To analyze the available information about continuous subcutaneous insulin infusion (CSII) and continuous glucose monitoring (CGM) systems in the public health care system of the Community of Madrid.
Material and methodsA survey consisting of 31 items was sent to the 28 endocrinology department of the Madrid public hospitals. Items focused on CSII and CGM and included patients’ registrations, as well as data regarding healthcare, administrative, and logistic aspects. Responses from a total of 20 hospitals where these procedures are used were received from March 2013 to May 2014. Data about pediatric patients were obtained from adult endocrinology departments, except for two hospitals which directly reported the information.
ResultsA total of 1256 CSII pumps were recorded in the Madrid region, of which 1089 were used by adults, and the remaining 167 by pediatric patients. During 2013, 151 new CSII systems were implanted (12% of the total), while 14 pumps were withdrawn. Availability of human resources (medical assistance) and the number of staff practitioners experienced in management of these systems widely varied between hospitals. Eighty-five percent of hospitals used retrospective CGM systems, and 40% routinely placed them before starting an insulin pump. Thirteen hospitals (65%) used long-term, real-time CGM systems in selected cases (a total of 67 patients).
ConclusionsUse of these technologies in diabetes is unequal between public health care hospitals in Madrid, and is still significantly lower as compared to other countries with similar incomes. However, there appears to be a trend to an increase in their use.
Analizar la información disponible sobre el estado de los sistemas de infusión subcutánea continua de insulina (ISCI) y de monitorización continua de glucosa (MCG) en la red pública sanitaria de la Comunidad Autónoma de Madrid (CAM).
Material y métodosSe remitió una encuesta a los 28 servicios de endocrinología de los hospitales públicos de la CAM con 31 preguntas sobre los sistemas ISCI y MCG, que incluían registros de pacientes y aspectos asistenciales, administrativos y logísticos. Entre marzo y mayo de 2014 se recibieron respuestas de los centros y se recabó la información de los 20 servicios que realizaban este tipo de procedimientos en nuestra comunidad. Los datos sobre pacientes pediátricos se recibieron mayoritariamente a través de los servicios de adultos, con la excepción de 2 servicios de pediatría de los que la información se recibió directamente.
ResultadosEn la CAM hay contabilizados un total de 1.256 sistemas ISCI en la población diabética. Los usuarios son mayoritariamente adultos (1.089 pacientes), mientras que 167 corresponden a pacientes pediátricos. Durante 2013 se instauraron 151 nuevos tratamientos (12% del total) mientras que se retiraron un total de 14 bombas. La disponibilidad de recursos asistenciales y la proporción de facultativos de plantilla encargados de estos tratamientos son muy desiguales entre distintos centros. Un 85% de los hospitales incluye entre sus prestaciones sistemas MCG retrospectivos, y un 40% los utiliza habitualmente al inicio de los tratamientos ISCI. Trece centros (65%) utilizan MCG a tiempo real (MCG-TR) a largo plazo en casos seleccionados, contabilizándose un registro acumulado de 67 pacientes.
ConclusionesLa implantación de las tecnologías en diabetes en la CAM es desigual en los distintos centros madrileños, y continúa siendo inferior a otros países de nuestro entorno, aunque parece observarse una discreta tendencia a recortar esas diferencias.
Current treatment of type 1 diabetes mellitus (T1DM) cannot be understood without the so-called intensive insulin regimens, consisting of multiple insulin injections or continuous subcutaneous insulin infusion (CSII) systems. Both methods, although they still have clear limitations, have been shown to be the best way of mimicking physiological insulin secretion in patients with T1DM, so preventing or decreasing the acute and chronic complications of this disease.
