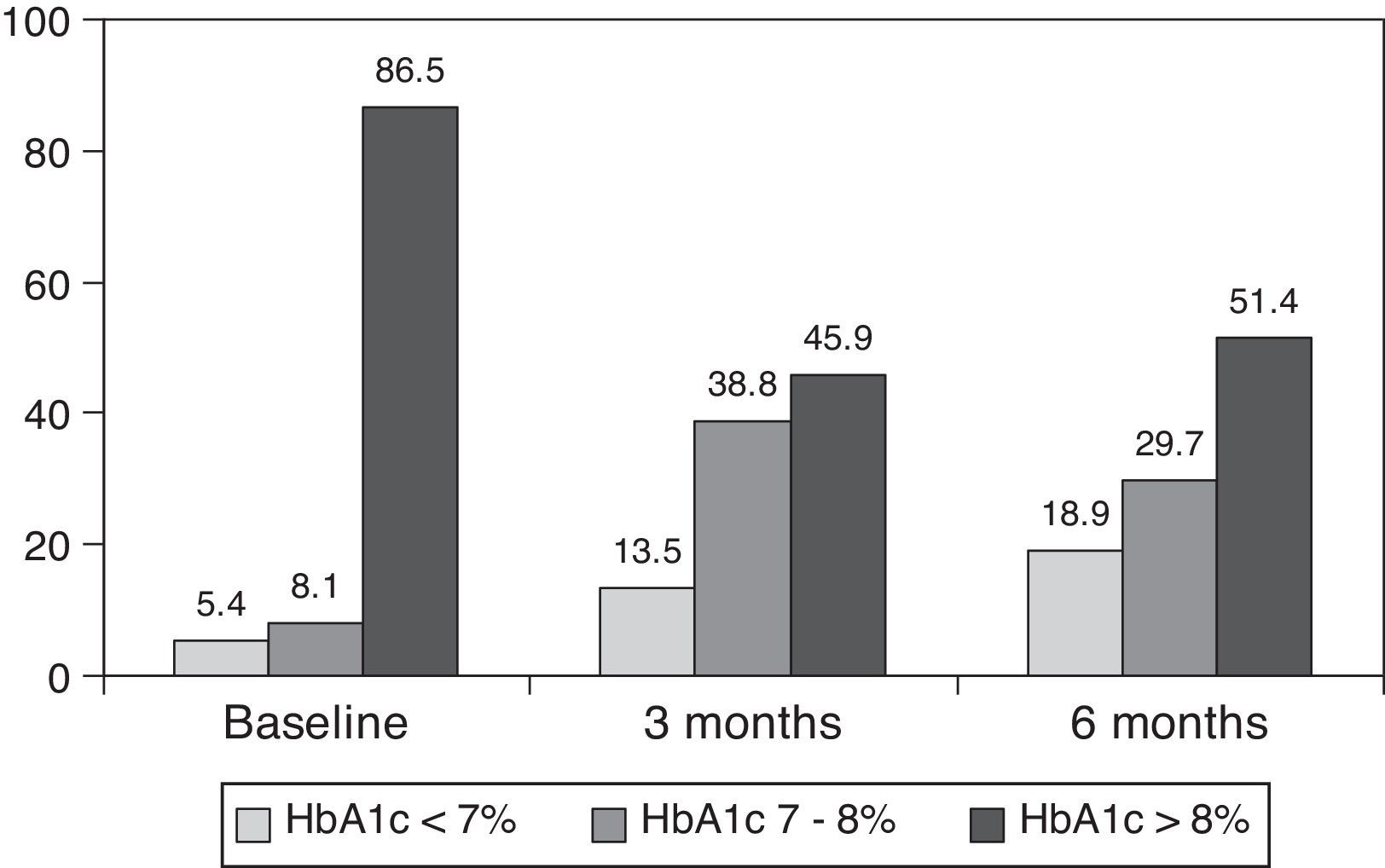
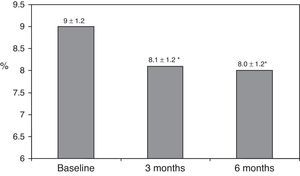
ResultsAfter switching to basal-bolus therapy, glycosylated hemoglobin (HbA1c) decreased from 9±1.2% to 8.1±1.2% (p<0.001) at 3 months and to 8.0±1.2% at 6 months (p<0.001) without changing total daily insulin dose. The proportion of patients with HbA1c≥9% decreased from 51% to 13.8% at 3 months and to 18.9% at 6 months respectively. There was a single episode of severe hypoglycemia. No changes were seen in body weight and quality of life. The size of LDL (low density lipoprotein) particles significantly increased at 3 and 6 months, while all other lipid parameters remained unchanged.
