The spread of multidrug-resistant Enterobacteriaceae related to the production of extended-spectrum β-lactamases and carbapenemases is a serious public health problem worldwide. Microbiological diagnosis and therapy of these infections are challenging and controversial.
Clinically relevant questions were selected and the literature was reviewed for each of them. The information from the selected articles was extracted and recommendations were provided and graded according to the strength of the recommendations and quality of the evidence. The document was opened to comments from the members from the Spanish Society of Infectious Diseases and Clinical Microbiology, which were considered for inclusion in the final version.
Evidence-based recommendations are provided for the use of microbiological techniques for the detection of extended-spectrum β-lactamases and carbapenemases in Enterobacteriaceae, and for antibiotic therapy for invasive/severe infections caused by these organisms. The absence of randomised controlled trials is noteworthy; thus, recommendations are mainly based on observational studies (that have important methodological limitations), pharmacokinetic and pharmacodynamics models, and data from animal studies. Additionally, areas for future research were identified.
La diseminación de Enterobacteriaceae multirresistentes en relación con la producción de β-lactamasas de espectro extendido y carbapenemasas es un importante problema de salud pública en todo el mundo. Tanto el diagnóstico microbiológico como el tratamiento de estas infecciones son complicados y controvertidos.
Los autores seleccionaron preguntas clínicamente relevantes, realizándose una revisión de la literatura para cada una de ellas; se obtuvo información de los artículos seleccionados y se realizaron recomendaciones que se clasificaron de acuerdo con la fuerza de la recomendación y la calidad de la evidencia. El documento estuvo abierto para los comentarios de los socios de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica, los cuales se consideraronpara su inclusión en la versión final.
Se proporcionan recomendaciones basadas en la evidencia para el uso de técnicas microbiológicas cara a la detección de β-lactamasas de espectro extendido y carbapenemasas en Enterobacteriaceae, y para el tratamiento antimicrobiano de las infecciones graves o invasivas causadas por estos microorganismos. Es llamativa la ausencia de ensayos aleatorizados, por lo que las recomendaciones se basan principalmente en estudios observacionales que tienen importantes limitaciones metodológicas, modelos farmacocinéticos y farmacodinámicos, y datos de estudios en animales. Además, se identificaron áreas prioritarias para la investigación futura.
The dramatic worldwide increase in the rate of infections due to Enterobacteriaceae showing resistance to several first-line antimicrobial families in most countries over the last decade1,2 is recognised as a public health crisis.3 The very limited therapeutic options available for these organisms are a real challenge. While several initiatives are being developed to facilitate the discovery and development of new antimicrobial agents and even non-antibiotic strategies for fighting infections due to multidrug-resistant (MDR) and extremely drug-resistant (XDR) organisms,3–6 the most urgent question to answer is what is the best available treatment for patients suffering these infections. Infections due to MDR Enterobacteriaceae are associated with increased mortality compared with their susceptible counterparts, which is mainly related to the intrinsic difficulties of therapy of MDR isolates.7,8 To the best of our knowledge, evidence-based guidelines with evidence-based recommendations on the treatment for infections caused by MDR and XDR Enterobacteriaceae have not been published.
The main objective of this guideline is to provide evidence-based recommendations for the microbiological diagnosis and treatment of invasive infections caused by MDR and XDR Enterobacteriaceae, and specifically those producing the most epidemiologically and clinically important mechanisms of resistance. Additionally, areas for future research are identified.
This guideline is intended to be useful for all clinical microbiologists, for clinicians in direct charge of patients with the infections covered, and for consultants such as infectious diseases specialists, clinical microbiologists, hospital epidemiologists, and pharmacists, as well as policy makers in the field of antibiotic stewardship and quality-of-care professionals. It is the intention of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) to review these guidelines in 2016 or before in case of substantial changes in evidence.
MethodologyThis guideline was committed by SEIMC to a multidisciplinary group of Spanish clinicians and clinical microbiologists expert in the field. The authors selected a number of clinical questions by consensus based on their perceived clinical importance. Then a systematic review of the literature was performed in PubMed for each of them. The abstracts of the selected articles were read, and those referring to the questions were selected for full review. References from these articles were also considered.
Because some of these pathogens may cause mild, non-invasive infections such as cystitis, the guideline is focused on invasive infections as described below, whatever their source. The document only targets microbiological diagnosis and antimicrobial therapy; therefore, other aspects of management of infection are excluded. Infections caused by bacteria other than Enterobacteriaceae are not considered. Only adult patients with these infections are covered. For this guideline, invasive infections are defined as focal or generalised infections causing a systemic inflammatory response syndrome (sepsis), including bacteraemic infections, needing hospital admission and/or intravenous antibiotic therapy.
Some of the mechanisms of resistance affecting broad-spectrum antibiotics in Enterobacteriaceae may cause heterogeneous level of resistance to some of these drugs, including low-level resistance or diminished susceptibility. Current susceptibility breakpoints are based on the concept that, given the pharmacokinetic/pharmacodynamic (PK/PD) properties of an antibiotic and the dose at which it is administered, it is the minimum inhibitory concentration (MIC) what is relevant for therapeutic decisions rather than the underlying mechanism of resistance.9–11 However, this is controversial; some authors consider that doubts about the precision of MIC determination, the potential different levels of expression of resistance genes in vivo, the inoculum effect, and further induction of resistance in bacteria when exposed to antimicrobial agents may need to be considered.12 Both aspects (MIC and mechanism of resistance) will be considered in this guideline according to available evidence. Definitions for MDR and extensively drug-resistant (XDR) organisms have been recently published13 and will be used here. Such definitions do not correlate with the presence of the most important mechanisms of resistance; however, all extended-spectrum β-lactamase (ESBL), AmpC or carbapenemase-producing Enterobacteriaceae are at least MDR according to those definitions.
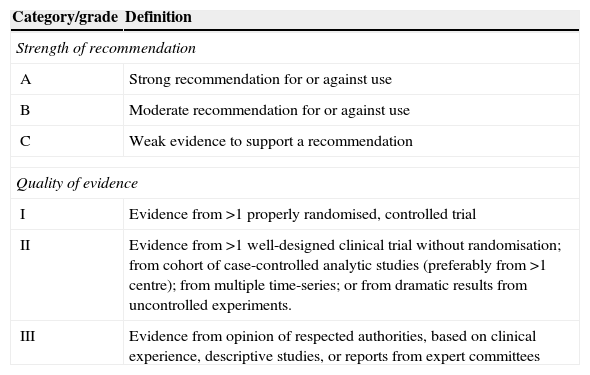
The data from each article related to each questions addressed were extracted using structured forms. For each topic, the quality of evidence and the strength of recommendations were evaluated and decided by the authors according to the methodology previously used by the Infectious Diseases Society of America (Table 1).14
Strength of recommendations and quality of evidence, modified from IDSA.
| Category/grade | Definition |
|---|---|
| Strength of recommendation | |
| A | Strong recommendation for or against use |
| B | Moderate recommendation for or against use |
| C | Weak evidence to support a recommendation |
| Quality of evidence | |
| I | Evidence from >1 properly randomised, controlled trial |
| II | Evidence from >1 well-designed clinical trial without randomisation; from cohort of case-controlled analytic studies (preferably from >1 centre); from multiple time-series; or from dramatic results from uncontrolled experiments. |
| III | Evidence from opinion of respected authorities, based on clinical experience, descriptive studies, or reports from expert committees |
The document was available to all SEIMC members during 4 weeks for their comments and suggestions, which were considered by the authors to write the final version.