In 2004, official approval for the financing of pumps by the different public health services was a significant milestone in the treatment of T1DM. This converted pumps into a therapy that could benefit a wide group of patients who had an adequate clinical profile, instead of only a few. However, contrary to the initial perspectives of growth, the dissemination of CSII in Spain has been slower than expected and quite heterogeneous, not only when the different autonomous communities are compared, but also within the different health areas of the communities. A study conducted in the Autonomous Community of Madrid (ACM) on the implantation of CSII systems after their public financing had been approved was published in 2006. This study analyzed the human and material resources allocated to CSII in the different healthcare centers and assessed its expectations for development.1
The purpose of this study was to provide a snapshot of the actual situation of CSII at the Madrid hospitals after a decade of use in our healthcare system, and to perform a critical analysis of its degree of clinical development. An analysis of continuous glucose monitoring (CGM) is also included because this is another technological support in diabetes, and because both procedures represent indicators of a qualified care for patients with T1DM.
Material and methodsTo conduct this study, a survey consisting of 31 items related to CSII and MCG systems, including available records and the care, administrative, and logistic aspects of these procedures, was sent to the 28 departments of endocrinology of the public hospital network of the ACM. The study questions are detailed in Annex 2.
Questionnaires were sent in March 2014 by electronic mail to the departments of endocrinology and pediatrics of the ACM public hospitals through the secretariat of the Society of Endocrinology, Diabetes, and Nutrition of (SENDIMAD). All hospitals–20 in all–using this procedure in the ACM sent their responses during the months of March to May of 2014. Information about CSII systems in pediatric patients was received from most hospitals through the survey conducted by the adult endocrinology departments, except in the cases of the Ramón y Cajal and Niño Jesús hospitals, in which information was directly received from the pediatric endocrinology departments. A basic descriptive statistical analysis was performed, recording quantitative and qualitative variables and percentages of all the responses obtained.
ResultsCSII systems were included in the portfolio of services of 20 (71%) of the 28 public hospitals considered. Hospitals and physicians participating in the survey are listed at the end of the article. The eight hospitals where CSII is not used yet include four of the six so-called “new hospitals” which came into operation in 2008, two each under private and public management. These hospitals have no healthcare area of their own and only have one endocrinologist on their staff.
Most hospitals started treatments with insulin pumps in 2004–2005, but six of the 20 hospitals (30%) only started to use CSII in 2011.
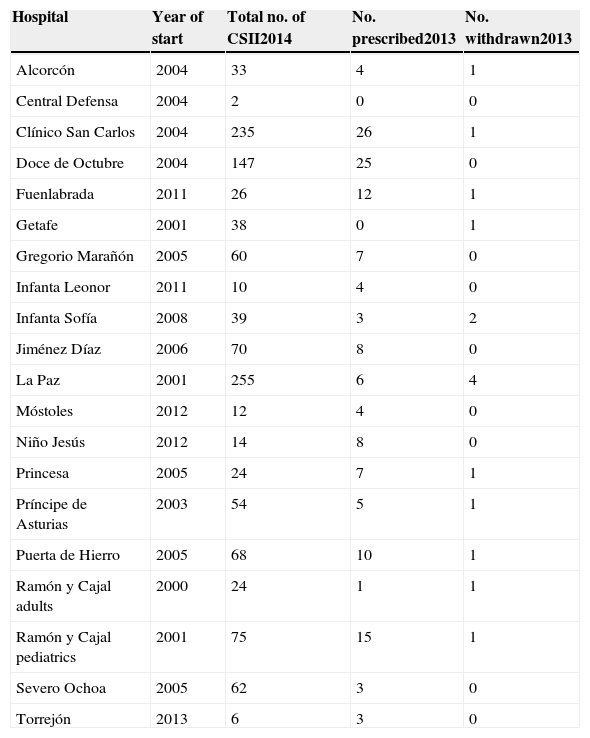
The survey shows that in the ACM there were 1256 patients using CSII systems (Table 1). Most users were adults (1089 patients), and only 167 pediatric patients were recorded. The vast majority of treatments in children were administered by five pediatric departments (those from the Ramón y Cajal, La Paz, Doce de Octubre, Severe Ochoa, and Niño Jesús hospitals). A total of 151 new treatments were started in 2013. These represented 12% of all treatments and were usually indicated because of poor metabolic control and/or high blood glucose variability. By contrast, 14 pumps were withdrawn, in most cases due to patient dissatisfaction.
