Oral testimonies from North Africa attribute anti-diabetic effects to medicinal preparations of the lizard Uromastyx acanthinura (UA). No scientific evidence of such effects is currently available. The acute effects of oral administration of UA to C57Bl/6J mice with diet-induced diabetes were tested and, if effectiveness was shown, the effect of subchronic UA administration was assessed in the same model.
MethodsMice were fed a diet containing 60% fat for at least 12 weeks. To assess acute effects, different doses of UA or saline were orally administered with 2g of glucose/kg during an oral glucose tolerance test (OGTT) on different days in a randomised crossover design. The most effective dose was then fed together with the high-fat diet for 90 days and compared to high-fat diet alone in a parallel design. Body weight (BW), food consumption, welfare, and external appearance were assessed weekly. HbA1c, OGTT, and intraperitoneal insulin tolerance tests (IPITT) were performed at baseline and after treatment. Severity of neuropathy was evaluated by cold allodynia response in the acetone test.
ResultsUA significantly decreased glucose levels as compared to saline 15min after administration. After 90 days of treatment, no differences were seen in OGTT or HbA1c between the groups, while IPITT showed higher glucose levels in UA-treated animals. Although weight increase was similar in both groups, weight tended to be higher in the treated group, which had a significantly higher daily food consumption. Cold allodynia response improved in frequency and intensity in the UA group.
ConclusionsOrally administered UA acutely decreased blood glucose in diabetic mice. Paradoxically, long-term administration of UA increased food consumption, weight, and insulin resistance. Improved nociceptive response suggested an effect on pain and/or neuropathy. Although additional studies are needed to elucidate the properties and potential applications of UA, our results highlight the value of ethnomedical approaches to African traditional medicine as starting point to evaluate new bioactive components.
Testimonios orales Norteafricanos atribuyen efectos hipoglucemiantes a preparados medicinales del lagarto Uromastyx acanthinura (UA), para los que no existen evidencias científicas actualmente. El objetivo de este trabajo fue el de investigar los efectos agudos de UA administrado oralmente en ratones diabéticos C57Bl/6J inducidos por dieta grasa, y si se demostrase su efectividad evaluar el efecto de su administración subcrónica en el mismo modelo animal.
MétodosFue administrada una dieta a los animales con un contenido graso del 60% durante al menos 12 semanas. Para evaluar los efectos agudos diferentes dosis de UA o suero salino fueron administrados conjuntamente con 2g/kg de glucosa durante sobrecargas orales de glucosa (SOG), en diferentes días, siguiendo un diseño cruzado aleatorizado. La dosis más efectiva en esta fase fue entonces administrada mezclada en la dieta durante 90 días y comparada con dieta solo en un diseño paralelo. El peso corporal y el consumo de alimento fueron evaluados semanalmente. HbA1c, SOG, y test de tolerancia intraperitoneal a la insulina (TTIPI) fueron realizados al inicio y tras el tratamiento. La gravedad de la neuropatía fue determinada mediante la evaluación de la alodinia al frío.
ResultadosEl UA redujo significativamente las concentraciones de glucosa de manera aguda en comparación con el control a los 15min tras su administración. Tras 90 días de tratamiento no se observaron diferencias en las SOG o HbA1c entre grupos, mientras que para los test de tolerancia intraperitoneal a la isulina valores más altos de glucosa fueron determinados en los animales tratados con UA. Aunque ambos grupos aumentaron su peso, este tendió a ser mayor en los tratados, que a su vez consumieron significativamente más comida por día. La respuesta a la alodinia al frío mejoró en frecuencia e intensidad en los tratados con UA.
ConclusionesEl UA administrado oralmente redujo de manera aguda la glucosa en sangre en ratones con diabetes. Paradójicamente, su administración crónica aumentó el consumo de alimento, el peso y la resistencia a la insulina. La mejora en la respuesta nociceptiva sugiere un efecto en el dolor y/o la neuropatía. Aunque son necesarios más estudios para aclarar las propiedades y posibles aplicaciones de este producto, nuestros resultados subrayan el valor de los enfoques etnomédicos hacia la medicina tradicional africana como origen para la evaluación de nuevos compuestos bioactivos.
Metformin and exenatide, originally developed from natural sources, are widely used to treat diabetes.1,2 Indeed, an ethnomedical approach can be used as the basis for drug discovery3 and, once a bioactive product is identified, scientific screening and confirmation should follow. In this context, ancient, northern African, oral testimonies, reported by a patient, attribute glucose-lowering effects to a desert lizard [Uromastyx acanthinura, Bell, 1985; Black Spiny-tailed Lizard, Dab] (UA). Culinary testimonials described that the Uromastyx genus lizards are occasionally eaten by nomads,4,5 but, to our knowledge, the only medicinal uses previously reported are otitis and earache, skin infections and burns.6,7 The aim of this study was to assess the effect of an UA extract on glucose metabolism in an animal model of type 2 diabetes.
