Cushing's syndrome results from a prolonged exposure to excessive concentrations of free circulating glucocorticoids. ACTH dependent Cushing's syndrome or Cushing's disease (CD) represents approximately 80–85% of the total cases, and around 80% of them correspond to ACTH secreting pituitary adenomas.1 Transesphenoidal surgery remains the first line of treatment for CD.1 Other therapeutic approaches such as medical treatment, pituitary radiotherapy or bilateral adrenalectomy constitute the second-line treatment, and are normally indicated in case of surgical failure.
Cabergoline is a D2 dopamine receptor agonist (DA). The drug has proven effectiveness in the treatment of prolactinomas. There is also encouraging information on its therapeutic value in pituitary GH-producing adenomas.2 In 2001, Petrossians et al. reported a positive response of a silent ACTH-producing pituitary adenoma to the treatment with cabergoline.3 In the mechanistic analysis the authors demonstrated the presence of D2 dopamine receptors in the primary tumor and its expression was similar to what is found in prolactinomas. This case-report opened a new horizon in the treatment of patients with CD. However, very few cases have been reported to date.
We present a 45-year-old woman with CD who started treatment with cabergoline as the first and only treatment for her disease. After 24 months of treatment, the patient is clinically asymptomatic, her 24h urinary free cortisol (UFC) levels are within the reference values, and her pituitary adenoma has shrunk to the extent of being undetectable by magnetic resonance imaging (MRI).
The patient, who lives overseas, came to our clinic in the summer of 2013 with a bizarre history of unproven diseases. She had been diagnosed of probable hypopituitarism eight years ago because of amenorrhea, osteoporosis and hypothyroidism. Her main complaint was a severe asthenia associated with impaired night rest, weight gain, nocturia, and decreased of libido. She also had noticed facial hirsutism, an increase in the posterior cervical fat volume and easily bruising with minimal trauma. She received therapy with l-thyroxine for a short period of time, calcium supplements and ibandronic acid for the previous 3 years. However, the last two treatments were withdrawn after an episode of nephrolithiasis. On physical examination her BP was 135/90mmHg, and we found an atrophic skin, facial hirsutism and a slightly enlarged thyroid gland.
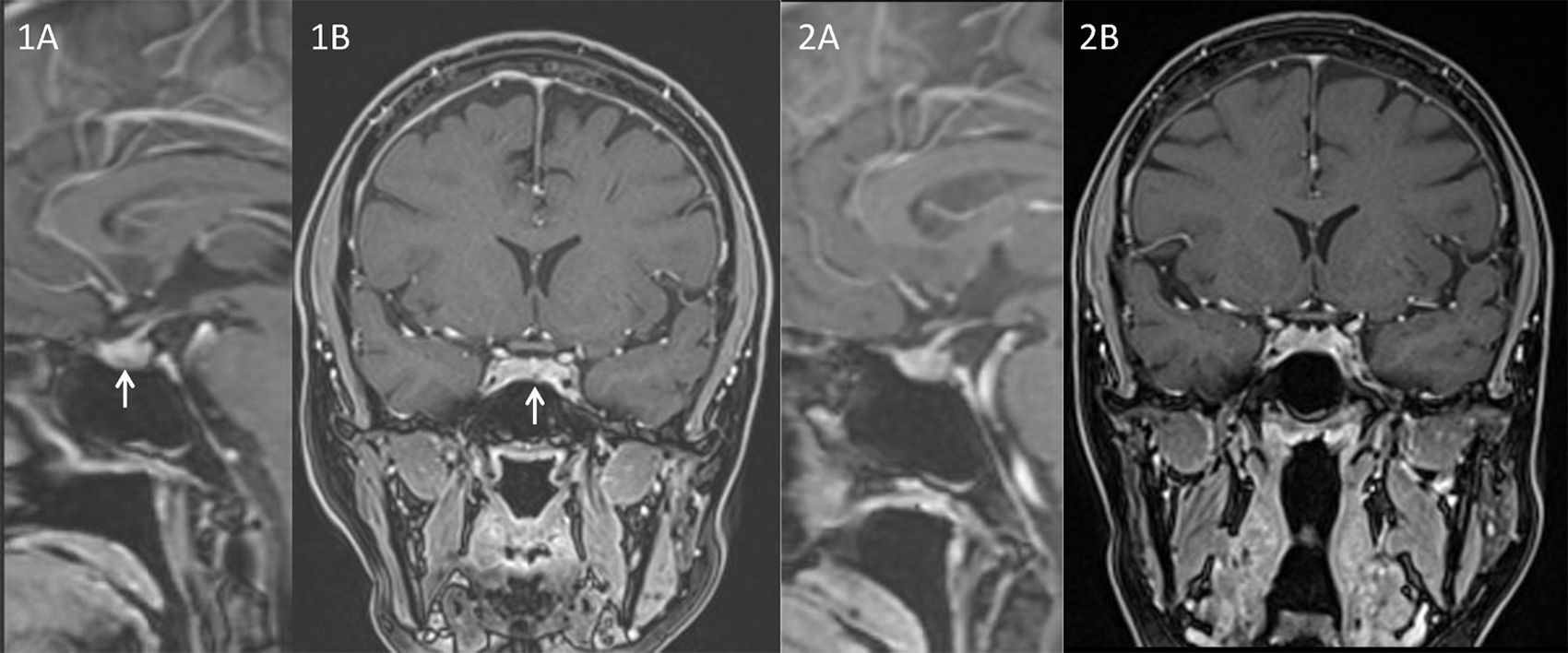
The history and physical features raised the suspicion of CD. Baseline hormonal investigations were: prolactin: 8.9ng/mL; LH: 1.4UI/L; FSH: 7.5UI/L; estradiol: 36.0pg/mL; IGF1: 223.1ng/mL; TSH: 0.1μU/mL and free T4: 0.67ng/dL. Screening laboratory studies revealed an UFC of 248μg/24h (RR 28.5–213.7). Cortisol at 8:00ha.m. after the administration of 1mg of dexamethasone was 16.8μg/dL. In order to confirm the diagnosis of CD we measured a plasma cortisol level at 23:00h that was 17.5μg/dL associated to a serum ACTH level of 85pg/mL. An 8mg dexamethasone nocturnal suppression test displayed a basal (8:00a.m.) cortisol concentration of 1.5μg/dL with a concomitant ACTH of 5.6pg/mL. An MRI focused on the sella, showed a lesion of 0.22cm of diameter in the pars tuberalis of the pituitary gland (Fig. 1A and B). Inferior petrosal sinus catheterization showed: basal ACTH levels (pg/mL) from right femoral vein (P), Right petrosal sinus (RPS) and left petrosal sinus (LPS) were, 64, 276 and 1940, respectively. ACTH levels 5min after 100μg CRH stimulation were: P: 97, RPS: 1980 and LPS: 5558, respectively. And 10min after the stimulus: P: 108, RPS: 899 and LPS: 2130, respectively.
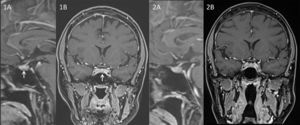