Continuous subcutaneous insulin infusion (CSII) systems implanted up to 2014 at hospitals of the public healthcare network of the Autonomous Community of Madrid and the number of systems started and withdrawn in 2013.
| Hospital | Year of start | Total no. of CSII2014 | No. prescribed2013 | No. withdrawn2013 |
|---|---|---|---|---|
| Alcorcón | 2004 | 33 | 4 | 1 |
| Central Defensa | 2004 | 2 | 0 | 0 |
| Clínico San Carlos | 2004 | 235 | 26 | 1 |
| Doce de Octubre | 2004 | 147 | 25 | 0 |
| Fuenlabrada | 2011 | 26 | 12 | 1 |
| Getafe | 2001 | 38 | 0 | 1 |
| Gregorio Marañón | 2005 | 60 | 7 | 0 |
| Infanta Leonor | 2011 | 10 | 4 | 0 |
| Infanta Sofía | 2008 | 39 | 3 | 2 |
| Jiménez Díaz | 2006 | 70 | 8 | 0 |
| La Paz | 2001 | 255 | 6 | 4 |
| Móstoles | 2012 | 12 | 4 | 0 |
| Niño Jesús | 2012 | 14 | 8 | 0 |
| Princesa | 2005 | 24 | 7 | 1 |
| Príncipe de Asturias | 2003 | 54 | 5 | 1 |
| Puerta de Hierro | 2005 | 68 | 10 | 1 |
| Ramón y Cajal adults | 2000 | 24 | 1 | 1 |
| Ramón y Cajal pediatrics | 2001 | 75 | 15 | 1 |
| Severo Ochoa | 2005 | 62 | 3 | 0 |
| Torrejón | 2013 | 6 | 3 | 0 |
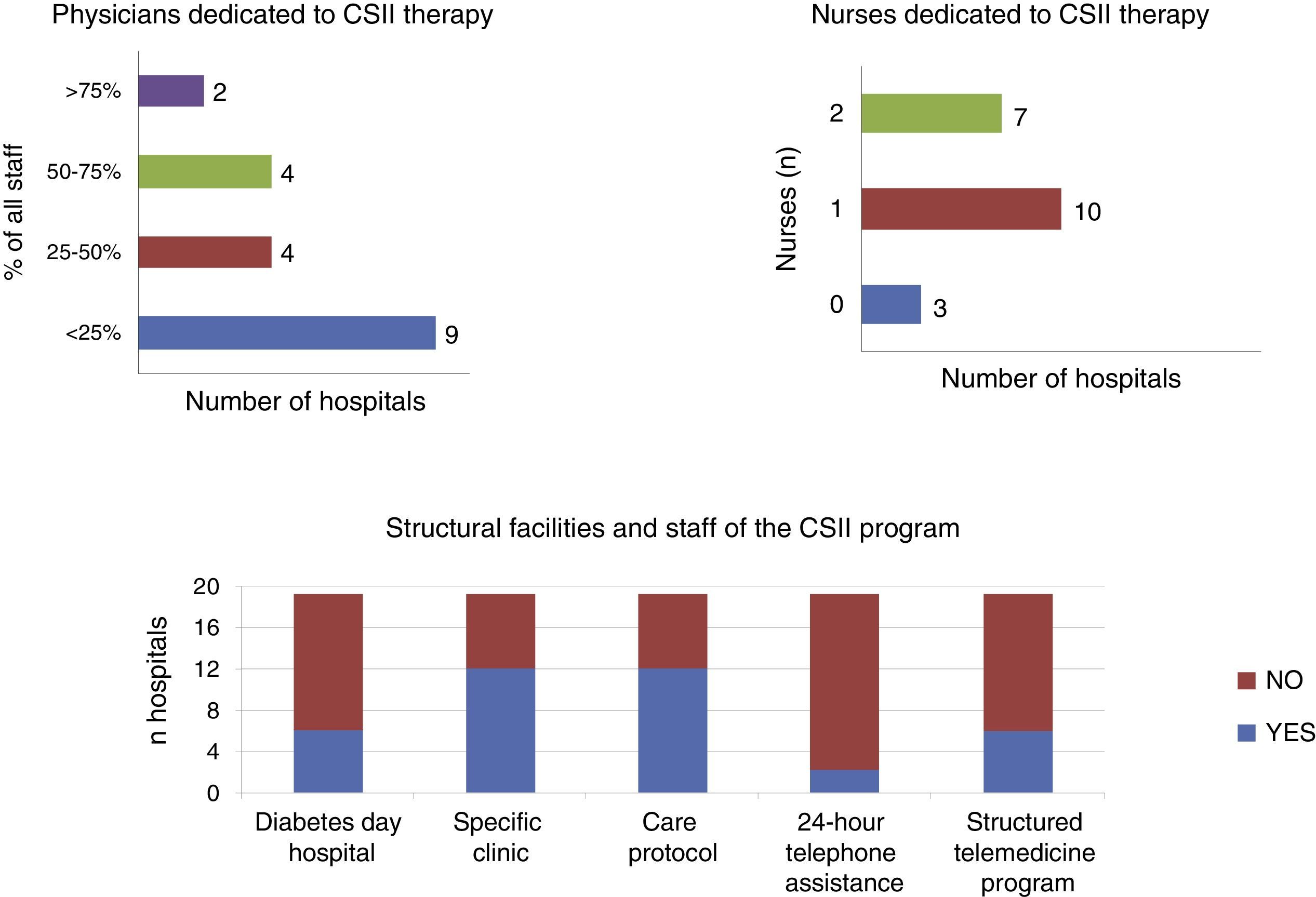
As regards the care resources available for the use of CSII, only 30% of the centers had a day care hospital with special facilities for diabetic patients, while 85% had diabetes education nurses (3 hospitals had none, 10 had 1 nurse, 7 had 2, and 1 had 3 nurses). Only 60% of the hospitals considered that they had adequate facilities for providing therapeutic education in CSII. A CSII-specific care protocol was available in 60% of the departments, and 30% had developed a structured telemedicine program. As regards continuous telephone assistance to patients with CSII, this was only provided by two departments (10%) (Fig. 1).