Materials and methodsAn UA preparation, elaborated in Mauritania, was donated to our research group by a patient. Following an ethnomedical approach, an interview was performed and, after pharmacological information was obtained, UA's potential acute and chronic glucose-lowering bioactivity was evaluated in vivo in C57BL/6J mice with diet-induced diabetes.
In vitro testing was initially considered, but the lack of detailed pharmacological information, the heterogeneous nature of the product and the complexity of diabetes itself, made cell-assays a poor solution.
The interview was recorded and dissected to define: product acquisition and preparation, dosage and frequency and route of administration. The whole carcass of UA (head excluded) is grilled and dried, and then minced (0.3–3cm) and incorporated into the diet. People traditionally take a handful of UA once or twice/day with food or water. The oral route was also used in the mice and doses were adjusted to body weight. A dose of 7.66g was estimated for a 60kg-person (0.13g/kg), based on handful measurements of four different people and a range (0.13–1.56g/kg) was tested in the mice.
The different doses of UA diluted in saline or saline alone (saline solution 0.9%, Braun Medical SA, Barcelona), were administered to each animal per body weight, from the starting dose 0.13g/kg, with 2g glucose/kg (Glucose G7528, Sigma–Aldrich Chemie GmbH, Steinheim, Germany) during an oral glucose tolerance test (OGTT; fasting period from 8:00h to 14:00h) (Glucocard G+ metre, GT1280, Arkray-Menarini Diagnostics, Shiga, Japan),8,9 on two different days, following a randomised, crossover design (Fig. 1a).10 C57BL/6 mice were used fed a 60%-fat diet (n=13, 8 males, 16 weeks of age) during the previous 12 weeks (D12492, Brogaarden, Lynge, Denmark), a well established model of type 2 diabetes.11 Doses were increased until the desired effect was noted. In the case of absent effects, the highest dose which was feasible to be administered, based on density, concentration or volume, was selected as an endpoint. The same doses were evaluated simultaneously in the whole group. In the long-term evaluation, the dose which had been most effective in the short-term experiments, was administered daily for 90 days in the high-fat diet (UA group) and compared to high-fat diet alone (control group) (n=10 per group: 50% males, 24 weeks of age, 20 weeks with 60%-fat diet). Body weight (BW), food and water consumption and welfare state were assessed weekly. Morning blood glucose was assessed every 1–2 weeks. HbA1c (DCA, Siemens Healthcare Diagnostics, Deerfield, IL), OGTT and intraperitoneal insulin tolerance tests (IPITT) were performed at baseline and after 3 months’ treatment. Neuropathy was assessed at 3 months by cold allodynia response to acetone,12 where higher nociceptive response scores indicate more severe neuropathy. Regarding sample size, to detect glycaemic variations of at least 30% (α=0.05) with a statistical power of 80%, nine animals per group were deemed necessary.13 The crossover design applied in the acute phase allowed us to reduce this number. In the sub-chronic phase, ten animals per group were used, allowing for a 10% loss and to harmonise sex-distribution.
