To evaluate expression of somatostatin receptor subtypes 2 and 5 (SSTR 2 and 5) by RT/PCR and immunohistochemistry (IHC) in GH-secreting adenomas, seeking correlations with response to octreotide.
MethodsSSTR2 and 5 expression was tested by IHC (n=37), RT/PCR (n=36) or both (n=13) in GH-secreting adenomas from 60 patients with acromegaly who had undergone pituitary surgery; 36 had been treated preoperatively with octreotide LAR for 3–6 months, and were categorized as responders (achievement of GH <2.5ng/mL and a normal age-adjusted IGF-1), partial responders (GH and IGF-1 reduction >50% and >30%, respectively) or non-responders. IHC was performed on a tissue microarray using specific antibodies directed to the carboxyl terminus of SSTR2 and 5.
ResultsSSTR5 was the predominantly expressed receptor subtype by both IHC and RT/PCR in all tumors tested, regardless of whether they came from octreotide-naïve, octreotide-responsive, or octreotide-resistant patients. Immunostaining was concentrated in the cytoplasm. Neither SSTR2 nor SSTR5 expression correlated with baseline or post-octreotide GH or IGF-1 levels or tumor volume by either method. The agreement rate between RT/PCR and IHC was 77% in all 13 adenomas in which both methods were used.
ConclusionExpression of these receptors does not guarantee an adequate response to somatostatin analogs; other functional aspects of this interaction, such as receptor homo- and heterodimerization, and the resulting signaling cascade, probably play a role in determining whether a patient will respond or not to these agents.
Evaluar la expresión de los receptores somatostatinérgicos 2 y 5 (SSTR 2 y 5) por RT/PCR e inmunohistoquímica (IHQ) en adenomas productores de GH, buscando correlaciones con la respuesta a octreótido.
MétodosSe analizó la expresión de SSTR2 y SSTR5 mediante IHQ (n = 37), RT/PCR (n = 36) o ambas (n = 13) en adenomas productores de GH de 60 pacientes con acromegalia sometidos a cirugía; 36 habían recibido tratamiento preoperatorio con octreótido LAR durante 3-6 meses y fueron categorizados como respondedores (GH <2.5 ng/mL e IGF-1 normal para edad), respondedores parciales (reducción de GH e IGF-1 a >50% y >30%, respectivamente) y no respondedores. La IHQ se realizó en una micromatriz de tejido, usando anticuerpos dirigidos contra el extremo carboxilo de SSTR 2 y 5.
ResultadosSSTR5 fue el receptor predominante en todos los tumores, tanto por IHQ como por RT/PCR, independientemente de si provenían de pacientes tratados (sensibles o resistentes) o no tratados con octreótido. La inmunotinción se localizó fundamentalmente en el citoplasma. No encontramos correlaciones entre la expresión de estos receptores por ninguno de los dos métodos con los niveles basales y post-octreótido de GH e IGF-1 ni con el volumen tumoral. La concordancia entre RT/PCR e IHQ fue del 77% en los 13 adenomas en los que ambos métodos fueron utilizados.
ConclusionesLa expresión de estos receptores no garantiza la respuesta farmacológica a análogos de la somatostatina; otros aspectos funcionales como la homo y heterodimerización, y la cascada de señalización producida, probablemente están involucrados en la determinación de la respuesta de cada paciente a estos agentes.