ConclusionsOur study confirmed that basal-bolus insulin therapy is feasible, effective, and safe in patients with long-standing T2DM, and does not impair their quality of life.
Evaluar en la práctica clínica habitual la factibilidad, la eficacia y la seguridad de un programa ambulatorio de paso a pauta bolus-basal en pacientes diabéticos de tipo 2 (DM2) con mal o inestable control glucémico.
Material y métodosEstudio prospectivo de 37 sujetos con DM2 (edad 65±8 años, 62,2% varones, índice de masa corporal 28,8±6,2kg/m2, tiempo de evolución de la diabetes 18±8 años) en los que se transfirió a una pauta bolus-basal (una dosis de glargina y 3 de aspártica o lispro) según el criterio de su médico. El tratamiento se instauró en un programa ambulatorio y el seguimiento se realizó durante 6 meses. Los parámetros clínicos y analíticos se recogieron a los 0, 3 y 6 meses.
ResultadosTras el cambio a la pauta bolus-basal, la hemoglobina glucosilada (HbA1c) se redujo de 9±1,2% al inicio a 8,1±1,2% (p<0,001) a los 3 meses y a 8,0±1,2% a los 6 meses (p<0,001) sin modificarse la dosis diaria total de insulina. El porcentaje de pacientes con HbA1c≥9% cayó del 51% inicial al 13,8% a los 3 meses y al 18,9% a los 6 meses, respectivamente. Se registró una hipoglucemia grave. El peso y la calidad de vida no mostraron cambios. El tamaño de las partículas de LDL (lipoproteínas de baja densidad) aumentó significativamente a los 3 y 6 meses, mientras que otros parámetros lipídicos no se modificaron.
ConclusiónEste estudio confirma que las pautas bolus-basal son factibles, eficaces y seguras en pacientes con DM2 de larga evolución y no alteran su calidad de vida.
Type 2 diabetes mellitus (T2DM) is characterised by an insulin resistance, which remains relatively stable throughout the course of the disease, and a progressive loss of β-cell function with an inadequate insulin secretion. Due to this progressive evolution, most patients with T2DM will eventually require insulin to achieve and maintain glycemic control, using a stepwise approach beginning with basal insulin combined with oral agents. When pre-prandial and postprandial glycemia is not adequately controlled, a twice-daily insulin regimen with NPH (Neutral Protamine Hagedorn) or premixed insulin preparations is preferred as the next step. As in type 1 diabetic subjects, basal-bolus insulin therapy should be indicated in T2DM patients with severe insulin deficiency that are unable to achieve and maintain glycemic targets with twice-daily regimen.1 However, this insulin regimen is clearly underused probably because of the reluctance of patients and physicians due to the complexity involved in its establishment as well as the limited information available about the feasibility, specially in elderly subjects, and its efficacy in patients previously treated with two insulin doses.2
In the present study, we evaluated the feasibility, effectiveness and safety of basal-bolus insulin therapy in patients with long-term type 2 diabetes and poor or unstable glycemic control.
Material and methodsIn this prospective, single centre study, we enrolled 37 patients who were switched to basal-bolus insulin regimen from October 2006 to October 2007 and had had unstable or poor glycemic control (glycated hemoglobin (HbA1c)≥8%) in the prior six months, despite intervention to improve it. The study protocol was approved by the institutional ethics review boards and informed written consent was obtained from all patients.
In the initial treatment, carbohydrates were distributed throughout the three main meals. The initial insulin glargine dose was calculated as 50% of the previous total daily dose and the initial prandial insulin (aspart or lispro) as the remaining 50% of the total daily dose, which was divided equally to cover the three meals. Patients who were taking metformin before switching the therapy and did not have any contraindication to it, continued using it at the same dose. The other oral antidiabetic drugs were stopped.