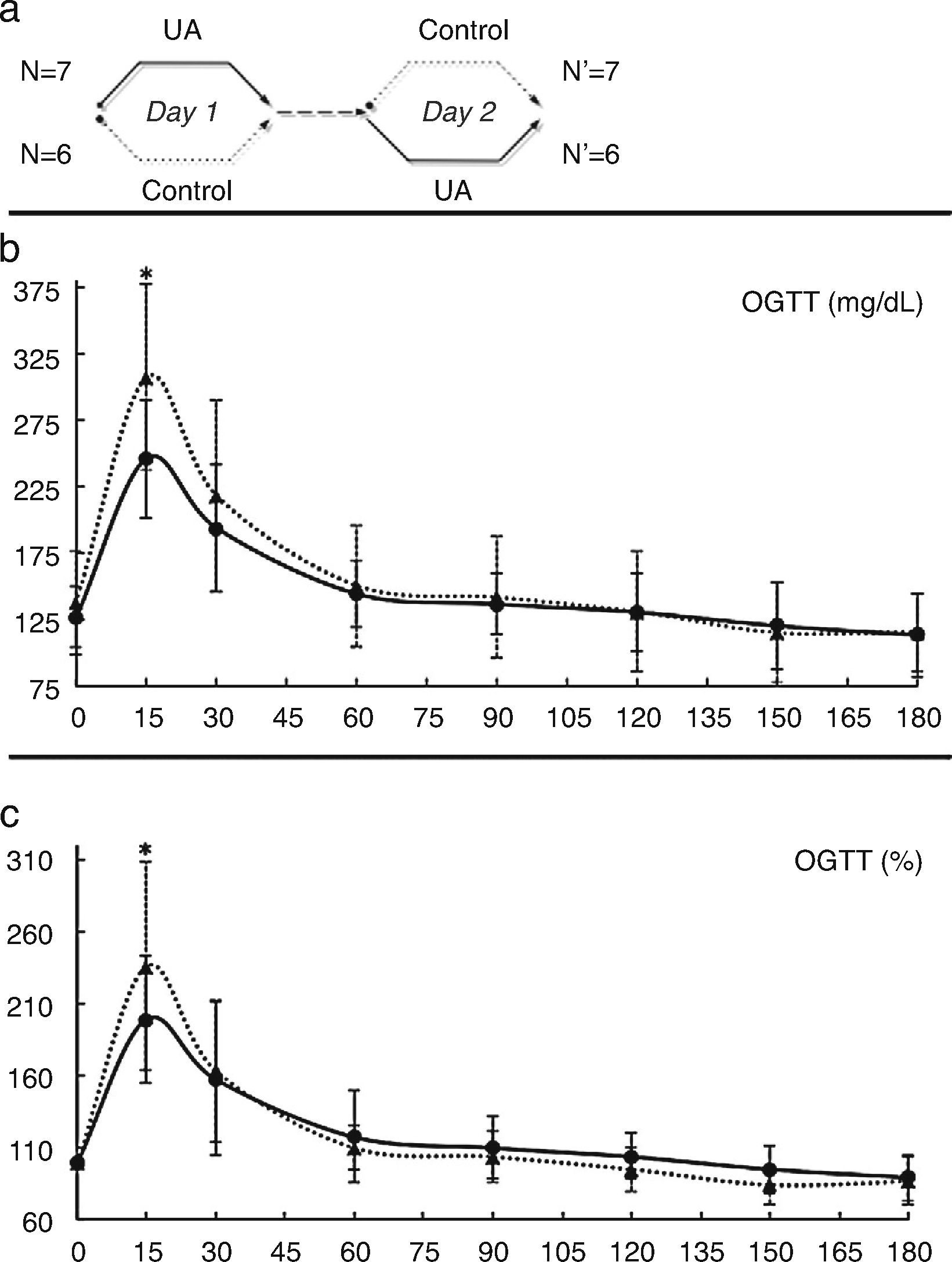
Results from the acute evaluation of Uromastyx acanthinura (UA) (solid line, UA; broken line, control). Crossover design of the experiment (a): on day 1, half of the group served as control while on day 2, after a 48h rest, the same group received the treatment. The other half of the group received treatment on day 1 and served as control on day 2. Thus, each treated animal served as its own control. Glucose concentrations (mean and SD) during the oral glucose tolerance test (b) and percentage glucose change from baseline (mean and SD) during the oral glucose tolerance test (c) (*=p<0.05).
The area under the curve (AUC) of glucose was calculated by the trapezoid rule. For comparisons between groups, Wilcoxon's or Student's tests were used and results were presented as mean (standard deviation) or median [range]. A two-tailed p<0.05 was considered significant (IBM SPSS Statistics Version 18, SPSS Inc., Chicago, IL). Animals were housed in groups of four-five per cage and provided with environmental enrichment. The protocol was performed following National and European requirements (RD 1201/2005, Law 32/2007, EU Directive 2010/63/EU) and was approved by the Animal Welfare Ethics Committee (Comité Ético de Experimentación Animal de la ULPGC; 009/2011).
ResultsEthnomedical approach and short-term evaluationDifferent traditional preparation methods of UA (basically, cooking methods) were described. Doses were rather approximate, since the person's hand is the most commonly used measure. The most effective dose was 0.048gUA/mouse, which showed a glucose-lowering effect 15min (′) after its administration [UA 246.15 (44.48)mg/dL vs. Control 307.39 (70.12)mg/dL; p=0.004]. The AUC of glucose tended to be lower in the treatment arm [UA 203.27 (35.18) vs. 222.90 (54.48)mg/dL/min; p=0.073] (Fig. 1b). Similar effects were observed at 15′ when results were defined as percentage variation from fasting glucose (Fig. 1c).