The number of physicians involved in CSII therapy varied depending on the organization of each hospital. In six of the 20 departments (30%), most physicians (≥60% of total staff) cared for patients with CSII. In four centers (20%) the percentage ranged from 30% to 60%, and in the remaining 50% this activity was discharged by only 30% of the staff, which in five of the hospitals meant a single physician.
With regard to improvements in human resources allocated to this care activity, only one of the 20 hospitals reported an additional physician, while two hospitals had hired an additional nurse.
Clinical monitoring consisted of visits every 2–4 months in 70% of hospitals, every 1–2 months in 20%, and every 4–6 months in 10%.
As regards administrative actions, the responders reported no refusal by the hospital managements of any CSII requested during 2013 (except for a specific case where a request for the replacement of a stolen pump was refused). New pump indications were automatically approved in two hospitals, while individualized approval was requested in four hospitals. Most hospitals (14/20) had a tender procedure that established a maximum number ranging from five to 30 pumps annually.
When asked if hospitals accepted patients living in other health areas of the ACM for their CSII programs, two centers said they did not accept them and another two only accepted such patients if this benefit was not available in their area, while most hospitals (13/20) accepted such patients. In these cases, pumps were financed by the hospitals accepting them (17 of 20 responses). This question was not answered by two centers, while a final one ticked both possibilities. When asked about the inclusion of patients from other autonomous communities, four hospitals said that this was acceptable if the user was registered in the ACM, nine denied this possibility, and six stated that they had had no such cases.
The logistic strategy for the regular dispensing of consumables was variable: direct shipping to the patient's home (4/20), centralized delivery at the diabetes unit/supply department (11/20) or both (4/20).