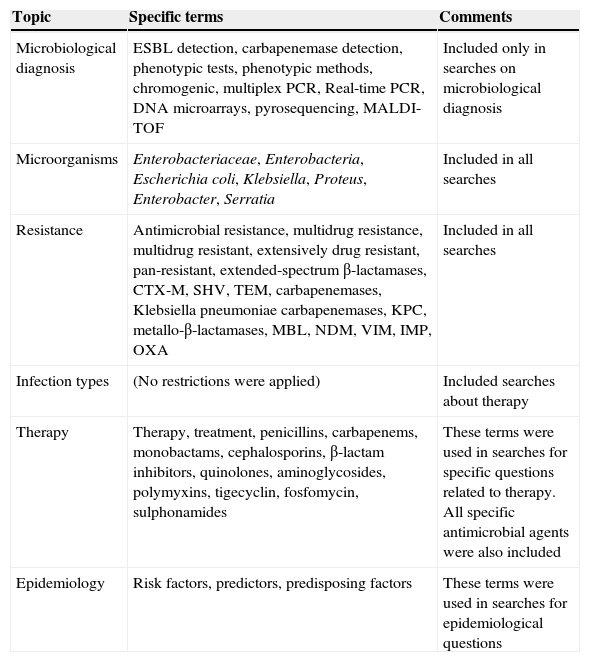
Microbiological diagnosisThe document will focus on ESBL- and carbapenemase-producing Enterobacteriaceae because these were considered the most relevant resistance mechanisms from both clinical and epidemiological points of view. The terms used in the literature review are specified in Table 2. No universal phenotypic or genotypic method exists which precisely embrace all ESBLs or carbapenemases types. The selected method to use will depend on the sample (surveillance or clinical), local prevalence, microorganism, resistance phenotype and resources.
Terms used in the literature searches.
| Topic | Specific terms | Comments |
|---|---|---|
| Microbiological diagnosis | ESBL detection, carbapenemase detection, phenotypic tests, phenotypic methods, chromogenic, multiplex PCR, Real-time PCR, DNA microarrays, pyrosequencing, MALDI-TOF | Included only in searches on microbiological diagnosis |
| Microorganisms | Enterobacteriaceae, Enterobacteria, Escherichia coli, Klebsiella, Proteus, Enterobacter, Serratia | Included in all searches |
| Resistance | Antimicrobial resistance, multidrug resistance, multidrug resistant, extensively drug resistant, pan-resistant, extended-spectrum β-lactamases, CTX-M, SHV, TEM, carbapenemases, Klebsiella pneumoniae carbapenemases, KPC, metallo-β-lactamases, MBL, NDM, VIM, IMP, OXA | Included in all searches |
| Infection types | (No restrictions were applied) | Included searches about therapy |
| Therapy | Therapy, treatment, penicillins, carbapenems, monobactams, cephalosporins, β-lactam inhibitors, quinolones, aminoglycosides, polymyxins, tigecyclin, fosfomycin, sulphonamides | These terms were used in searches for specific questions related to therapy. All specific antimicrobial agents were also included |
| Epidemiology | Risk factors, predictors, predisposing factors | These terms were used in searches for epidemiological questions |
Depending on the goal to achieve, screening or confirmatory, different tests can be used.
Screening. For surveillance samples, the use of chromogenic media, designed for rapid detection and identification of ESBL-producing Enterobacteriaceae, is probably the best option. Although there are scarce comparative studies, Brilliance ESBL agar® (Thermofisher, UK) and ChromID ESBL® (bioMérieux, France) seem to offer similar sensitivity and specificity for this purpose.15 These media reduce the need for bacterial identification in comparison with non-chromogenic selective media. Their most important limitation is the lack of specificity due to growth of AmpC-overproducing organisms (11–44%), K1/OXY-overproducing Klebsiella oxytoca and OXA-30-producing Escherichia coli.15 Other selective media such as MacConckey agar supplemented with cefotaxime or ceftazidime (1–2mg/L) had been used.16
There is no agreement about the best antimicrobial indicator and cut-off values for screening of ESBL in Gram-negative isolates both from clinical and surveillance samples. Cefpodoxime offers higher sensitivity than cefotaxime, ceftriaxone or ceftazidime. Nevertheless, the use of ceftazidime along with cefotaxime (see MIC and zone diameter breakpoints at specific documents following the European Committee on Antimicrobial Susceptibility Testing [EUCAST]9 and Clinical Laboratory Standards Institute [CLSI] criteria10) or ceftriaxone is still recommended because of their higher specificity.17
Confirmation. Phenotypic ESBL confirmation is evaluated by ESBL inhibition by clavulanic acid.17 This has limitations since some isolates can contain other resistance mechanisms than can mimic an ESBL (SHV-1 or K1 overproduction, Klebsiella pneumoniae-carbapenemase [KPC] carbapenemases) or mask the presence of ESBL (porin loss or AmpC-co-producers).16 Inhibition by clavulanic acid may be studied by a double-disk synergy test (DDST) or by the combined-DDST (CDDST), which uses disks containing cephalosporins and clavulanic acid.10 The activity of cephalosporins with or without clavulanic acid can be also evaluated by microdilution (3-dilutions difference)10; many automatic or semiautomatic susceptibility methods offer this possibility. The chromogenic Cica-Beta-Test® (Kanto Chemical, Japan) requires further evaluation for ESBL confirmation.18 ESBL Etest® strips are less efficacious and more expensive than CDDST since MIC values of cephalosporins in some ESBL-producing isolates can be out of the range of the strip19 or yield false positive results.19 For ESBL confirmation, the best option is probably the use of CDDST with cefotaxime, ceftazidime, and cefepime. In AmpC co-producers the best method to confirm ESBL production is CDDST assay in agar containing cloxacillin17 or the use of commercially available cefotaxime and ceftazidime disks containing 3-aminophenylboronic acid (APBA).16,20,21 However, both methods require further evaluation.
Which phenotypic methods should be used to detect carbapenemase enzymes?Screening. For surveillance samples the use of chromogenic media is also probably the best option. CHROMagar KPC® (Hylabs, Israel) detects bacteria only with high-level resistance to carbapenems, but has poor sensitivity with low level of carbapenem resistance displayed by OXA-48-producers and some metallo-β-lactam (MBL) producers.15 CRE Brilliance® (Thermofisher) detects KPC and MBL but not all OXA-48 producers.22 Recently, an in-house medium containing ertapenem, cloxacillin and zinc named SUPERCARBA® has been described; it seems to improve the sensitivity for all types of carbapenemases including OXA-48.22,23 ChromID OXA-48 medium (bioMérieux, France) is specific for detection of OXA-48 producers24. Also, chromID CARBA SMART (bioMérieux) is a media bi-plate that combines of ChromID CARBA (to detect KPC and MBL) on one side and ChromID OXA-48 media on the other but probably need further evaluation.