All patients attended a structured out-patient diabetes training programme consisting of three 2-h group sessions in one week for 5–8 patients. In general, they were taught to follow a diet assuming qualitative carbohydrate intake at each meal, although for patients who wished to vary it, carbohydrate counting had to be done. Patients also learned the management of the basal-bolus therapy and how to adjust basal insulin doses according to fasting self-monitoring blood glucose (SMBG) every 7 days. Adjustment of prandial insulin dose was performed according to pre-meal values using a simple algorithm with set doses of rapid-acting insulin. Patients followed up visits provided by the nurse at 1 and 3 weeks, 3 and 6 months, and by the endocrinologist at 2, 4 and 7 months, where diet was checked and diary with SMBG values was reviewed to adjust insulin doses. Anthropometric data (weight, body mass index and waist circumference) as well as treatment and biochemical variables were obtained at baseline and at 3 and 6 months in all the patients. We quantified insulin requirements and the number of severe hypoglycemia (defined as requiring assistance) by anamnesis and a review of the patients’ diaries.
HbA1c was determined by high-performance liquid chromatography (HPLC) (Bio-Rad Laboratories, Munich, Germany), with a reference range of 4.6–5.8%. Cholesterol and triglycerides were determined by standardized enzymatic methods and high-density lipoprotein cholesterol (HDLc) by a direct method (Roche Diagnostics, Basel, Switzerland). Low-density lipoprotein cholesterol (LDLc) was estimated by the Friedewald formula (if triglyceride levels were<3.39mmol/l) or by ultracentrifugation. Apolipoprotein (Apo) B was determined by an inmunoturbidimetric method (Tina-quant, Roche Diagnostics) and LDL size by electrophoresis (2–16%). Quality of life was measured using a disease-specific questionnaire adapted in Spain from the Diabetes Quality of Life (DCCT) at baseline and repeated at 6 months after basal-bolus insulin therapy.3,4
Data were analyzed by the statistical programme SPSS 15.0 (SPSS Inc.). The changes in anthropometrical variables, HbA1c, insulin requirements and lipid profile were evaluated by t of Student test. They were considered significant values of p≤0.05.
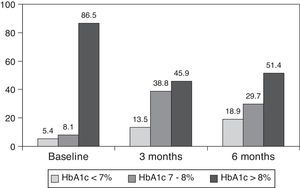
ResultsThe baseline clinical characteristics are summarized in Table 1. Seventy-eight per cent of patients were under treatment with 2 doses of NPH or premixed insulin preparations, 11% of patients were taking oral drugs and bedtime insulin (glargine, detemir or NPH) and the remaining 11% of patients were using other regimens with 3 doses of NPH and regular insulin. Eighty-seven per cent of patients had an HbA1c concentration >8% and 51% had HbA1c>9%. After switching to basal-bolus therapy, HbA1c dropped from 9±1.2% to 8.1±1.2% (p<0.001) at 3 months and to 8.0±1.2% at 6 months (p<0.001) (Table 1, Fig. 1). The percentage of patients with HbA1c≥9% fell from an initial 51% (19 subjects) to 13.8% and 18.9%, at 3 and 6 months respectively. Fig. 2 shows the proportion of patients with an HbA1c<7%, 7–8% and >8% before and after switching to basal-bolus therapy. Five of the patients showed a worsening in the HbA1c values. There was only one episode of severe hypoglycemia registered. At 6 months all patients were able to adjust the basal insulin dose but only 10 patients modified the prandial dose according to the patterns of SMBG autonomously.
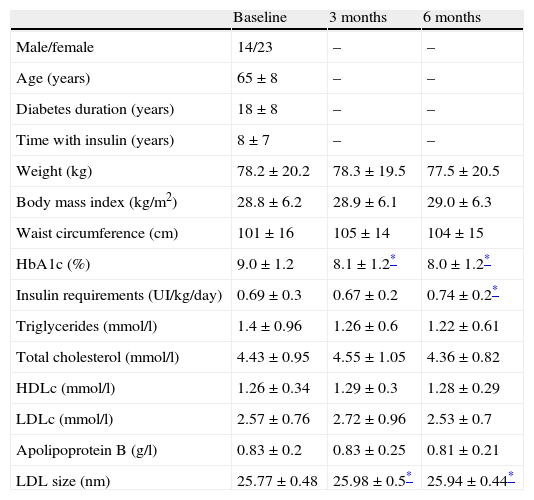
Baseline characteristics of the study population and change on anthropometric variables, insulin requirements, glycemic control and lipid profile at baseline and 3 and 6 months after switching to basal-bolus regimen.