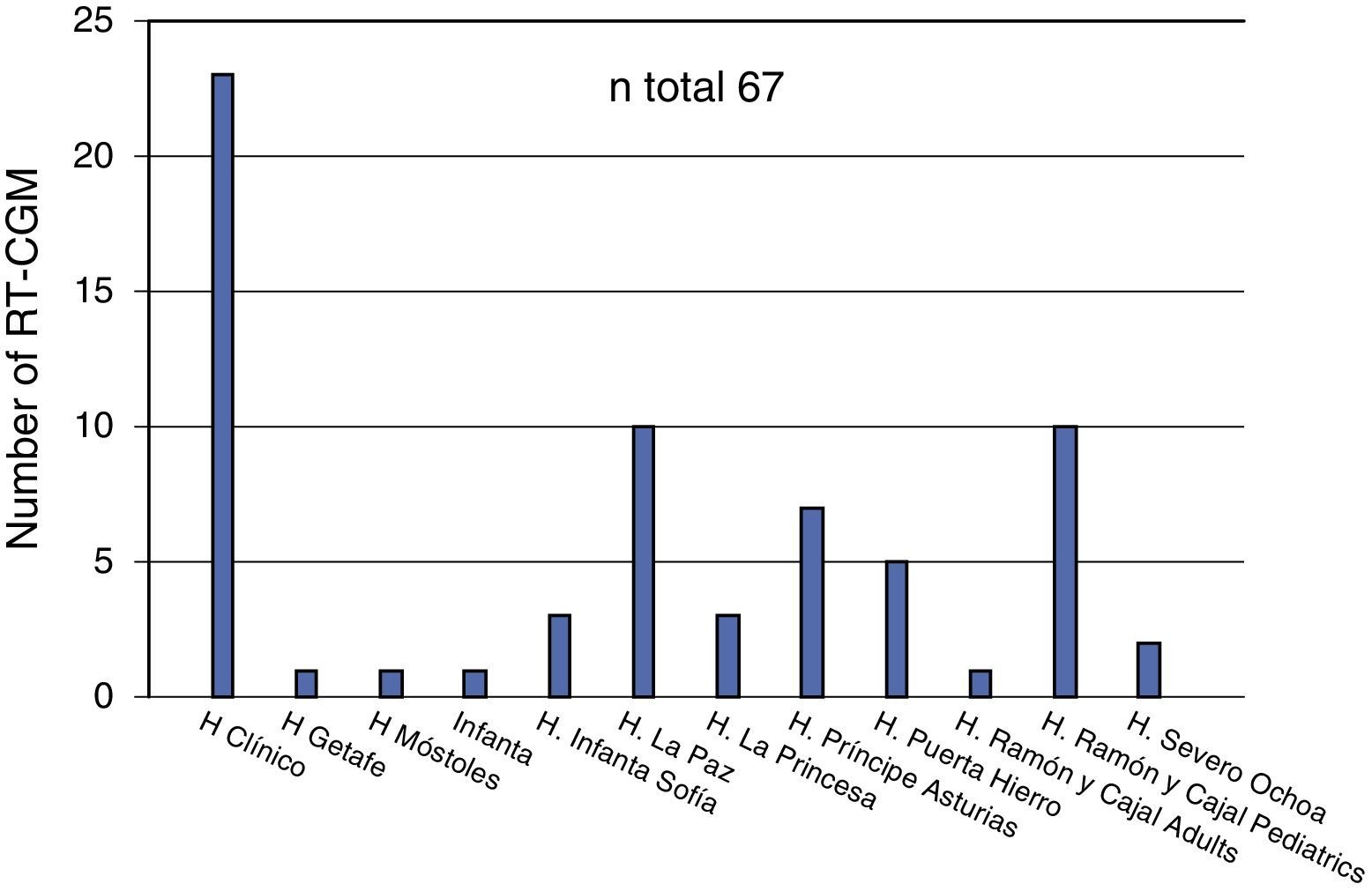
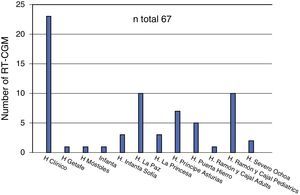
With regard to CGM, three of the 20 hospitals (15%) did not include retrospective reading systems in their benefits. The number of receiving devices available at the departments offering this technique ranged from one to five (median 2). Forty percent of departments surveyed recognized the routine use of blinded or open CGM at the start of CSII treatment. Thirteen hospitals (65%) used real time CGM (RT-CGM). Except for cases where the indication was due to a blood glucose assessment for therapeutic adjustment, most patients on RT-CGM (60%) usually used the system for longer than 12 months. According to the survey, a total of 67 patients used the system (Fig. 2), of whom 44 were using it at the time of the survey.