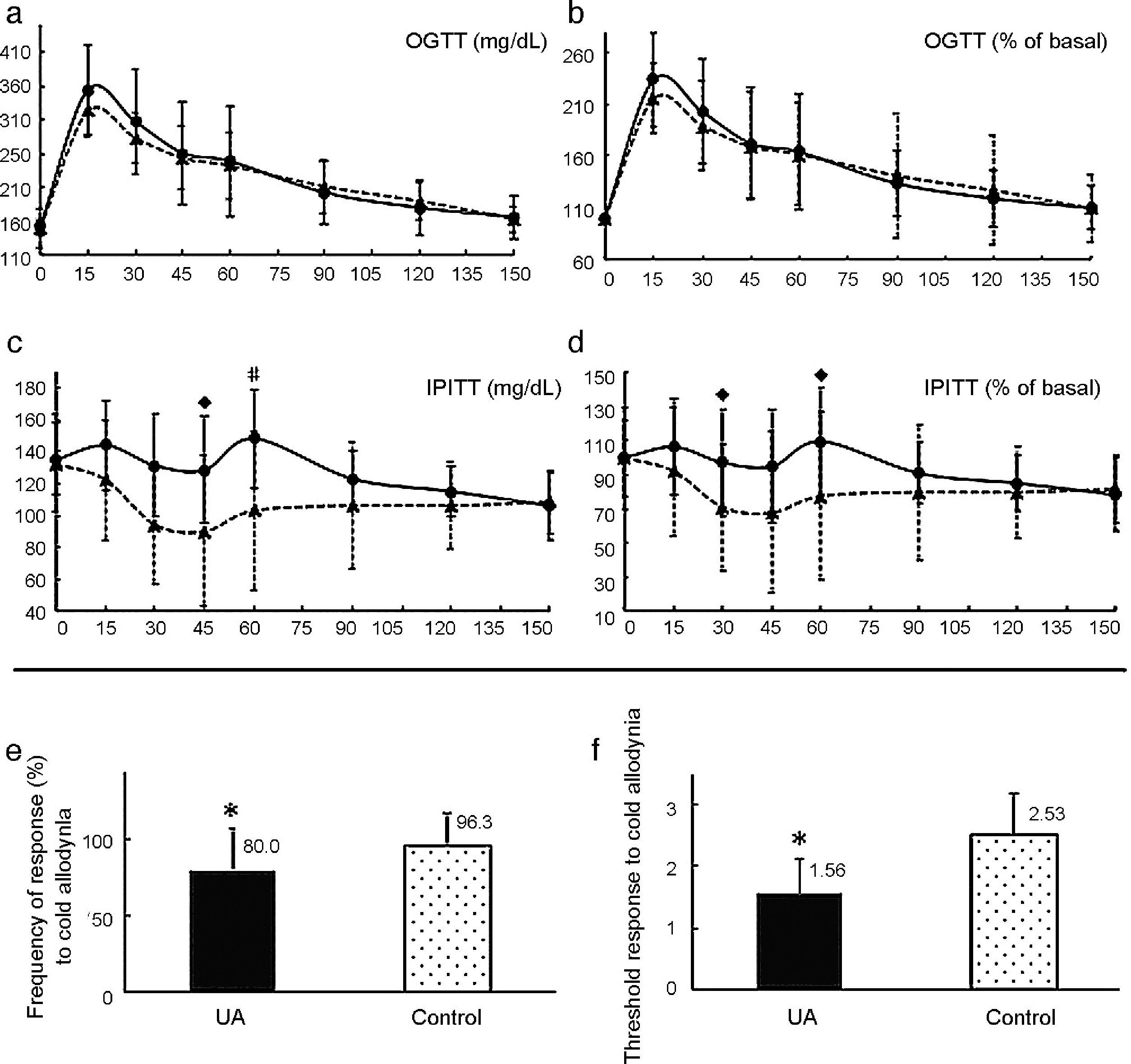
Long-term evaluationIn the post-treatment evaluation (UA n=8, Control n=10), no significant differences were observed for OGTT (Fig. 2a and b) or HbA1c [UA 5.06 (0.48), Control 4.78 (0.29) %; p=0.134]. In the IPITT, there was a trend towards higher glucose values for UA at 60′ [148.12 (33.47) vs. 102.9 (52.40)mg/dL; p=0.051], and 45′ [128.56 (36.29) vs. 89.95 (49.82)mg/dL; p=0.086] (Fig. 2c and d). During follow-up, morning plasma glucose concentrations were significantly lower in the active treatment group only transiently: at weeks three (UA 132 [85–165], Control 167 [118–233]mg/dL; p=0.004) and five (UA 129 [119–139], Control 150 [111–201]mg/dL; p=0.012) after treatment start. Although both groups increased their BW, the increment tended to be higher in the UA group ΔBW 6.45 (8.18) vs. 5.06 (3.94)g, p=0.064], which consumed more food [2.86 (2.83–2.86) vs. 2.52 (2.40–2.64)g/mouse/day, p<0.001]. Cold allodynia response was less frequent [75 (37.5–100) vs. 100 (87.5–100)%; p=0.034] and intense in the treated group [0.81 (0.35) vs. 1.22 (0.33); p=0.022] (Fig. 2e and f). During long-term evaluation, idiopathic ulcerative dermatitis (pruritus, alopecia and ulcers) developed in both groups, associated with a certain level of distress (unplanned increment in light brightness). The source of distress was removed and animals were treated [enrofloxacin (7.5mg/kg, 8 days); topic hydrogen peroxide and povidone-iodine]. All but two mice (2 females in the UA group, which were sacrificed due to their worsened condition) recovered their normal health status and stress level.