After discussion with the patient, the lack of a clear image encouraged us to look for alterative therapeutic options to surgery despite the convincing biochemical data. Due to ketoconazole potential severe side effects we discarded its long-term use. However, some published data supported the treatment of CD with cabergoline.4 Additionally some investigators had shown that 80% of ACTH-producing tumors express dopamine D2 receptors.5 Based on these considerations and because of the extensive experience on the use of DA in prolactinomas, we made the decision of starting treatment with cabergoline. The initial dose was 0.5mg per week. In the follow-up we tested UFC levels periodically. After 4 weeks of treatment, UFC was two times over the upper reference range showing that the cabergoline dose was insufficient. Thus the dose was tripled to 1.5mg per week. Three months later UFC fell within the normal rage, but it was next to the superior level of the reference range. Therefore, cabergoline dose was increased to 2mg per week. After 14 months since the beginning of the treatment (that corresponds to a treatment of 2 months on 2mg per week), the value of UFC decreased to 60.96μg/24h. A second pituitary MRI performed one year after the diagnosis was not able to identify the structural lesion (Fig. 2A and B). The patient was asymptomatic with no clinical symptoms suggestive of hypercortisolism, regular menses, and normal biochemical results. In her last visit (24 months after the diagnosis) the patient remained under treatment with 2mg of cabergoline per week. She had no complaints, her menses continued to be regular and with no physical features of CD. The biochemical investigations showed a depressed serum prolactin level (0.2ng/mL) and a normal UFC of 77.4μg/24h.
Although the beneficial effect of cabergoline in the pituitary ACTH-secreting adenomas could be evident (in view of this and others results), the clinical experience is limited although not entirely new. Early studies from the eighties6,7 showed response to bromocriptine in some patients with past medical history of CD. Since then DA have been frequently listed among the pharmacological options for CD.8 Investigating this route, Pivonello et al. studied the expression of D2 receptors in 20 ACTH-producing pituitary tumors and the effect of treatment with cabergoline in the secretion of cortisol. After 3 months of treatment, the inhibition of cortisol secretion was observed in 60% of patients, achieving a normalization of cortisol levels in 40% of the cases.9 Administration of cabergoline in a higher dose than commonly used in patients with hyperprolactinemia leads to a decrease in UFC in up to 75% of the patients,10,11 with a normalization of cortisol levels in 36.6%10 and 35%11 of the cases; however, up to 20% of the subjects can escape from long-term beneficial effects.10,11 Interestingly, the use of this drug has not only shown a reduction of systemic levels of ACTH, but also a reduction in tumor size.12,13
At present the patient is doing well under cabergoline (2mg weekly) with no complains and free of Cushing's clinical stigmata. Her UFC has been consistently within the normal range for the last 18 months although with no perfect circadian rhythm (in June 2015 her serum cortisol levels at 8:00a.m. and 8:00p.m.: were 17.6μg/dL and 11.7μg/dL, respectively). In her last follow-up visit (June 2015) the echocardiogram was normal and the densitometry display a stable osteopenia under Denosumab treatment. She has a 20% chance of escape to this therapy, but there is still a broad margin for intensify the DA treatment, and her response is likely to be dose-dependent. Currently we are following her biannually with blood and urine test (hormonal and general), and annually with a pituitary MRI.
In conclusion, cabergoline may be a good therapeutic alternative in the medical treatment of CD, given its modulatory effect on the release of ACTH and its possible impact on reducing tumor size.