There was no financing of RT-CGM by the Madrid health service (SERMAS), and the system was paid for by the users themselves in most cases. Only four of the hospitals surveyed had provided financial assistance for a patient. These were isolated cases, except in one hospital (Hospital Universitario Clínico de San Carlos) that included pregnant women with CSII as a clinical indication.
DiscussionTreatment of T1DM has greatly changed over the past two decades. It should be kept in mind that in the Diabetes Control and Complications Trial, the study with the greatest impact conducted on this disease, only a minority of patients used an insulin pump, while most were treated with rapid- and intermediate-acting human insulins.2 Basal-bolus regimens with multiple dose injections (MDI) of insulin analogs are used by virtually all patients with T1DM because of their greater flexibility and clinical benefit,3,4 while CSII has become established as a viable alternative to MDI.5–7
The weight of scientific evidence5,6 was determinant for the different Spanish public health systems when it came to authorizing reimbursement for the costs of CSII.7 Today, medical societies encourage the use of CSII in adequately selected patients.8–11
This study analyzes implementation of CSII in the ACM, while reporting its disparate distribution in the different hospitals. While CSII is widely used in some hospitals, it is still not being used in other centers. The most plausible reason usually given for the absence of CSII programs is the lack of sufficient resources for its implementation. However, hospitals with similar characteristics and resources do include CSII in their portfolio of services, probably due to the initiative and commitment of professionals who want to provide a service they consider essential. The introduction of CSII systems in several hospitals over the past two or three years is encouraging, as it appears to mark a positive trend that we expect to continue as professionals more and more accept CSII as a standard therapy, despite the barriers undoubtedly posed by the scarce resources available.
In line with the increasing use of technical devices in T1DM therapy, CGM systems have gradually been introduced in public health. On the one hand, “blind” CGM, as a retrospective procedure to test blood glucose behavior and variability, has been used for some time in most hospitals. On the other hand, RT-CGM is used much less commonly by some in the short term as a support for treatment adjustment or at the start of therapy with a pump. The level of evidence for the clinical benefits of RT-CGM12 is still not as high as for CSII, but the combined use of the two systems appears to be becoming more established in clinical practice, especially when CGM is frequently and continuously used in patients with repeated or inadvertent hypoglycemia episodes that hinder their metabolic improvement.13 The survey shows that the use of RT-CGM is very limited in ACM hospitals, which is undoubtedly due to the lack of public reimbursement, except in some individual cases and in specific clinical situations in isolated centers.
The results of our survey demonstrate that, with few exceptions, the development of CSII programs has not been supported, by the corresponding reinforcement of medical and nursing staffs which was initially expected after approval for public reimbursement had been given. On the contrary, the trend in recent years has been to freeze and reduce resources.
Once CSII is included in the budgets of hospital managements, there appear to be, according to the responses of the departments surveyed, no major obstacles to new indications for CSII being approved within the limits of the total number of pumps approved, which is usually updated in the annual or biannual tenders.
The survey shows that hospitals usually assume responsibility for the reimbursing of treatment for patients referred by other hospitals, especially if these have no CSII programs. This has been facilitated by the recent creation of a single area in the ACM, as compared to the old division of the community into several healthcare areas. However, the budgets assigned to each hospital and current cost containment policy have led to a certain degree of competition for resources among some hospitals.