Detection of carbapenemase production in isolates based only on MIC values has low sensitivity and specificity. According to EUCAST and CLSI guidelines9,10 carbapenemase detection is only recommended for epidemiological purposes, although many authors disagree with this recommendation because of the scanty clinical data on the efficacy of carbapenems against carbapenemase-producing isolates with low MIC values and the low intermethod reproducibility for carbapenem MIC determination.22 Detection of carbapenemase activity has been recommended on any enterobacteria yielding MIC >0.12μg/ml or <25mm for ertapenem and/or meropenem and/or >1μg/ml or <23mm for imipenem (imipenem should not be used for genera Proteus spp., Providentia spp., and Morganella spp.).16 If disks are used, a zone diameter of <23mm for imipenem and/or meropenem and/or <25mm for ertapenem would suggest the presence of a carbapenemase. Ertapenem has low specificity because of high MIC in some ESBL and/or AmpC producers; specifically, ertapenem should not be used as a marker for carbapenemase in Enterobacter spp.25
Confirmation. The clover leaf method or modified Hodge test (MHT) is still used in many laboratories and it has been the only method of detection so far recommended by the CLSI.10 The MHT is still used in many laboratories; it is time-consuming, offers a low sensitivity for some enzymes (OXA-48) and species as Enterobacter and it does not distinguish among different classes of carbapenemases.17 Several phenotypic methods based on the inhibitory capacity of several compounds may provide information with respect to the molecular class of the carbapenemase. For instance, boronic acid compounds have shown availability to inhibit not only class C enzymes but also KPC and others class A carbapenemases26; cloxacillin inhibition may help to differentiate class A carbapenemase (no inhibition for cloxacillin) with respect to other mechanisms that also confer carbapenem resistance such as AmpC production plus reduction in permeability. To differentiate between classes A and C enzymes, others inhibitors such as chelating agents including ethylene diamine tetra-acetic acid (EDTA), 2-mercaptopropionic acid (2-MPA) or dipicolinic acid (DPA) can be used for inhibition-based MBL confirmation by DDST (double-disk-synergy test) or CD (combined-disk) methods. Besides, 30μg temocillin disk can be added to differentiate OXA-48 producers (zone diameter ≤10mm).27 A new test based on in vitro hydrolysis of imipenem named Carba NP® has been described.28 Hydrolysis of imipenem is detected by colorimetric measurement of changes in pH value. This rapid method (<2h) yielded high sensitivity and specificity and has the capability of detecting carbapenemases belonging to Ambler classes A, B and D. Although promising, further evaluation is still needed. Nevertheless, regardless to the phenotypic method used, genetic confirmation is usually required for accurate confirmation of carbapenemase production.
Other methods such as spectrophotometric measurement of carbapenem hydrolysis are considered to be the reference standard methods for detection of carbapenemase production although they should be performed in reference laboratories.16
Rapid tests to detect ESBL and carbapenemase enzymes in specific situationsRapid detection of Enterobacteriaceae expressing either ESBL and/or carbapenemases-type may be very important in specific situations, such as for infection control purposes or in life-threatening infections. We consider rapid test as those providing the results on the same day; using this criteria, the spectrum of tests fulfilling this requirement is reduced to molecular (or genotypic) methods and those based on proteomic methods.
Molecular methods. Several rapid molecular approaches can be used:
- (a)
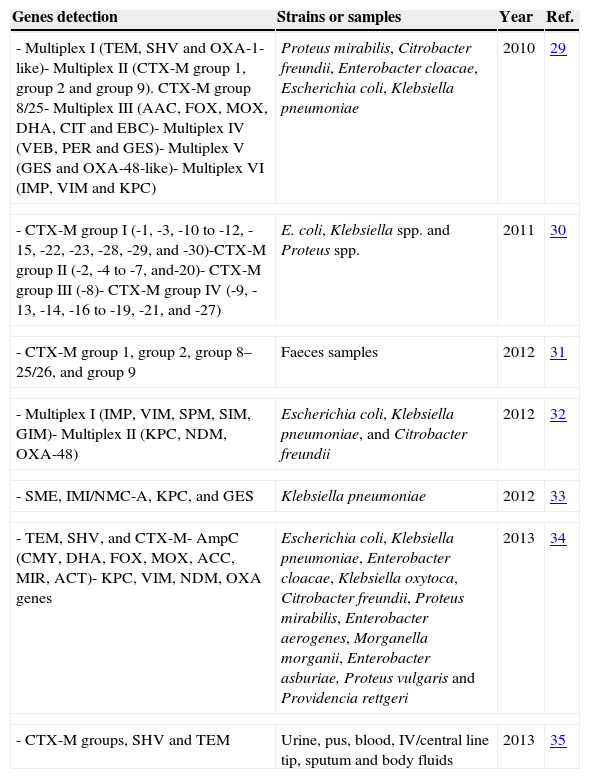
There are many published studies of the application of multiplex PCR for detection of ESBLs and/or carbapenemases in Enterobacteriaceae29–35 (Table 3). With respect to MBL, a commercial multiplex PCR (Hyplex-MBL ID Multiplex PCR-ELISA®), which was proven reliable in detecting blaVIM genes in blood, urine, exudate, and sputum samples, is available.36 Overall, these methods lack clinical validation with multicentre studies.
Table 3.Multiplex PCR for detection of the ESBLs and carbapenemases genes.
Genes detection Strains or samples Year Ref. - Multiplex I (TEM, SHV and OXA-1-like)- Multiplex II (CTX-M group 1, group 2 and group 9). CTX-M group 8/25- Multiplex III (AAC, FOX, MOX, DHA, CIT and EBC)- Multiplex IV (VEB, PER and GES)- Multiplex V (GES and OXA-48-like)- Multiplex VI (IMP, VIM and KPC) Proteus mirabilis, Citrobacter freundii, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae 2010 29 - CTX-M group I (-1, -3, -10 to -12, -15, -22, -23, -28, -29, and -30)-CTX-M group II (-2, -4 to -7, and-20)- CTX-M group III (-8)- CTX-M group IV (-9, -13, -14, -16 to -19, -21, and -27) E. coli, Klebsiella spp. and Proteus spp. 2011 30 - CTX-M group 1, group 2, group 8–25/26, and group 9 Faeces samples 2012 31 - Multiplex I (IMP, VIM, SPM, SIM, GIM)- Multiplex II (KPC, NDM, OXA-48) Escherichia coli, Klebsiella pneumoniae, and Citrobacter freundii 2012 32 - SME, IMI/NMC-A, KPC, and GES Klebsiella pneumoniae 2012 33 - TEM, SHV, and CTX-M- AmpC (CMY, DHA, FOX, MOX, ACC, MIR, ACT)- KPC, VIM, NDM, OXA genes Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Klebsiella oxytoca, Citrobacter freundii, Proteus mirabilis, Enterobacter aerogenes, Morganella morganii, Enterobacter asburiae, Proteus vulgaris and Providencia rettgeri 2013 34 - CTX-M groups, SHV and TEM Urine, pus, blood, IV/central line tip, sputum and body fluids 2013 35 - (b)
Real-time polymerase chain reaction (RT-PCR) for the detection of ESBLs and/or carbapenemases by SYBR Green is less common that using TaqMan probes. Brolund et al. developed a real-time SYBR Green PCR assay for rapid detection of acquired AmpC in Enterobacteriaceae strains.37 RT-PCR with probes genes encoding SHV-type ESBL38 and KPC carbepenemases39 have been described. Five types of class A and D enzymes (including GES, IMI/NMC, KPC, OXA-48 and SME) were rapidly detected by real-time TaqMan PCR in clinical isolates.40 More importantly, some studies evaluated real-time PCR with probes in clinical samples. Detection of blaCTX-M genes was studied in 810 urine samples and 36 ESBLs-producing Enterobacteriaceae, mostly E. coli, were found with this technique.41 In rectal samples from enrichment broth, RT-PCR for detecting KPC carbapenemase genes was compared with two selective screening agar plates (CHROMagar or VACC plates). RT-PCR showed higher sensitivity than both cultures (97% vs 77%).42 Detection of KPC carbapenemase gene by quantitative RT-PCR TaqMan was carried out in BACTEC blood culture bottles in another study.43 The sensitivity, specificity, positive and negative predictive value of this quantitative RT-PCR assay compared to the results of culture were all 100%. Also, in an outbreak situation caused by organisms producing an OXA-48-like enzyme, a RT-PCR has been used in stools samples with promising results.44 A duplex RT-PCR assay for blaKPC and blaNDM (D-PCR) performed directly on perianal and perirectal swabs and stool was compared to PCR after broth enrichment (BE-PCR) and two culture methods (HardyCHROM ESBL® agar and CDC screening). Overall, D-PCR showed excellent sensitivity when specimens with visibly stool underwent preparatory extraction.45 Also, a non-commercial multiplex PCR (with a new TaqMan probe) has been designed to detect all different allelic variants of blaKPC from easily available clinical specimens in less than 2h.46 Finally, it is worth mentioning the commercial EasyQ KPC® test (bioMérieux, Marcy l¿Etoile, France), a novel RT-PCR assay that has recently been developed for blaKPC detection which showed no false positives.47