| Baseline | 3 months | 6 months | |
| Male/female | 14/23 | – | – |
| Age (years) | 65±8 | – | – |
| Diabetes duration (years) | 18±8 | – | – |
| Time with insulin (years) | 8±7 | – | – |
| Weight (kg) | 78.2±20.2 | 78.3±19.5 | 77.5±20.5 |
| Body mass index (kg/m2) | 28.8±6.2 | 28.9±6.1 | 29.0±6.3 |
| Waist circumference (cm) | 101±16 | 105±14 | 104±15 |
| HbA1c (%) | 9.0±1.2 | 8.1±1.2* | 8.0±1.2* |
| Insulin requirements (UI/kg/day) | 0.69±0.3 | 0.67±0.2 | 0.74±0.2* |
| Triglycerides (mmol/l) | 1.4±0.96 | 1.26±0.6 | 1.22±0.61 |
| Total cholesterol (mmol/l) | 4.43±0.95 | 4.55±1.05 | 4.36±0.82 |
| HDLc (mmol/l) | 1.26±0.34 | 1.29±0.3 | 1.28±0.29 |
| LDLc (mmol/l) | 2.57±0.76 | 2.72±0.96 | 2.53±0.7 |
| Apolipoprotein B (g/l) | 0.83±0.2 | 0.83±0.25 | 0.81±0.21 |
| LDL size (nm) | 25.77±0.48 | 25.98±0.5* | 25.94±0.44* |
HbA1c: glycated hemoglobin; HDLc: high-density lipoprotein cholesterol; LDLc: low-density lipoprotein cholesterol.
Body weight, insulin requirements and lipid parameters at baseline and during follow-up are shown in Table 1. Body weight remained stable during the 6 months of follow-up and insulin requirements (UI/kg/day) did not change at 3 months and increased slightly at 6 months. The size of the LDL particles increased significantly at 3 (25.77±0.48nm vs 25.98±0.5nm, p<0.05) and 6 months (25.77±0.48nm vs 25.94±0.44nm, p<0.05), while the other lipidic parameters did not change.
The diabetes quality of life (DQOL) questionnaire showed no changes at 6 months in the scores for satisfaction (2.22 vs 2.14), impact of diabetes (2.28 vs 2.15), social concern (1.81 vs 1.75) and concern related to diabetes (2.69 vs 2.35).
DiscussionIn the present study, we showed that basal-bolus insulin therapy allows glycemic control to improve without compromising security and quality of life in subjects with long-term type 2 diabetes, previously treated with one or more insulin doses. We also demonstrate the feasibility of the implementation of these insulin regimens through a structured out-patient training program.
Consensus guidelines for the management of type 2 diabetes consider that the primary goals of treatment are to achieve HbA1c concentrations as low as possible without causing unacceptable hypoglycemia, especially in older patients or with coronary disease, and to prevent the development of microvascular and macrovascular complications.1,5 Unfortunately, recent surveys indicate that a large proportion of patients with diabetes fail to meet the recommended glycemic goals.6,7 Although in the National Health and Nutrition Examination Survey (NHANES) the proportion of patients with HbA1c<7% increased from 37% in 1999–2000 to 56.8% in 2003–2004, rates of suboptimal glycemic control are especially high in individuals with similar characteristics to those studied by us, who have long-standing diabetes or insulin treatment.6,7 Twice-daily dosing with NPH or premixed insulin are used to simplify insulin regimens, but have limited flexibility, require rigid adherence to regular mealtimes, limit the ability to adjust the dosages of the individual components and increase the possibility of hypoglycemia. Thus, although many patients initially will achieve adequate glycemic control with this regimen,8 when insulin secretory capacity of beta cells is lost and insulin deficiency is severe, glycemic control becomes poor and unstable as in most of the patients included in the present study.1,8 We showed that in patients with long-term type 2 diabetes, poorly controlled with other regimens of insulin and unstable profile, the basal-bolus insulin regimen reduces HbA1c one point over 6 months and the proportion of patients with HbA1c≥9% from 51% to 14% at 3 months and 19% at 6 months. This is probably because it is a more physiological therapy; while prandial insulin replaces first-phase endogenous insulin secretion, basal insulin decreases the level of fasting hyperglycemia. Thus, in addition to the level of HbA1c 9 the way treatment is intensified greatly alters the relative contributions of basal and postprandial hyperglycemia to the overall hyperglycemia of T2DM patients. Recently, Riddle et al.10 showed that after treatment intensification with insulin, the contribution of basal hyperglycemia drops but still accounts for about one-third of the remnant hyperglycemia. Hence, according to the findings of the present study, the use of insulin regimens combining basal with prandial