The number of pumps withdrawn over the past year was quite low, which may be related to the suitability of therapeutic indications and adequate patient adaptation to and satisfaction with the system. However, the results of the survey show that the total number of pumps implanted in this decade was lower than initially expected. In 2004 there were 109 pumps in the ACM before public reimbursement was approved, and the number had increased to 213 pumps one year later.1 The future annual growth of CSII was therefore expected to be similar. However, a national survey conducted in 2006 showed a lower than expected increase in CSII use, as only 274 patients were being treated with pumps in ACM hospitals.14
This may be attributed to the initial inertia which occurs whenever new technology appears. Unfortunately, this does not seem to have been the only reason because six years later, in January 2012, the Spanish Federation of Healthcare Technology Companies (FENIN) stated that 981 CSII systems were being used in the ACM (data not published), i.e. they had been implanted in only 6% of the estimated population with T1DM. This represented a 40% lower increase than had been initially expected.
Subsequent studies have found a somewhat higher pump implantation rate in the ACM as compared to the mean national rate, lower than 5% according to international publications.15
This study showed some increase as compared to the above figures. Based on the few epidemiological registers of T1DM available in Spain, the prevalence of this disease ranges from 0.17% to 0.3%.16,17 As the total population of the ACM during 2014 was 6,448,272 habitants,18 the data from our survey show the total number of patients with T1DM currently being treated with CSII in the Madrid area to be approximately 10%. This represents an increase in the use of CSII in recent years and partly reduces the differences to be seen when these figures are compared to those of countries similar to ours.19
This study was not intended to make a comprehensive analysis of the underuse of CSII in Spain, but a self-critical reflection suggests that part of the problem may lie in our own reluctance to make the professional effort required for this task, which is not merely ignored, but even appears to be discouraged by management. However, CSII is currently a consolidated treatment which will undoubtedly be used in the future in an increasing number of patients with T1DM as a real alternative to multiple dose insulin therapy in selected patients, rather than as a last resource. And because of the specific weight of this disease in our specialty, endocrinology departments or units should consider CSII to be an integral part of their services on offer and should therefore require healthcare managers, with the support of our medical associations, to provide the human and material resources needed for the adequate implementation of these care systems.
In conclusion, this study was intended to reflect the current status of CSII and CGM as the main technologies used in diabetes in the public healthcare system of the ACM, to update the available registers, and to analyze various structural and logistic factors possibly related to the degree of introduction of these systems. A significant heterogeneity was seen in treatment implementation at the different hospitals, with indicators that are still lower as compared to countries similar to ours. However, there are signs of a trend toward a slight reduction of this difference.
Conflicts of interestThe authors state that they have no conflicts of interest.
Francisca Almodovar (Hospital Universitario Fundación de Alcorcón)
Macarena Alpañés (Hospital Universitario Ramón y Cajal)
Victor Andía (Hospital General Universitario Gregorio Marañón)
Teresa Antón (Hospital Universitario de Móstoles)
Jesús Argente (Hospital Infantil Universitario del Niño Jesús)
Alfonso Arranz (Hospital Universitario de la Princesa)
Sharona Azriel (Hospital Universitario Infanta Sofía)
Raquel Barrio (Hospital Universitario Ramón y Cajal, Department of Pediatrics)
Marta Botella (Hospital Universitario Príncipe de Asturias)
Miguel Brito (Hospital Universitario Puerta de Hierro)
José Ramón Calle (Hospital Universitario Clínico de San Carlos)
Gloria Cánovas (Hospital Universitario de Fuenlabrada)
María Durán (Hospital Universitario de Getafe)
Icíar Galicia (Hospital Universitario de Torrejón)
Noemí González (Hospital Universitario de La Paz)
Arturo Lisbona (Hospital Central de la Defensa Gómez Ulla)
Purificación Martínez de Icaya (Hospital Universitario Severo Ochoa)
Inmaculada Moreno (Hospital Universitario Infanta Leonor)
Celestino Rodríguez (Hospital Universitario Doce de Octubre)
Clotilde Vázquez (Hospital Fundación Jiménez Díaz)
Please cite this article as: Arranz Martín A, Calle Pascual A, del Cañizo Gómez FJ, González Albarrán O, Lisbona Gil A, Botella Serrano M, et al. Estado actual de los sistemas de infusión subcutánea continua de insulina y monitorización continua de glucosa en la Comunidad de Madrid. Endocrinol Nutr. 2015;62:171–179.