- (c)
DNA microarrays for detection of the CTX-M, TEM and SHV genes ESBLs in Enterobacteriaceae strains have been described showing a sensitivity of 95% and specificity of 100%, using molecular characterisation of ESBLs by PCR and sequencing as reference.48 Several commercial microarrays have been studied in clinical isolates. Check-KPC ESBL® microarray (Checkpoints, Wageningen, the Netherlands), using sequencing as reference method, showed a sensitivity of 97%, a specificity of 98%, a positive predictive value of 99% and a negative predictive value of 92%.49 Also, Check-MDR CT101® microarray (Checkpoints, Wageningen, the Netherlands) has been studied for detection of the ESBLs.50,51 One of the most promising approaches is the Check-MDR CT102® microarray (Checkpoints, Wageningen, the Netherlands), aimed at identifying bacteria producing several β-lactamases such as ESBL (SHV, TEM, and CTX-M) and carbapenemases (KPC, OXA-48, VIM, IMP, and NDM-1); the test showed a sensitivity and specificity of 100% for most of the tested genes.52,53 Currently, this technology has only been used for bacterial isolates rather than clinical samples. Recently Bush et al. reported the need to include the gene of SME serine carbapenemase in the detection systems of carbapenemases arrays, especially when carbapenem-resistant Serratia marcescens isolates are suspected.54
- (d)
Pyrosequencing is a so far a promising tool based on real-time sequencing by synthesis approach that has been applied to the single-nucleotide polymorphism detection for TEM- and SHV-type ESBL identification in clinical strains.55 Further work is still needed to implement it in clinical practice.
Proteomic methods. The matrix-assisted laser desorption ionisation-time of flight mass spectrometry (MALDI-TOF MS) is a potentially useful tool for the detection of antimicrobial resistance, especially β-lactamases in Enterobacteriaceae and non-fermenting rods, with a turnaround time of 2.5h.56 Carbapenemase activity was identified in all carbapenemase-positive isolates studied, showing neither false-positive nor false-negative results.57 Moreover, MALDI-TOF MS was applied to detect ESBL-producing Enterobacteriaceae directly from positive blood culture bottle with promising results.58
TherapyWhen should empirical treatment of MDR Enterobacteriaceae be considered?It is well known that initial inappropriate antimicrobial therapy of severe infections leads to an increased morbidity and mortality.59,60 Adequate therapy of severe infections caused by ESBL, plasmid-mediated AmpC or carbapenemase-producing Enterobacteriaceae is challenging, as many of the main agents typically used for infections caused by susceptible microorganisms are inactive. This may lead to extensive use of broad spectrum antibiotics, which would contribute to selection of further resistance and may also expose patients to unnecessary toxicity. Thus, selection of patients who should receive empirical treatment covering MDR enterobacteria is important.
First, clinical scenarios in which Enterobacteriaceae are likely pathogens should be considered. Second, the risk of MDR is related to the epidemiological situation (e.g., local prevalence, including community, long term care facilities or hospital environments) and to individual factors. While genes codifying for ESBL are found in the community and hospitals, carbapenemases are still mainly found in hospitalised patients, although OXA-48 may be already disseminating in the community.61 Long term care facilities are well known as reservoirs for ESBLs, but are recently also being recognised as potential reservoirs also for carbapenemase-producing Enterobacteriaceae (CPE).62,63 The updated percentage of isolates showing resistance to fluoroquinolones, third-generation cephalosporins and aminoglycosides among invasive isolates of E. coli and K. pneumoniae in European countries can be accessed at the EARS-Net website (www.earsnet.eu); although helpful, it should be noted that these data include only bacteraemic episodes and merge community and hospital infections; also, for microorganisms which frequently cause outbreaks (such as K. pneumoniae), the data from one country may not be representative of the situation in a single hospital.
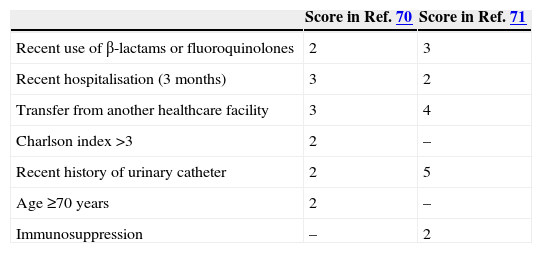
Individual risk factors have been investigated in case-control and cohort studies of patients with bloodstream infections (BSI) caused by MDR Enterobacteriaceae. Regarding ESBL-producers in community-onset infections, risk factors were previously reviewed64; several multicentre studies have been developed in Spain, including different populations65–67; finally, a multinational study was also reported.68 Of note, in spite of heterogeneity in study designs, populations and analysis, the results are quite similar across studies. The most frequently found risk factors for ESBL producers are age, healthcare-associated infection, long-term care facility admission, recurrent or obstructive urinary tract infections (UTI), urinary catheter, and previous antibiotic use (specifically, fluoroquinolones and cephalosporins). Two studies developed predictive scores for ESBL-producers. One of them, performed in Italy, included a derivation and a validation group69; the other, performed in the US, was based on the previous study data.70 Both were case–control studies, which is not the most appropriate design for developing predictive models. The variables selected and their punctuations are shown in Table 4. Anyway, a score value ≥3 in both studies showed high sensitivity (≥94% in both models) and negative predictive value but a poor specificity; our interpretation is that this cannot be used for all patients but are probably useful for patients with severe sepsis or septic shock. On the other hand, a score value ≥8 showed a high specificity (≥95% in both models) and positive predictive value with lower sensitivity, suggesting that it would be useful for not severe patients. Of note, another study challenged the predictive utility of the Tumbarello model.71 Additionally, it should also be considered that recent travel to areas in which the prevalence of these mechanisms of resistance is high is also a risk factor, such as western Asia and the Indian subcontinent.72
The risk of nosocomial infection caused by ESBL-producers varies greatly according to the local epidemiology, even to a ward level. On the basis of local epidemiology and according to studies performed in Spain, individual risk factors include previous duration of hospital stay, exposure to invasive procedures (mainly mechanical ventilation), previous antibiotics (mainly cephalosporins and fluoroquinolones) and previous colonisation with these microorganisms.73–77 Predictive scores have not been developed. Risk factors for infections caused by Enterobacteriaceae producing AmpC have been less studied.78–81 Overall, the risk factors are similar to those for ESBL-producers; previous use of antibiotics selecting for these organisms (mainly cephalosporins) is also important.