The depot somatostatin analogs (SSA) octreotide LAR and lanreotide autogel are currently the mainstay of the pharmacological treatment of acromegaly.1,2 Early studies reporting efficacy rates as high as 60% suffered from a significant selection bias.3,4 More recent multicentric trials without such a selection bias have established more realistic efficacy rates that range from 25% to 30%; thus, a considerable number of patients do not respond to SSA and remain with clinically and biochemically active disease.5,6 Predicting the pharmacological response to SSA has been an elusive goal. The value of the subcutaneous octreotide test in identifying those patients with acromegaly who will eventually respond to long-term SSA therapy has been a matter of controversy; some studies claim that this test is highly predictive of the pharmacological response,7–9 while others conclude that it should only be used to establish tolerance.10–12 Similarly, 111In-Octreotide scanning cannot discern responsive from resistant patients, due to the background uptake by the normal pituitary gland.13 More recently, hypointense lesions on T2-weighted magnetic resonance images (MRI) have been associated with a favorable response to SSA; yet, the positive predictive value of such a finding is only 62% and thus cannot be used by itself to decide whether or not to treat a patient with SSA.14
These SSA bind preferentially to the subtype 2 of somatostatin receptors (SSTR2) and to a lesser extent to subtype 5 (SSTR5).15,16 In fact, they were designed to bind these receptor subtypes precisely because they are the most abundantly expressed in the vast majority of GH-secreting adenomas.15,16 SSTR2 expression by the tumor at both the mRNA (by RT/PCR)17,18 and protein (by IHC)19–23 levels has been positively associated with the response to SSA. However, a significant number of patients with tumors expressing high levels of SSTR2 are resistant to these pharmacological agents.24 Compared to SSTR2, SSTR5 is expressed by a greater proportion of these tumors; yet its significance in terms of predicting response to SSA is less well understood.18–24 Although in vitro SSTR5 appears to be fundamental for SSA-induced GH inhibition,25 a low SSTR2/SSTR5 mRNA ratio was found to be associated with a poor pharmacological response to octreotide LAR.18 At the protein level, while SSTR2 immunostaining is found in SSA-responsive patients, SSTR5 is found in SSA-resistant patients as well.20 Despite these observations suggesting a negative effect of SSTR5 expression in determining pharmacological response to SSA, subsequent IHC studies have focused mainly on SSTR2.21,22
In the present study we evaluated the expression of SSTR 2 and 5 by both RT/PCR and IHC in a large group of patients with acromegaly, with the aim of finding patterns associated with the pharmacological response to SSA.
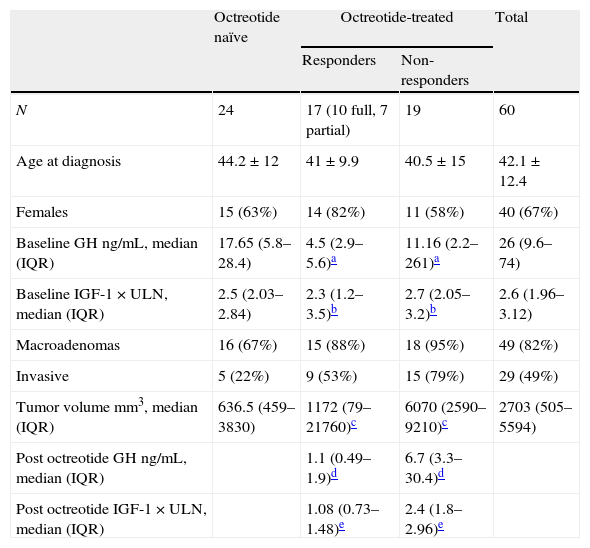
Materials and methodsPatientsSixty patients with acromegaly (20 males, 40 females, mean age 42.1±12.4 years) who had undergone transsphenoidal surgery (TSS) for removal of GH-secreting adenomas were included in the study (Table 1). The study was approved by our local ethics and scientific committees and all subjects signed an informed consent. Acromegaly had been diagnosed preoperatively on the basis of classical clinical symptoms and signs of the disease, as well as by the biochemical evidence of hypersomatotrophism (a glucose suppressed GH above 1ng/mL and an elevated age-adjusted IGF-1 level). MRI showed a microadenoma in 18% and a macroadenoma in 82% of the patients (Table 1).
Demographic, biochemical and tumoral characteristics.
