Candida auris is a yeast that was first described in Japan in 2009 from an exudate of the ear and which, in recent years, for unknown reasons, has emerged simultaneously in several continents.1–3 Therefore, health alerts have been issued in different countries due to its resistance to multiple antifungals.4,5
Recently, at the Hospital La Fe in Valencia (Spain), the first cases in Europe were described of nosocomial fungemia caused by C. auris.6 The objective of this letter is to communicate one case of candidemia and one of colonisation by C. auris in another Spanish hospital, geographically very far from the other, and with the peculiarity that in our centre the characteristics of transmissibility and virulence of this emerging pathogen did not coincide totally with those previously described in the literature.
In October 2016, a yeast was isolated in the blood cultures, catheter tip and urine of a patient admitted to the intensive care unit (ICU) of the Hospital Universitario Río Hortega of Valladolid, who presented with seizures during the course of a pneumonic process. The growth in chromogenic medium ID (bioMérieux) showed a colour similar to that of Candida parapsilosis. When trying to identify it through mass spectrometry (Vitek® MS MALDI-TOF, bioMérieux), although a quality spectrum was obtained, no results were obtained, since this yeast was not included in the database of the version used. Identification with the Vitek® 2 system (bioMérieux) was also attempted, with this Candida haemulonii with a 95% reliability. The strain was sent to the Microbiology Department of the Hospital Universitario La Paz in Madrid, where, with MALDI-TOF Bruker®, C. auris was obtained. However, the score was unsatisfactory (1.4). This identification was repeated several times using cultures of different ages (24 and 48hours). Since neither of the two mass spectrometry systems provided adequate reliability, the strain was sent to the National Microbiology Centre for study by molecular methods.
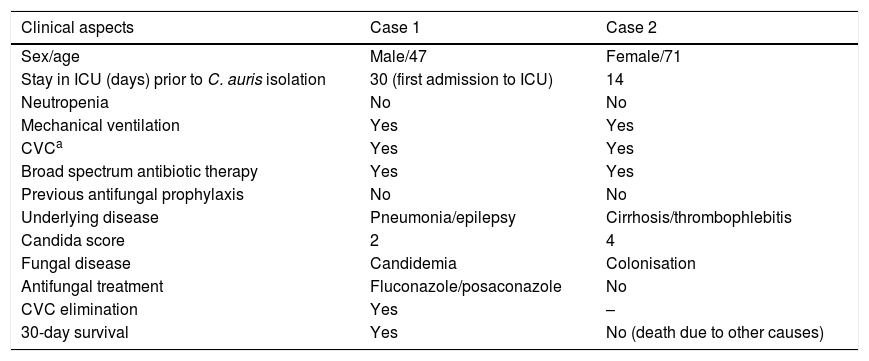
Six weeks after the detection of the first isolates, a yeast of similar characteristics was isolated in a surveillance sample (axillary smear) of a patient also admitted to the ICU. It also could not be identified with Vitek® MS, and the Vitek® 2 system identified it as C. haemulonii. The strain was resent to the National Reference Centre and to the Hospital Universitario La Paz in Madrid. In this latter centre, the MALDI-TOF Bruker® system corroborated the isolate as C. auris yet again. This second patient presented with liver cirrhosis of autoimmune origin and had already died due to liver failure and septic shock when the yeast grew in the surveillance culture. Finally, at the National Microbiology Centre, the identification of both isolates was confirmed by sequencing as C. auris.Table 1 summarises the clinical characteristics of both patients.
Clinical characteristics of patients with isolations of Candida auris.
| Clinical aspects | Case 1 | Case 2 |
|---|---|---|
| Sex/age | Male/47 | Female/71 |
| Stay in ICU (days) prior to C. auris isolation | 30 (first admission to ICU) | 14 |
| Neutropenia | No | No |
| Mechanical ventilation | Yes | Yes |
| CVCa | Yes | Yes |
| Broad spectrum antibiotic therapy | Yes | Yes |
| Previous antifungal prophylaxis | No | No |
| Underlying disease | Pneumonia/epilepsy | Cirrhosis/thrombophlebitis |
| Candida score | 2 | 4 |
| Fungal disease | Candidemia | Colonisation |
| Antifungal treatment | Fluconazole/posaconazole | No |
| CVC elimination | Yes | – |
| 30-day survival | Yes | No (death due to other causes) |
We investigated retrospectively, from the month of admission of the first patient until the appearance of the second case, the rare Candida species identified by the Vitek® 2 system in our laboratory, and two other isolates from surveillance cultures were found, the result of which was C. haemulonii. Both belonged to patients admitted to the ICU, had been detected before the last case and were no longer hospitalised, so the identifications could not be confirmed, since the strains had not been stored. However, it is possible that both isolates were mistakenly taken as C. haemulonii and that they were actually two other cases of colonisation by C. auris. This assumption is based on the fact that the Vitek® MS system (MALDI-TOF) did not identify the yeasts and therefore the Vitek® 2 card was used, so the sequence of events was the same as in the two confirmed cases.