Regarding CPE, most studies investigating risk factors were performed in the context of individual hospital outbreaks; age, severity of illness, admission to intensive care unit (ICU), previous use of antibiotics (mainly carbapenems, fluoroquinolones, cephalosporins), and invasive procedures (endoscopy, length of venous catheter use) have been identified.82–90
Are carbapenems the drugs of choice for the treatment of invasive infections caused by ESBL-producing Enterobacteriaceae?Carbapenems are not affected by ESBLs and therefore, unless another mechanism of resistance affecting these drugs is expressed, ESBL-producers are fully susceptible to carbapenems.1,64,91 We did not find any randomised clinical trials comparing carbapenems with other drugs for the treatment of infections caused by ESBL-producing organisms. A meta-analysis including 21 observational studies published until January 2012 on BSI concluded that carbapenems were associated with lower mortality when compared with cephalosporins in both empirical and definitive therapies, and with lower mortality than fluoroquinolones in empirical therapy; the differences were not significant for β-lactam/β-lactam inhibitor combinations (BLBLI).92 Specific analyses by source of infection or organism (E. coli or Klebiella) could not be performed. It should be noticed that appropriate therapy was associated with reduced mortality, and that comparisons among antibiotics were not controlled by susceptibility.
We found three observational studies on BSI published after this meta-analysis in which carbapenems were observationally compared to other in vitro active drugs using multivariate analysis; in one of them, cefepime was dependently associated with increased mortality in comparison with carbapenems93; in another, patients with haemodialysis access-related bacteraemia also showed higher mortality when treated with flomoxef than with carbapenems94; and in the third one, the use of carbapenems was also associated with lower mortality compared with all other antibiotics.95 Thus, the available data suggest that carbapenems should be considered as the drugs of choice for the treatment of severe infections caused by ESBL-producing Enterobacteriaceae including bactereamic infections with the potential exception of BLBLI (discussed below), but data for specific types of infections, microorganisms or isolates showing susceptibility to other antibiotics are limited.
Which carbapenem should be used for ESBL-producers?Among carbapenems, most studies evaluated imipenem or meropenem.64,91 The available data are limited for doripenem; a recently study performed a post hoc analysis of 6 randomised clinical trials evaluating doripenem in infections caused by ESBL-producers (1 in complicated UTI [cUTI] in comparison with levofloxacin, 2 in complicated intraabdominal infections [cIAI] in comparison with meropenem and 2 in hospital-acquired pneumonia [HAP], one compared to piperacillin/tazobactam and the other in comparison with imipenem); overall, 20/30 (61.7%) of patients treated with doripenem had a favourable outcome at test of cure, similar to comparators (24/39, 61.5%).96 The numbers were too low to make comparisons with specific drugs or for specific indications.
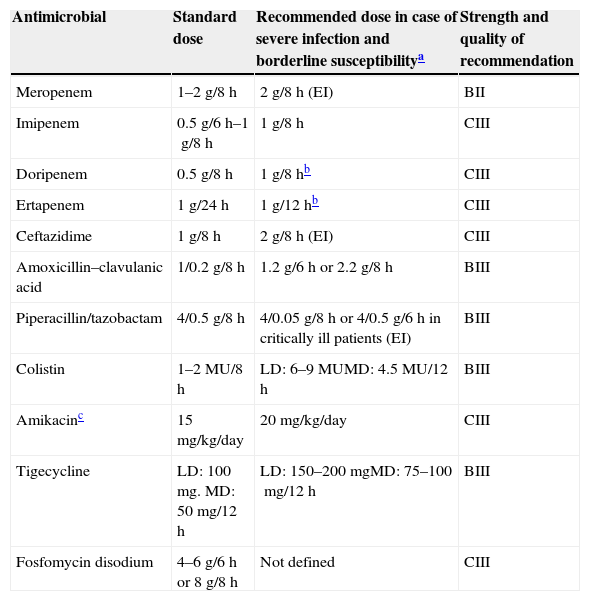
Ertapenem is an interesting alternative because it has no activity against Pseudomonas aeruginosa or Acinetobacter baumannii and thus may contribute to reduce the pressure posed by other carbapenems against these pathogens.97–100 We found 4 observational studies in which ertapenem was compared to other carbapenems in the treatment of BSI due to ESBL-producing E. coli or K. pneumoniae95,101–103; in none of them ertapenem was found to be associated with increased risk of death, either as empirical or definitive therapy. In one of those studies, mortality was higher if ertapenem MIC was ≥0.5mg/L, suggesting that present CLSI breakpoints are appropriate.103 Development of ertapenem resistance or failure during therapy of ESBL- or AmpC-producing Enterobacteriaceae has been reported anecdotally104–108; by reviewing these cases, development of ertapenem resistance seems to be related with borderline MICs and/or complex infections; whether increasing the dose of ertapenem would avoid this has not been studied in clinical trials. Ertapenem has also been used successfully in patients with ventilator-associated pneumonia (VAP)109 and as outpatient-antimicrobial therapy for UTI caused by ESBL-producers.110 Recommended dosing regimens are shown in Table 5.
Dose regimens recommended for the most frequently used drugs in the treatment of multidrug-resistant and extensively drug-resistant Enterobacteriaceae.
| Antimicrobial | Standard dose | Recommended dose in case of severe infection and borderline susceptibilitya | Strength and quality of recommendation |
|---|---|---|---|
| Meropenem | 1–2g/8h | 2g/8h (EI) | BII |
| Imipenem | 0.5g/6h–1g/8h | 1g/8h | CIII |
| Doripenem | 0.5g/8h | 1g/8hb | CIII |
| Ertapenem | 1g/24h | 1g/12hb | CIII |
| Ceftazidime | 1g/8h | 2g/8h (EI) | CIII |
| Amoxicillin–clavulanic acid | 1/0.2g/8h | 1.2g/6h or 2.2g/8h | BIII |
| Piperacillin/tazobactam | 4/0.5g/8h | 4/0.05g/8h or 4/0.5g/6h in critically ill patients (EI) | BIII |
| Colistin | 1–2MU/8h | LD: 6–9MUMD: 4.5MU/12h | BIII |
| Amikacinc | 15mg/kg/day | 20mg/kg/day | CIII |
| Tigecycline | LD: 100mg. MD: 50mg/12h | LD: 150–200mgMD: 75–100mg/12h | BIII |
| Fosfomycin disodium | 4–6g/6h or 8g/8h | Not defined | CIII |
EI, extended infusion; LD, loading dose; MD, maintenance dose.
In order to avoid the overuse of carbapenems, alternatives for the treatment of ESBL-producers are needed. Potential alternatives include BLBLI, temocillin, cephalosporins, fluoroquinolones, trimethoprim-sulphametoxazole, aminoglycosides, tigecycline, fosfomycin, and colistin.