| Octreotide naïve | Octreotide-treated | Total | ||
| Responders | Non-responders | |||
| N | 24 | 17 (10 full, 7 partial) | 19 | 60 |
| Age at diagnosis | 44.2±12 | 41±9.9 | 40.5±15 | 42.1±12.4 |
| Females | 15 (63%) | 14 (82%) | 11 (58%) | 40 (67%) |
| Baseline GH ng/mL, median (IQR) | 17.65 (5.8–28.4) | 4.5 (2.9–5.6)a | 11.16 (2.2–261)a | 26 (9.6–74) |
| Baseline IGF-1×ULN, median (IQR) | 2.5 (2.03–2.84) | 2.3 (1.2–3.5)b | 2.7 (2.05–3.2)b | 2.6 (1.96–3.12) |
| Macroadenomas | 16 (67%) | 15 (88%) | 18 (95%) | 49 (82%) |
| Invasive | 5 (22%) | 9 (53%) | 15 (79%) | 29 (49%) |
| Tumor volume mm3, median (IQR) | 636.5 (459–3830) | 1172 (79–21760)c | 6070 (2590–9210)c | 2703 (505–5594) |
| Post octreotide GH ng/mL, median (IQR) | 1.1 (0.49–1.9)d | 6.7 (3.3–30.4)d | ||
| Post octreotide IGF-1×ULN, median (IQR) | 1.08 (0.73–1.48)e | 2.4 (1.8–2.96)e | ||
According to the usual clinical practice in our center, approximately half of the acromegaly patients who are considered good surgical candidates receive treatment with octreotide LAR 20mg every 4 weeks during 3–6 months while waiting for their TSS, and the rest undergoes surgery without prior pharmacological treatment. Among the 60 patients included in the study, 36 received preoperative octreotide LAR treatment and 24 did not. In patients receiving preoperative octreotide LAR, a full biochemical response was defined as the achievement of GH<2.5ng/mL and IGF-1<1.2×ULN (times the upper limit of normal); patients were categorized as partial responders if their GH and IGF-1 levels were reduced by at least 50% and 30% respectively when compared to baseline values; those who did not achieve these targets were considered non-responders.
Immunohistochemistry and RT/PCR methodologyOf the 60 tumors included in the study, 24 were evaluated by IHC, 23 by RT/PCR and 13 by both methods.
- •
Immunohistochemistry methodology and TMA (tissue microarray) construction: Tissue removed at the time of surgery was fixed in 10% buffered formaldehyde, dehydrated in graded ethanol, and embedded in paraffin. At least three representative areas of tumor were selected for each case under light microscopy of hematoxilin-eosin stained slides. When available, surrounding normal tissue was also included. A TMA was built using the TMArrayer from Pathology Devices, including triplicate 0.6mm cores of each type of tissue per case. Manual IHCs were standardized using appropriate negative controls as suggested by the manufacturer for each antibody. Each TMA slide allows for additional intra-assay controls (different structures). All TMAs were stained in the same run for each antibody to avoid inter-assay variability. All slides were reviewed by light microscopy to confirm the type of tumor, the specificity of the staining in the pituitary adenoma cells and the expected sub-cellular localization. IHC was carried out in the microarray, using the following specific antibodies: SSTR2 (antibody 9550, 1/500 Abcam, Cambridge MA, US); SSTR5 (antibody 28618, 1/500, Abcam, Cambridge MA, US); GH (antibody A0570, 1/500, Dako, Carpinteria, CA). Both the SSTR2 and the SSTR5 antibodies are directed against the intracellular, carboxyl terminus of the receptor; therefore, immunostaining for these receptors was mainly localized in the cytoplasm. All stained TMAs were digitally scanned with the Aperio ImageScopeXT at 40× magnification and then analyzed with the Spectrum XS 10 Image Analysis software using two independent algorithms – nuclear and color deconvolution – each one developed for nuclear- and cytoplasmic-specific analysis. After registering pixel count and intensity of staining using red, blue, green and brown and establishing the percentage of cells and nuclear count, the different algorithms can generate specific scores for each cell compartment. For the cytoplasmic algorithm or color deconvolution, each percentage of cells is multiplied by the intensity of its stain and a constant adjusting the mean for the strongest staining [(Score=1.0×(%Weak)+2.0×(%Medium)+3.0×(%Strong)].26 We calculated the SSTR5/SSTR2 ratio in order to ascertain if the relative expression of the two receptor subtypes was associated with response or resistance to SSA treatment.
- •
RT/PCR methodology: Total RNA was extracted from frozen tumor tissues obtained during surgery, using the MinElute RNA purification kit (Qiagen, Alameda, CA). Reverse transcription was carried out in 2μg of RNA (Trancriptor First Strand cDNA Synthesis Roche Diagnostics GmbH 68298 Mannhein, Germany). SSTR 2 and 5 mRNA expression was determined by means of real time PCR on a Fast Start Universal Probe Master Light Cycler Real-Time PCR System (Roche Diagnostics GmbH 68298 Mannhein, Germany). Primers were selected using genomic sequences obtained from Genebank (Accession numbers, for SSTR2 NM_001050 and for SSTR5 NM_001053 XX). For SSTR2: sense primer GGCATGTTTGACTTTGTGGTG, antisense primer GTCTCATTCAGCCGGGATTT (product size 185bp); for SSTR5: sense primer CTGGTGTTTGCGGGATGTT, antisense primer GAAGCTCTGGCGGAAGTTGT (product size 183bp). Amplification protocol consisted of a preincubation step (5min at 94°C), followed by 35 cycles of denaturing (10s at 95°C), annealing (20s at 50°C) and extension (30s at 72°C). The resulting mRNA copy number for each SSTR in each individual sample was corrected by a normalization factor derived from the expression of three housekeeping genes (HPRT, beta-actin and GAPDH) as previously described. The relative expression of the two receptors mRNA was estimated by means of a SSTR5/SSTR2 ratio.