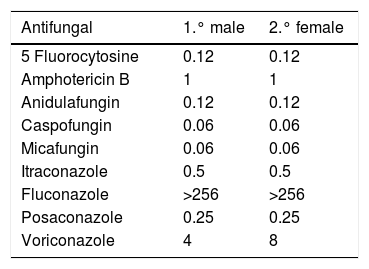
The determination of antifungal sensitivity of the two strains was carried out by the marketed broth microdilution method (Sensititre® Yeast One). The reading was made by changing the colour after 24hours of incubation. The results are presented in Table 2. Of note are the high MICs of fluconazole obtained with respect to both strains (at present no cut-off points have been established by the Clinical and Laboratory Standards Institute or by the European Committee on Antimicrobial Susceptibility Testing for C. auris).
The first patient presented candidemia probably associated with the central catheter and was empirically treated with fluconazole for two days. Despite the resistance of the yeast to fluconazole, the clinical response was satisfactory, probably because the catheter was also removed. Subsequently, at discharge and for eleven days, posaconazole was administered orally. There were no positive blood cultures, since the candidemia cleared up when the central line was removed. The empirical antifungal treatment was established by clinicians of the Neurology Department, and when the final diagnosis was made, it had been days since the patient had received the medical discharge. After three months, the patient went to a follow-up consultation for his epilepsy. He was in good general condition and was given a new appointment for a six-month follow-up.
After seven months since the last case, no other case has been detected, yeasts with similar characteristics have not been isolated, and the incidence of candidemia has not increased. Likewise, in the clinical samples and in surveillance samples, none of the species that can be confused with C. auris (C. haemulonii, Candida famata, Candida sake, Rhodotorula glutinis or Saccharomyces cerevisae) have been identified. In this period, only one case of Candida guillermondii was detected in a surveillance culture (also included in the Candida species that can lead to erroneous identification5), a result obtained with Vitek® MS (MALDI-TOF) with a reliability of 99.9%. Both the phenotypic characteristics of the culture and the proteomic technique have ruled out C. auris, and according to previous references, MALDI-TOF is a reliable method for identification of this yeast.4,5,7,8
In the literature, the described cases of hospital outbreaks caused by C. auris have lasted for months despite the measures adopted to eradicate them.7,8 The mode of transmission of C. auris is unknown, although it is confirmed that it is isolated in the environment of the affected patient, that it forms biofilms and that outbreaks subside or decrease when the control measures are increased (hand hygiene, cleaning of the environment, etc.).3,4,7,9,10 In our centre, we have not been able to learn the reason why only two cases have been detected to date, especially considering that no additional control measures have been implemented.
The surveillance from the Microbiology laboratory should be based on the suspicion of yeasts that cannot be identified by proteomics or that do so as C. haemulonii or other species (C. famata, C. sake, S. cerevisae, etc.) with a low score.6 In our case, these suspicious strains were not identified by Vitek® MS. In addition to the phenotypic characteristics of the colony, the sensitivity profile to fluconazole is also helpful for the diagnosis, since the MICs of this antifungal agent against C. auris are generally very high. Based on our findings, it may be the case that the presence of this yeast is underdiagnosed, that it may not always cause outbreaks and that our case (low transmissibility and virulence) is not unique.
When the laboratory does not have a mass spectrometry system that includes C. auris in its database, it is crucial to send the strain to a reference centre for proper identification. The molecular techniques that use sequencing are useful for the identification of these yeasts, although they delay the diagnosis. The methods based on biochemical tests (API 20 C, Vitek® 2, etc.) do not provide conclusive results and are currently not recommended, although they could be used for presumptive identification in the absence of other confirmation systems. Therefore, the use of proteomic methods seems the most appropriate for the health care field because of its speed,4,5,7,10 but when the confidence level is low, sequencing is essential.
It would be desirable to establish reliable surveillance and identification protocols to define the dissemination of this pathogen in the health care setting of our environment.
To Rut Oneizat Cortijo.
Please cite this article as: Viñuela-Sandoval L, Falces-Romero I, García-Rodríguez J, Eiros-Bouza JM. Candidemia y colonización por Candida auris, un reto diagnóstico. Enferm Infecc Microbiol Clin. 2018;36:253–255.