As previously reviewed, cephalosporins are associated with higher mortality than carbapenems.92 However, most of the available data come from studies in which cephalosporins were not active according to current breakpoints.9,10 These breakpoints are supported mainly by PK/PD data111; however, available clinical data evaluating the efficacy of “active” cephalosporins in invasive infections caused by ESBL-producers are still limited and sometimes contradictory.112–117 As regards cefepime, higher mortality than carbapenem was observed considering CLSI breakpoints, but mortality was lower for isolates with MIC ≤1mg/L than for isolates with higher MIC.117 As regards cephamycins, which are typically active against ESBL producers if no other mechanism of resistance is present, there are scarce data. Two small observational studies evaluated flomoxef in comparison with carbapenems with contradictory results.118,94 Also, a retrospective cohort study showed similar efficacy of cefmetazole and carbapenems in patients with pyelonephritis due to ESBL-producers (cure rate, 9/10 vs 12/12, respectively).119
Regarding BLBLI, a post hoc analysis of prospective cohorts of patients with bloodstream infections due to ESBL-producing E. coli showed similar results for empirical or definitive therapy with in vitro active BLBLIs and carbapenems after controlling for confounders120; the sources of BSI in this study were mostly urinary and biliary tracts, and high doses were used (amoxicillin/clavulanic acid: 2/0.2g/8h; piperacillin/tazobactam, 4/0.5g/6h). The same group also assessed the importance of piperacillin-tazobactam MIC in the outcome of 39 episodes of BSI due to ESBL E. coli; all 11 patients with urinary tract infections survived, irrespective of the MIC. For other sources, 30-day mortality was lower for isolates with a MIC of ≤2mg/L than for isolates with a higher MIC (0% vs 41.1%; P=0.02).121 As explained above, a recent meta-analysis of observational studies could not find that mortality was lower in patients who received empirical or definitive therapy with carbapenem in comparison with BLBLI.92 Administration of piperacillin/tazobactam in extended infusion (4/0.5g every 8h in 3–4h) is associated with better PK/PD target achievement, and has been associated with improved outcomes.122
Temocillin, which is not commercialised in Spain, is stable against ESBLs and AmpC enzymes123; its efficacy did not seem to be affected by ESBL or AmpC-production in an observational study,124 but studies comparing temocillin with other drugs are lacking.
As stated above, empirical therapy with fluoroquinolones was associated with higher mortality than carbapenems.92 However, this may be due to the fact that ESBL-producers are frequently resistant to fluoroquinolones. Tumbarello et al. described a high mortality date (50%) in 8 episodes of BSI due to ESBL-producing Enterobacteriaceae who were treated with ciprofloxacin60; in all these patients, the ciprofloxacin MICs were in the limit of susceptibility or intermediate according to EUCAST (0.5–1mg/L).9 There is no reason to suspect a lower efficacy for the rate isolates with lower MICs.
Data on aminoglycosides, tigecycline, fosfomycin and colistin will be discussed in the specific sections addressing these drugs.
Should combination therapy be used for invasive infections caused by CPE?Resistance to carbapenems in Enterobacteriaceae is most frequently caused by the production of carbapenemases, although resistance may arise as a consequence of combined production of other β-lactamases and reduced permeability, augmented efflux, and alterations in PBP.125,126 Carbapenemases may confer low to high level resistance to carbapenems and resistance to all other commercially available β-lactams, with the exception of aztreonam in the case of metallo-β-lactamases (MBL) and oxy-imino-cephalosporins in the case of OXA-48 (but OXA-48 producers frequently co-produce ESBLs).2,126 Also, temocillin may retain partial activity against KPC-producers.127 Beyond that, most carbapenemase-producers are also resistant to quinolones and trimethoprim-sulphamethoxazole. Thus, the more frequently active drugs usually are colistin, fosfomycin, tigecycline and some aminoglycosides, but prevalence of susceptibility to these drugs is heterogeneous depending on location, genetic environment of the carbapenemase, and species.2,126–141 Most reports have revealed a high proportion of treatment failures among patients with invasive infections caused by CPE with reported mortality rates ranging from 18% to 72%.2,126,128–143 Patients in published series were treated with different regimens of monotherapy and combined therapy with variable results, which may be influenced by other prognostic factors such as age, severity of underlying conditions, severity of infection, site of infection, and administration of inappropriate antimicrobial therapy. Thus, adequate control of confounders is essential.
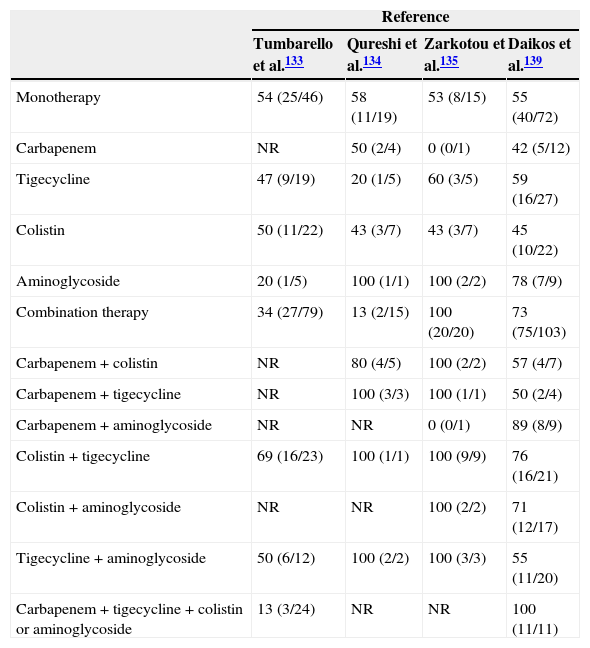
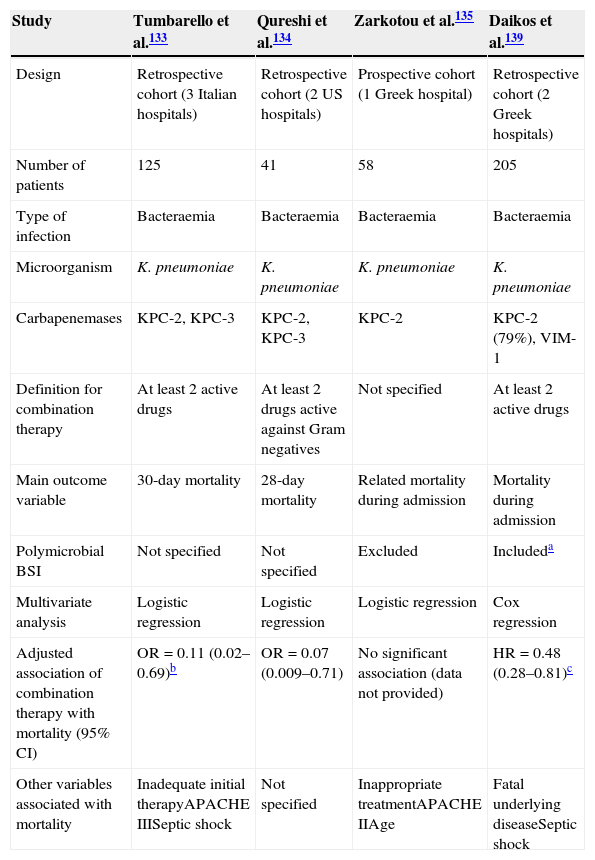
After a literature search, we could not find randomised trials comparing combination therapy with and monotherapy in infections caused by CPE. We found 4 observational cohort studies analysing the impact of combination therapy on outcome, and using multivariate analysis133–135,139; the data are summarised in Tables 6 and 7. All but one135 were retrospective studies, and all included patients with BSI due to KPC-producing K. pneumoniae (one of them also included VIM-producers139). In three of them, combination therapy was associated with lower mortality in comparison with monotherapy. In the study by Tumbarello et al. all active antimicrobials were used at high daily doses (colistin 6–9 millions of international units [MU], tigecycline 100–200mg, gentamycin 4–5mg/kg), and meropenem was administered by extended infusion at a dose of 2g every 8h. Monotherapy was used more frequently in cases with a urinary tract source whereas combination therapy was more common in ICU patients and in patients with suboptimal/borderline tigecycline and colistin MICs.133 The study by Qureshi et al. did not control for presentation with shock.134 The study by Zarkotou et al. found lower mortality among patients who were treated with combination therapy in the univariate analysis, but the multivariate analysis showed that it was appropriate therapy which had a protective effect on infection-related mortality.135 Finally, the study by Daikos et al. included the highest number of patients; polymicrobial BSI were included, although this variable was included in the multivariate analysis; combination therapy was associated with lower mortality, particularly among patients with septic shock and rapidly fatal underlying disease.139
Clinical response and/or survival rate among patients with invasive infections caused by carbapenemase-producing Enterobacteriaceae treated with different antibiotic regimens from different cohorts. Data are expressed as percentage (no. of patients who survived/no. of patients treated).