Both GH and IGF-1 values are expressed in mass units. To convert to SI units, multiply by 0.13 in the case of IGF-1 and by 2 in the case of GH. GH was measured using a two-site chemiluminescent enzyme assay (DiaSorin-Liaison, Salugia, Italy), with a detection limit of 0.009ng/mL and intra- and inter-assay CVs of 2.5% and 5.8%, respectively; the IRP used in the calibration of the GH assay was WHO second 95/574. IGF-1 was separated from its binding proteins by means of an acid-ethanol extraction step, and the hormone levels were quantified in the extracted samples by a chemiluminescent assay (DiaSorin-Liaison, Salugia, Italy), with advertised intra- and inter-assay CVs of 3.8% and 5.5%, respectively; the IRP used in the calibration of the IGF-1 assay was WHO second 02/254. We established our own normative IGF-1 data analyzing serum samples from 340 healthy individuals and thus calculated the real intra- and inter-assay CVs as 3% and 4%, respectively. The resulting normal age-adjusted reference values are as follows: 18–30 years: 150–430ng/mL; 31–40 years: 110–310ng/mL; 41–50 years: 78–200ng/mL; 51–60 years 60–170ng/mL; 61 and older 60–150ng/mL.
Statistical analysisData are presented as measurements of central tendency and dispersion. The categorical variables were described as percentages and frequencies. Quantitative data distribution was established by means of the Shapiro Wilks method. Normally distributed data were presented as means±SD, whereas non-normally distributed data as medians with interquartile ranges (IQR). Differences in categorical variables were analyzed by x2 test, and two-tailed, unpaired T-test or Wilcoxon rank-sum test were used for continuous variables depending on their distribution. Analysis of variance (ANOVA) was used to compare three or more treatment groups for SSTR expression by IHC or RT/PCR. Pearson's test was used to establish correlations between SSTR expression and baseline and post-octreotide GH, and IGF-1. STATA, version 11.2 was used as statistical software. A p value of <0.05 was considered as statistically significant.
ResultsTable 1 depicts the biochemical and imaging characteristics of the patients. Although GH and IGF-1 levels were similar in patients who were treated preoperative with octreotide and in those who did not, subjects who received the SSA before surgery had smaller and less invasive tumors (Table 1). Of the 36 patients who received octreotide LAR treatment, 10 (27%) achieved full biochemical control targets (GH <2.5ng/mL and IGF-1<1.2×ULN), 7 (19%) responded partially (>50% decrease in GH and >30% decrease in IGF-1, compared to baseline levels) and 19 (52.7%) were non-responders. GH at baseline was significantly lower among octreotide responsive patients (complete and partial responders, analyzed together) compared to those who did not respond to the SSA (p=0.008). Although IGF-1 levels also tended to be lower, the difference did not reach statistical significance (p=0.08). As a group, patients responding to octreotide had smaller adenomas than those in whom the SSA was not effective (median tumor volume [IQR]: responders 1172mm3 [79–21760], non-responders 6070mm3 [2590–9210], p=0.004).