insulin will often be needed to achieve glycemic goals. In fact, these findings are consistent with the vast evidence for the advantages of basal-bolus therapy in type 1 diabetes 11 and are supported by the limited data from observational studies in patients with type 2 diabetes switching from premix to basal-bolus glargine-based regimen 12,13 and by a randomised comparison of a premix-based regimen versus a basal-bolus regimen in type 2 diabetic patients.2,14,15 In patients previously treated with glargine plus oral anti-diabetic drugs, the difference in HbA1c was 0.22% in favour of the basal-bolus glargine-based regimen, compared to a premixed insulin regimen15. In the PREFER study,14 the subgroup of patients previously on a basal insulin regimen showed a greater HbA1c reduction with detemir/aspart basal-bolus regimen compared to biphasic insulin aspart (−1.21% vs −0.75%). Finally, in premix treated type 2 diabetic patients, Fritsche et al. showed that a basal-bolus glargine/glulisine-based insulin regimen was superior to a premix insulin regimen in the reduction of HbA1c (−1.31% vs 0.8%).2 Therefore, although there are differences in the magnitude of improvement between studies, probably due to the different baseline characteristics of the studied population, the superiority of a basal-bolus regimen in selected patients with long-standing disease seems demonstrated. The reduction of more than one point of HbA1c achieved by switching to a basal-bolus insulin regimen can be considered clinically significant because it may result in the reduction of clinical outcomes. Unfortunately, basal-bolus therapy is underused in patients with T2DM because physicians consider it complex to implement, it is time consuming and there are fears of the increased number of injections, risk of hypoglycemia, weight gain and worsening quality of life. In this and previous studies body weight and rate of severe hypoglycemia were not increased,2,12 which could be related to the more physiologic insulin substitution with basal-bolus regimen and the flexibility that this therapy may offer to patients. Thus, fear of hypoglycemia should not be a barrier to start this kind of therapy in T2DM, but it has to be considered in order to establish glycemic control targets as it can cause morbidity and increased mortality.16 Regarding the impact on quality of life, in concordance with the report of Ménard et al.,17 our study did not support the view that basal-bolus regimens lead to a decreased quality of life.
According to a previous report,12 in the present study all patients were able to titrate their basal insulin dose according to the fasting SMBG of the last 3–7 days. In contrast, few patients were able to adjust prandial insulin doses according to the patterns of SMBG and most needed the support of a simple algorithm with set dose depending on premeal blood glucose. This is not surprising since establishing the optimal mealtime insulin dose often involves calculations that consider multiple factors and is difficult for some patients. Moreover, using a simple algorithm to adjust mealtime rapid-acting insulin each week based on SMBG patterns is as effective as adjusting mealtime insulin using insulin-to-carbohydrate ratios in T2DM subjects.18
Limitations of the study are related to the prospective observational design and lack of control group. These aspects and the short follow-up of patients, difficult to interpret the findings and their applicability to patients with T2DM followed in other centers. However, although future studies in larger groups of patients should be performed to confirm these findings, the study provides information that can be useful for the management of a common and poorly treated clinical situation.
In conclusion, the present study demonstrated that a 9-hour out-patient program allowed long-duration T2DM patients poorly controlled with other insulin regimens to switch to basal-bolus insulin regimen. We also confirmed that basal-bolus insulin regimen is effective, safe and does not alter the quality of life in this subgroup of T2DM patients. Thus basal-bolus therapy might be offered to T2DM subjects inadequately controlled with other insulin strategies.
Conflict of interestIrene Vinagre has received lecture fees from Eli Lilly, Novo Nordisk and Sanofi Aventis. Antonio Perez has received con sulting and lecture fees from Eli Lilly, Novo Nordisk and Sanofi Aventis.