| Reference | ||||
|---|---|---|---|---|
| Tumbarello et al.133 | Qureshi et al.134 | Zarkotou et al.135 | Daikos et al.139 | |
| Monotherapy | 54 (25/46) | 58 (11/19) | 53 (8/15) | 55 (40/72) |
| Carbapenem | NR | 50 (2/4) | 0 (0/1) | 42 (5/12) |
| Tigecycline | 47 (9/19) | 20 (1/5) | 60 (3/5) | 59 (16/27) |
| Colistin | 50 (11/22) | 43 (3/7) | 43 (3/7) | 45 (10/22) |
| Aminoglycoside | 20 (1/5) | 100 (1/1) | 100 (2/2) | 78 (7/9) |
| Combination therapy | 34 (27/79) | 13 (2/15) | 100 (20/20) | 73 (75/103) |
| Carbapenem+colistin | NR | 80 (4/5) | 100 (2/2) | 57 (4/7) |
| Carbapenem+tigecycline | NR | 100 (3/3) | 100 (1/1) | 50 (2/4) |
| Carbapenem+aminoglycoside | NR | NR | 0 (0/1) | 89 (8/9) |
| Colistin+tigecycline | 69 (16/23) | 100 (1/1) | 100 (9/9) | 76 (16/21) |
| Colistin+aminoglycoside | NR | NR | 100 (2/2) | 71 (12/17) |
| Tigecycline+aminoglycoside | 50 (6/12) | 100 (2/2) | 100 (3/3) | 55 (11/20) |
| Carbapenem+tigecycline+colistin or aminoglycoside | 13 (3/24) | NR | NR | 100 (11/11) |
NR, not reported.
Studies investigating the impact of combination therapy in patients with invasive infection due to carbapenemase-producing Enterobacteriaceae; only studies using multivariate analysis are included.
| Study | Tumbarello et al.133 | Qureshi et al.134 | Zarkotou et al.135 | Daikos et al.139 |
|---|---|---|---|---|
| Design | Retrospective cohort (3 Italian hospitals) | Retrospective cohort (2 US hospitals) | Prospective cohort (1 Greek hospital) | Retrospective cohort (2 Greek hospitals) |
| Number of patients | 125 | 41 | 58 | 205 |
| Type of infection | Bacteraemia | Bacteraemia | Bacteraemia | Bacteraemia |
| Microorganism | K. pneumoniae | K. pneumoniae | K. pneumoniae | K. pneumoniae |
| Carbapenemases | KPC-2, KPC-3 | KPC-2, KPC-3 | KPC-2 | KPC-2 (79%), VIM-1 |
| Definition for combination therapy | At least 2 active drugs | At least 2 drugs active against Gram negatives | Not specified | At least 2 active drugs |
| Main outcome variable | 30-day mortality | 28-day mortality | Related mortality during admission | Mortality during admission |
| Polymicrobial BSI | Not specified | Not specified | Excluded | Includeda |
| Multivariate analysis | Logistic regression | Logistic regression | Logistic regression | Cox regression |
| Adjusted association of combination therapy with mortality (95% CI) | OR=0.11 (0.02–0.69)b | OR=0.07 (0.009–0.71) | No significant association (data not provided) | HR=0.48 (0.28–0.81)c |
| Other variables associated with mortality | Inadequate initial therapyAPACHE IIISeptic shock | Not specified | Inappropriate treatmentAPACHE IIAge | Fatal underlying diseaseSeptic shock |
Other previous publications reviewed data from previous studies on combination therapy, including case series. Hirsch and Tam reviewed 15 articles on infections caused by KPC-producing Enterobacteriaceae.140 Among the 55 cases included in the review (most of them UTIs), they found high rates of clinical success in patients who had received combination regimens including a polymyxin, or monotherapy with aminoglycosides or tigecycline. Similar results were reported by Lee and Burgess, who reviewed 38 articles comprising 101 infections (most of them BSI and respiratory infections).141 They also found higher rates of clinical success in patients who received combination regimens. Polymyxin and carbapenem monotherapy were associated with higher rates of treatment failures compared with polymyxin or carbapenem-based combination therapy. Overall treatment failures were not significantly different in the three most common antibiotic-class combinations: polymyxin plus carbapenem, polymyxin plus tigecycline or polymyxin plus aminoglycoside. Akova et al. reviewed the data from 9 studies that included 234 patients, most of them BSI (132 due to MBL-producing K. pneumoniae and 102 due to KPC-producing K. pneumoniae).142 The overall success rate of combination therapy was significantly higher than that of monotherapy. In addition, the carbapenem-containing regimens were significantly more effective than the non-carbapenem-containing regimens. Tzouvelekis et al. compiled 34 articles comprising 301 patients with infections (161 infected with KPC-producing K. pneumoniae and 140 infected with MBL-producing K. pneumoniae).2 The vast majority of these patients had invasive infections such as BSI or pneumonia. They found that the lower failure rate was observed for patients who received combination therapies including a carbapenem, and that this regimen was superior to combined therapy not including a carbapenem and to monotherapy with tigecycline or colistin. Tigecycline and colistin monotherapy resulted in failure rates comparable to that observed for patients who received inappropriate therapy. Finally, a recent systematic evaluation of antimicrobial therapy of CPE infections showed a wide clinical heterogeneity of the clinical reports that precluded the statistical analysis of the available evidence.143 Overall, the data included in these reviews have important limitations because many of the infections evaluated belong to case reports or small series of cases, with high potential for publication bias, where precise definitions of outcome were not given. In addition, the impact of antibiotic therapy on the mortality has not been adjusted for confounding factors such as the underlying comorbidities, site and severity of infection, drug dosage, or use of inappropriate antimicrobial therapy.
Finally, it is important to remember that most of the clinical data published in the literature on therapy have been related to KPC-producing K. pneumoniae, while published analysis of the experience with infections due to other carbapenemase-producers such as MBL or OXA-48 is sparse. Also, experience with specific sources of BSI is very limited. For instance, whether monotherapy with an appropriate active agent can be used in cases of BSI with a urinary tract source has not been appropriately studied. Also, there are no reasons to support that results with an active aminoglycoside in UTI would be different than for non-carbapenemase-producing organisms.
The limitations of the evidence available supporting combination therapy were highlighted in a recent systematic review and meta-analysis including studies on carbapenem-resistant Gram negatives (therefore, not only studies on Enterobacteriaceae were included, but also on P. aeruginosa or A. baumannii).144 In that study, specific combinations could not demonstrate superiority against colistin monotherapy; only when combined combinations were included together, combination therapy was superior to colistin monotherapy.