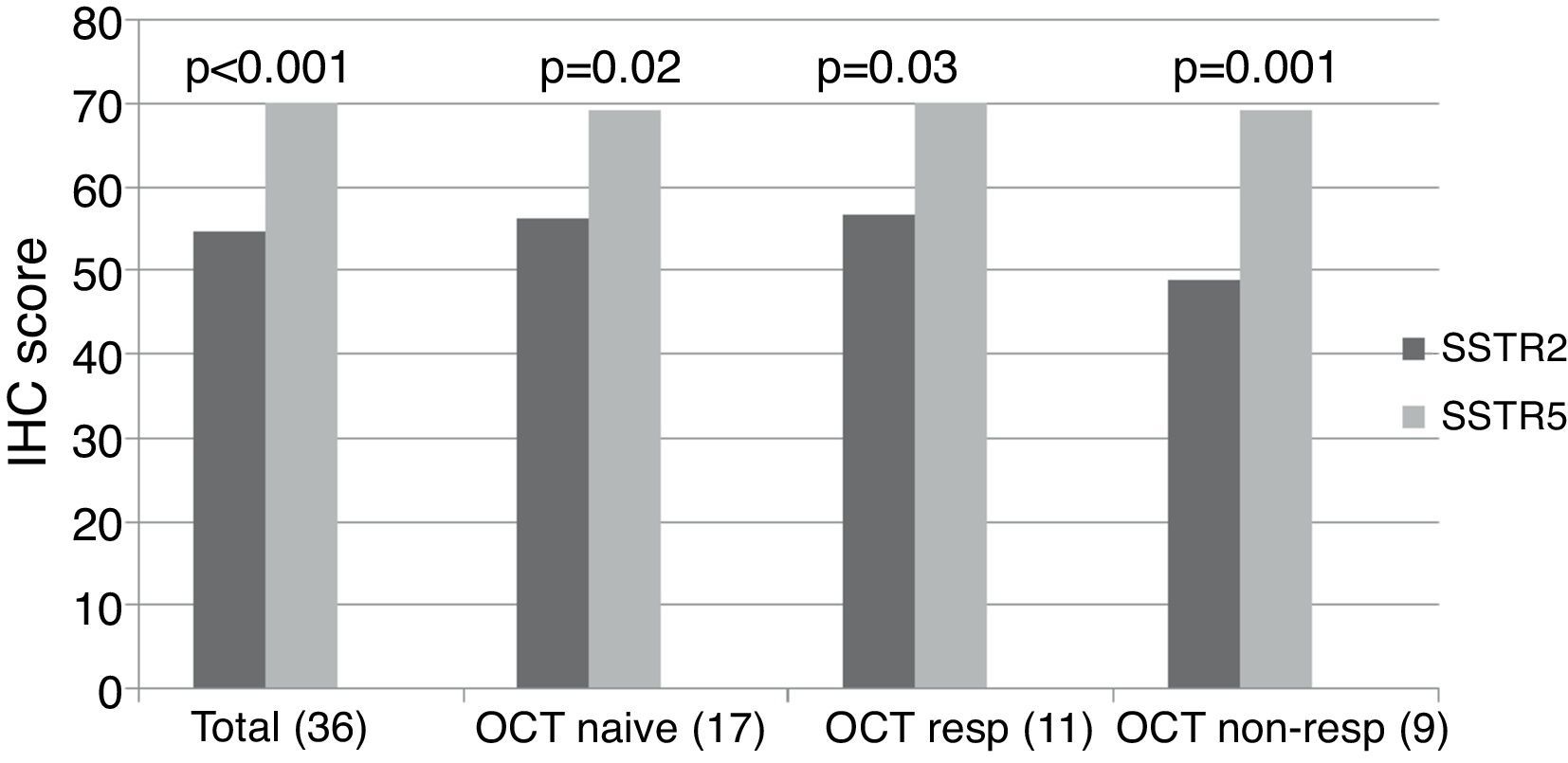
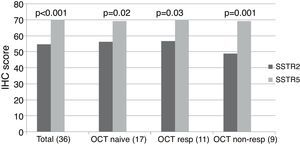
ImmunohistochemistryA total of 37 GH-secreting tumors were included in a TMA for immunohistochemical analysis; 17 (46%) were octreotide naïve, whereas 20 (54%) had been treated preoperatively with octreotide LAR (5 complete responders, 6 partial responders and 9 non-responders). Low to moderate intensity SSTR2 and SSTR5 immunostaining was found in all tumors studied taking into account the cytoplasmic algorithm. The mean SSTR5 immunostaining score was significantly higher than that of SSTR2 when the whole group was analyzed together (SSTR5 70.1±12.3 vs. SSTR2 54.6±14.9, p<0.001), and this remained so when we analyzed octreotide naïve (SSTR5 69.1±15.8 vs. SSTR2 56.3±15.2, p=0.02), octreotide responsive (SSTR5 70.18±10.1 vs. SSTR2 56.6±16.2, p=0.03) and octreotide non-responsive (SSTR5 69.1±8.6 vs. SSTR2 48.8±12.9, p=0.001) patients separately (Fig. 1, supplementary Table 1).