Results for the comparisons between the treatment (solid lines and columns) and control groups, after 3 months of Uromastyx acanthinura (UA): glucose concentrations during an oral glucose tolerance test (a) and percentage of baseline concentration during the OGTT (b); glucose values (mg/dL) per minute during the intraperitoneal insulin tolerance test (IPITT) (c) and percentage of baseline concentration during the IPITT (d); frequency (%) (e) and intensity threshold (f) of response to cold allodynia (*, p<0.05; #, p<0.065; , p≤0.093).
Oral administration of UA acutely reduced blood glucose in diabetic mice. Paradoxically, long-term administration of UA tended to increase insulin-resistance, food-consumption and mean BW. Symptoms of neuropathy were significantly attenuated in the treated group.
The acute glucose-lowering effects of UA might be explained by the persistence, in its carcass, of a bioactive compound, either synthesised by UA itself, as is the case of Heloderma suspectum (exendin),2 or included in its diet and stored. Indeed, some of the plant species eaten by UA14 have shown glucose-lowering effects.15,16
Food intake and BW increments could explain the discrepancy between the short- and long-term results and the lack of persistence of lower glucose values after the first 5 weeks of treatment. However, other causes can be suggested, including dose (insufficient for the long-term study), pharmacokinetics (short duration of action) or other, unknown metabolic effects.
Insulin-resistance significantly increased in the UA group when compared to controls. Therefore, the improved response to cold allodynia could be due to direct effects on pain or neuropathy itself, and not as side-effect from a better glucose control.
The effects detected in the acute evaluation indicate that the crossover design suited its purpose and allowed a reduction in the number of animals, which is one of the mandatory demands of the principles for laboratory animal handling.17–20 Randomisation of treatment order reduced period bias, as well as bias from possible cumulative effects of UA. The homogeneity of results regardless of the treatment sequence (saline-product vs. product-saline) confirmed that the time left for clearance between treatments was long enough. The crossover design,10 could be an answer to the claim to reduce animal numbers in the pre-evaluation of glucose-lowering and other bioactive compounds.
Only data obtained from animals that were healthy at the end of the study were considered. However, a confounding effect of the development of dermatitis and the distress level cannot be ruled out.21 This dermatitis was not attributed to UA, since both groups were affected by what is a common disorder in C57Bl/6J.21 Although a role of UA in the worse outcome (two losses) cannot be excluded, previous reports describe its topical effects as a treatment for, rather than a cause of dermatitis.6
Finally, our findings suggest direct effects of UA on glucose metabolism and appetite, as well as on pain and/or neuropathy, approaches to consider in further studies. They also support the value of African traditional medicine as a starting point for the screening of new bioactive components.
Conflict of interestsThe authors are not aware of any conflicts of interest related to the contents of this article.
We are grateful to Natalia Navarro and Fidela González (Menarini Diagnostics) for providing glucose test strips, Juan Carlos Hernández (MSD Healthcare) for providing cuvettes for HbA1c analysis and Natalia María Santana and Francisco Martín González for their commitment in the care of the animals. During the performance of this study, the authors were supported by a predoctoral fellowship (Fundación Canaria de Investigación y Salud, FUNCISID41/2008) (YBC) and grants from the European Foundation for the Study of Diabetes (EFSD/JDRF/Novo Nordisk Programme for Type 1 Diabetes 2008) and Instituto de Salud Carlos III (PI08/01113, PI11/02441, PI10/02310, ADE10/00032), from the Spanish National Research Programme.