SSTR2 and SSTR5 immunohistochemical score. The total group includes octreotide treated and naïve patients. The octreotide-responsive group consists of 5 complete responders and 6 partial responders. OCT NAÏVE: octreotide naïve group; OCT RESP: octreotide-responsive group; OCT NON-RESP: octreotide non-responsive group.
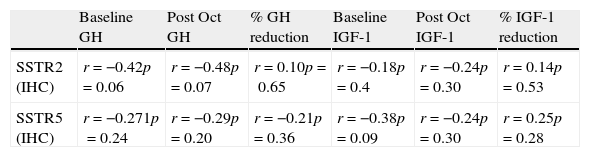
Among the 17 patients who did not receive octreotide preoperatively, SSTR5 was the predominantly expressed receptor in 8 (47%); in the remaining 9 patients, no clear predominance of any of the two receptors could be found. Of the 11 subjects who responded to octreotide, 8 (72.7%) expressed SSTR5 as the predominant receptor subtype, whereas SSTR2 immunostaining was more intense in two (27.3%). SSTR5 was clearly predominant in 8 out of the 9 (88.8%) patients who did not respond to octreotide; in the remaining non-responsive patient immunostaining scores for SSTR2 and SSTR5 were similar. Thus, although SSTR5 predominance tended to be more apparent in non-responders than in responders, the difference did not reach statistical significance. Neither SSTR2 nor SSTR5 protein expression correlated with baseline and post-octreotide GH, baseline and post-octreotide IGF-1, or tumor volume (Table 2). The SSTR5/SSTR2 ratio tended to be higher among octreotide non-responders, but the difference did not reach statistical significance (responders 1.3±0.32, non-responders 1.47±0.31, p=0.2).
Correlations between SSTR2 and SSTR5 protein (IHC) and mRNA (PCR) expression with baseline and post-octreotide GH and IGF-1.
| Baseline GH | Post Oct GH | % GH reduction | Baseline IGF-1 | Post Oct IGF-1 | % IGF-1 reduction | |
| SSTR2 (IHC) | r=−0.42p=0.06 | r=−0.48p=0.07 | r=0.10p=0.65 | r=−0.18p=0.4 | r=−0.24p=0.30 | r=0.14p=0.53 |
| SSTR5 (IHC) | r=−0.271p=0.24 | r=−0.29p=0.20 | r=−0.21p=0.36 | r=−0.38p=0.09 | r=−0.24p=0.30 | r=0.25p=0.28 |
SSTR mRNA expression data were available in 36 patients; 9 (25%) were octreotide naïve, whereas 27 (75%) had been treated preoperatively with octreotide LAR (7 complete responders, 4 partial responders and 16 non-responders). Median SSTR5 normalized mRNA copy number was higher than that of SSTR2 (SSTR5 63.5 [IQR 1–536] vs. SSTR2 8 [IQR 0.9–29], p=0.03), when the whole group was analyzed together. When analyzed separately, SSTR5 mRNA predominance remained statistically significant in octreotide treated but not in octreotide naïve patients (Supplementary Table 2).
Of the 9 subjects not receiving preoperative octreotide, SSTR2 expression was dominant in three and SSTR5 in other three. Among the octreotide responders, the proportion of subjects predominantly expressing SSTR2 and SSTR5 was 27.2% and 54.4%, respectively; in 3 patients SSTR2 and SSTR5 were equally expressed, while no SSTR mRNA expression was detected in one case in which RNA was considerably degraded. Among the 16 patients who did not respond to octreotide, the proportion of subjects predominantly expressing SSTR2 and SSTR5 was 18.75%, and 68.75%, respectively; one patient expressed equal amounts of SSTR2 and SSTR5 mRNA. The proportion of patients expressing predominantly SSTR5 was higher among non-responders than among responders, although the difference did not reach statistical significance (octreotide responders 54.5% vs. non-responders 68.75%, p=0.45). SSTR2 tended to be the predominant receptor subtype in 27.2% of responders and in 18.75% of non-responders (p=0.09). Neither SSTR2 nor SSTR5 mRNA expression correlated with baseline and post-octreotide GH, baseline and post-octreotide IGF-1, or tumor volume (Table 2). The relative SSTR5 to SSTR2 mRNA expression was not different between octreotide responsive and octreotide resistant patients.
Immunohistochemistry-RT/PCR concordanceIHC and RT/PCR were both carried out in 13 tumors, two from untreated patients and 11 from patients who received preoperative octreotide LAR (5 responders and 6 non-responders). No significant correlations were found between protein and mRNA expression of neither of the receptor subtypes (SSTR2 r=0.07, SSTR5 r=0.19, NS). The two methods were concordant in 10 (77%). SSTR5 was the predominant receptor subtype according to both mRNA and protein levels in the two octreotide naïve subjects (100%), in 5 out of 6 octreotide non-responders (83.3%) and in 3 out of 5 octreotide responders (60%). In two of the three subjects with mRNA-protein discordance (one octreotide responsive and one octreotide resistant), SSTR5 predominated by IHC and SSTR2 by RT/PCR. The remaining discordant patient, an octreotide responder, showed SSTR5 predominance by RT/PCR but by IHC, SSTR2 and SSTR5 were equally expressed.
DiscussionIn the present study we evaluated a large number of patients with acromegaly, of whom two-thirds had received preoperative treatment with octreotide-LAR for 3–6 months while one-third had not. We found SSTR5 to be the more frequently and abundantly expressed somatostatin receptor subtype in all studied tumors, both by IHC and by RT/PCR, but we found no significant correlations between any of the two receptor subtypes and the response to octreotide LAR treatment. It has been suggested that preoperative SSA treatment may modify receptor expression.21–24 More specifically, SSTR2 protein expression but not mRNA is reduced in tumors from patients who received octreotide prior to surgery, perhaps reflecting the presence of ligand-induced internalization of the receptor but not transcription down-regulation.23 In our study, both octreotide naïve and octreotide-treated patients had the same expression pattern of SSTR2 and SSTR5 by IHC. However, the clear predominance of SSTR5 over SSTR2 mRNA expression was found only in the octreotide-treated subjects. Taken together, our results agree with the previously mentioned studies.
Although SSTR5 predominance has been consistently reported in earlier studies evaluating somatostatin receptor subtype expression in GH-secreting adenomas,18,20,24 recent investigations seem to have neglected the study of this receptor, focusing mostly on SSTR2.21–22 A positive correlation between SSTR2 expression and the response to SSA has been found at both mRNA and protein levels.17–18,21–23 A high sensitivity and negative predictive value between SSTR2 protein expression and biochemical response to SSA have been recently reported.21–22 Yet, SSTR2 expression specificity and positive predictive value are considerably low.15–16 The latter arises from a large number of SSA-resistant patients whose tumors express large amounts of SSTR2. Thus, although SSTR2 expression appears to be necessary in order to achieve a pharmacological response to SSA, in a large number of patients it is not sufficient.
The discrepancy between our results and the aforementioned studies may result from a variety of factors. Studies evaluating the relationship between SSTR expression and SSA response have included diverse experimental designs, have used different criteria for pharmacological response and have variably included patients with and without preoperative treatment with SSA.17–23 IHC studies have used different antibodies against the SSTRs and immunoreactivity has been graded by different methods.19–23 Although we did not use the recently introduced monoclonal antibodies against SSTR2, the IHC technique was rigorously controlled and validated. Furthermore, the quantification of the individual immunostaining scores in the TMA was established by an automated technique which is less susceptible to the inherent observer bias than the visual interpretation performed by one or more pathologists. The antibodies we used for SSTR2 and SSTR5 IHC are targeted against the carboxyl terminus of the molecule, which is located in the cytoplasm. This is in contrast to other studies that use antibodies that predominantly detect membrane-bound receptor. Since these antibodies may be tracking receptors downstream from signaling, perhaps in the process of being recycled, they may not reflect the actual pool of receptors to bind to SSA.
In regards to the criteria for pharmacological response, one study has reported SSTR2 mRNA expression to correlate with IGF-1 decrement upon SSA treatment, but not with the achievement of GH control target; no data are provided concerning receptor expression and achievement of combined GH-IGF-1 therapeutic goals.18 Among the three most recently published studies evaluating SSTR2 protein expression with a highly specific monoclonal antibody,21–23 only one evaluates SSA response defined according to combined GH and IGF-1 targets.22 This study by Wildenberg et al. reports that among the 54 patients with high to moderate immunostaining scores for SSTR2, only 21 [38%] met the Cortina Consensus criteria for biochemical control.22 In our work, considering biochemical response as the achievement of both IGF-1 and GH targets, we could not find a difference in the SSTR2 or SSTR5 mRNA level between octreotide responsive and octreotide resistant patients. SSTR2 was the predominantly expressed receptor subtype by IHC in almost one-third of the octreotide responsive patients, while in none of the octreotide resistant patients. SSTR5 mRNA and protein expression tended to predominate more in non-responders than in responders; however, this did not reach statistical significance.
A consistent finding among studies evaluating SSTRs in pituitary adenomas by RT/PCR, including our own, has been the wide variability of mRNA expression, even after correction with a normalization factor derived from simultaneously amplifying different house keeping genes.17–18,22 In this regard, it must be emphasized that RT/PCR yields only partially quantitative results of the mRNA copy number. Thus, the absence of statistically significant correlations between IHC and RT/PCR data is not surprising.21,27 Among the 13 patients in our study in whom both IHC and RT/PCR data were available, concordance rate between the two methods was 77%. To our knowledge, only one study has evaluated SSTR2 expression at both mRNA and protein levels in patients with GH-secreting adenomas21; it reports a high concordance between the two methods but does not provide a concordance rate. Furthermore, only 3 of the 7 patients in this study who normalized IGF-1 after octreotide treatment had their SSTR2 mRNA expression analyzed.21
The response to SSA by GH-secreting adenomas depends on a complex array of mechanisms, and not only on the density of receptor expression at the cell membrane.24,28 A low Ki-67 proliferative index has been associated with a favorable response to SSA29–30 and, not unexpectedly, appears to be related to a densely granulated tumor phenotype as determined by both electron microscopy31 and CAM5.2 cytokeratin immunostaining.30,32 Somatostatin receptors are known to homo- and heterodimerize, and this phenomenon affects receptor signaling, internalization and recycling.28,33–34 Elegant in vitro studies using confocal microscopy have shown that although both SSTR2 and SSTR5 bind octreotide with high affinity, the internalization of the octreotide-SSTR2 complex is more rapid and efficient than that of the octreotide-SSTR5 complex.35 The latter is paralleled by a greater biological effect in terms of cAMP inhibition and ERK activation.35 The existence of two splice variants of the SSTR5 gene, encoding truncated but functional proteins with 4 and 5 transmembrane domains (SSTR5TMD4 and SSTR5TMD5, respectively), has been demonstrated.36 Both variants appear to be located at intracellular compartments, and although they are notoriously absent in normal pituitary tissue, SSTR5TMD4 is abundantly expressed in pituitary tumors, particularly somatotrophinomas and non-functioning pituitary adenomas.36 In somatotrophinomas SSTR5TMD4 expression positively correlates with SSTR5 but not with SSTR2 expression. The finding of a statistically significant negative correlation between the expression of SSTR5TMD4 and the GH response to octreotide in vivo and with the in vitro GH inhibitory response to a SSTR5-selective agonist suggests that the concomitant presence of SSTR5 and its truncated splice variant could interfere with the pharmacological response to SSA.37 The possibility that the SSTR5 antibody used in our study could be detecting SSTR5TMD4 is practically excluded since it is directed against carboxy terminus epitopes located distally from the truncation site, and therefore identifies only the full length SSTR5. Based on this in vitro evidence and the available in vivo data, we could speculate that SSTR5 acting in concert with its truncated splice variant may exert a regulatory role modulating the effect of somatostatin and SSA, sometimes inhibiting and sometimes promoting signaling and biological effect.38 Although D2R expression has not been positively associated with the pharmacological response to SSA, D2R/SSTR2 heterodimers have been shown in vitro to potentiate the GH inhibitory effect of SSA, perhaps explaining the benefit of adding cabergoline to SSA-resistant patients in reducing GH secretion.28,38
In conclusion, our results underscore the complexity of the interaction between SSA and its receptors. The mere expression of these receptors does not guarantee an adequate pharmacological response; rather, other not fully characterized functional aspects of this interaction, such as receptor homo- and heterodimerization, and the resulting signaling cascade, probably play a role in determining whether a patient with acromegaly will respond or not to these agents.
Conflict of interestThe authors have nothing to declare.
FundingFondo de Investigación en Salud, Instituto Mexicano del Seguro Social número 3601.