When and how should carbapenems be used for infections by carbapenemase-producing Enterobacteriaceae?Because of the very limited options for the treatment of CPE, and the fact that some CPE show low MIC of carbapenems, carbapenems are to be discussed as potential options. The MICs for carbapenemase-producing K. pneumoniae may vary within a broad range of values, from 0.12 to >256mg/L, depending on both the geographical location and the type of carbapenemase produced.2,126,128,130–132,145–150 Thus, although VIM enzymes have strong carbapenem-hydrolytic activity, some VIM-producing K. pneumoniae isolates have low carbapenem MIC.146 In contrast, the vast majority of isolates producing NDM show higher carbapenem MICs.147
Following the evaluation of MIC distributions of contemporary carbapenemase-producing isolates, PK/PD properties, and limited clinical outcome data, both EUCAST and CLSI recently revised their susceptibility breakpoints for carbapenems. EUCAST decided to set its clinical breakpoints for Enterobacteriaceae to ≤2mg/L for imipenem and meropenem, ≤1mg/L for doripenem, and ≤0.5mg/L for ertapenem,9 while CLSI reduced its previous breakpoint values to ≤1mg/L for imipenem, meropenem, and doripenem, and to ≤0.25mg/L for ertapenem.10 The results should be reported as susceptible to carbapenems irrespective of the existence of mechanism of resistance. This is a controversial issue; some authors consider that there is a higher risk of failure with carbapenems in monotherapy148,149 while lowering the breakpoints would allow the use of carbapenems in patients who may benefit from them.150
In the absence of randomised controlled trials, and because of the scarcity of clinical studies addressing this issue, we include a review of the literature for animal infection models and PK/PD studies.150–160 In relation to animal infection models, the minimum proportion of time for which free carbapenem should remain above the MIC in a dosing interval (T>MIC) was estimated to be 20–30% for a bacteriostatic activity and 40–50% for a bactericidal activity.151 Most of the studies showed a significant effect against VIM-1-producing isolates with low imipenem MICs (2–4mg/L) when imipenem was administered at high doses.150–153 Bulik et al. showed that a high dose of meropenem (2g every 8h) infused over 3h was bactericidal against KPC-producing K. pneumoniae isolates with MICs of 2mg/L.154 However, although the targeted 40% T>MIC exposure was achieved by this dosing regimen against KPC-producing K. pneumoniae isolates with meropenem MICs up to 16mg/L, this drug was not able to produce a reliable reduction in the bacterial density in 2 of the 3 isolates with MIC=8mg/L. In another study the efficacy of 1 and 2g doses, and extended infusions of doripenem against KPC-producing K. pneumoniae isolates with MICs ranging 4–32mg/L, in both immunocompetent and neutropenic mice, were evaluated.155 The 1g dose was able to produce only a bacteriostatic response with MICs of 4–8mg/L, whereas the 2g dose achieved a similar effect for isolates with MICs up to 16mg/L. Compared to the neutropenic mice, a significant reduction in bacterial density was observed in immunocompetent animals, with overall decreases of up to 1log with either the 1-g or the 2-g doripenem dose. A critical interpretation of the animal infection model data suggests that high-dosing, extended infusion regimens of carbapenems are able to achieve at least a bacteriostatic effect in severely compromised hosts and a modest bactericidal effect in immunocompetent animals infected with KPC-producing K. pneumoniae isolates with MICs up to 8mg/L. Some recent data from animal models found that the type of carbapenemase may affect the activity of carbapenems, even for isolates with the same MIC. The results of these studies suggested that carbapenems would be less effective against KPC- and OXA-48- than against NDM-producers or against isolates with non-carbapenemases-related resistance to carbapenems.156–159 Clinical experience with carbapenem monotherapy is limited to case reports, case series, retrospectives studies and one prospective observational study. However, most studies have found that it is associated with a high rate of clinical failure and/or increased mortality.2 A study compared the efficacy of carbapenems in monotherapy in 32 patients with infection caused by carbapenemase-producing K. pneumoniae according to MIC149; it increased from 29% for a MIC of >8mg/L to 60% for a MIC of 8mg/L and to 69% for a MICs of ≤4mg/L. Although the comparison between the groups was not statistically significant, it is worth noting that the efficacy of carbapenems in the last group was similar to that observed in 22 patients infected with non-carbapenemase-producing, carbapenem-susceptible K. pneumoniae (73%). Also, combination therapy including a carbapenem has been found to be associated with lower mortality than combinations without carbapenems in crude and adjusted observational comparisons when the carbapenem MIC was ≤8mg/L, in the case of meropenem.133,139 It should be remembered that meropenem was used at 2g every 8h, and administered by extended infusion in these studies. Of note, use of extended infusion of carbapenems may be associated with improve outcomes in critically ill patients.122
KPC enzymes show a high affinity for ertapenem; interestingly, the combination of ertapenem with doripenem showed enhanced activity in both in vitro and animal models, particularly for isolates with lower doripenem MIC.159–161 Clinical experience is limited to a few successful anecdotal cases.162,163
Which is the best combination for invasive infections caused by CPE?Again we did not find any randomised controlled trials to answer this question, and therefore the data from observational studies are analysed. In the retrospective cohort study by Tumbarello et al., 30-day survival was independently associated with the use of meropenem, colistin and tigecycline in combination.133 As previously stated, the doses for these antibiotics were optimised. Mortality rates were 0% (0/5) for meropenem MIC ≤2mg/L, 20% (2/10) for MIC=4mg/L, 25% (1/4) for MIC=8mg/L, and 35.2% for MICs ≥16mg/L. In the study by Qureshi et al., patients who received colistin/polymyxin B or tigecycline monotherapy had significantly higher mortality (66.7%) than those treated with colistin/polymyxin B or tigecycline combined with a carbapenem (12.5%).134 Also, Daikos et al. found a lower mortality in carbapenem-containing combinations than in carbapenem-sparing combinations (19.3% vs 30.6%), and also found that cases with a carbapenem MIC ≤8mg/L had a lower mortality than those with a MIC >8mg/L (19.3% vs 35.5%).139
Additionally, some reviews summarised data from case series; it should be noted that these analyses are crude and no meta-analysis technique was used to analyse the data. Daikos et al. found that the combination of carbapenem with another active drug, such as an aminoglycoside, colistin or tigecycline, was associated with significant lower mortality that combinations of non-carbapenem drugs if the isolate had a carbapenem MIC of ≤4mg/L150; Akova et al. found similar results.142 Also, Tzouvelekis et al. found patients receiving combinations therapy including a carbapenem had lower failure rate, and found a correlation between the response rate and carbapenem MICs among patients treated with carbapenem monotherapy, increasing from 25% (MIC >8mg/L) to 66.7% (MIC=8mg/L), 71.4% (MIC=4mg/L), and 72.4% (MIC ≤2mg/L).2
On the other hand, a recently published case series found a high rate of clinical response (92%) and low 30-day mortality (11.5%) among 26 polytrauma ICU patient treated with carbapenem-sparing combinations including tigecycline (100mg every 12h) in combination with gentamycin (19 patients) and/or colistin (12 patients, 4.5 millions IU every 12h), and some of them also with fosfomycin (13 patients, 4g every 8h); 16 patients had VAP (5 of them bacteraemic).164 The study does not include a control group treated with carbapenems in combination; it should be noticed that the mortality rate of VAP in this study is lower than expected.
When and how should colistin be used in the treatment of MDR Enterobacteriaceae?The role of colistin in the treatment of Enterobacteriaceae infections has become increasingly relevant as the threat of carbapenem-resistant strains has spread, including some European countries.2,165 The effectiveness of polymyxins in comparison to β-lactams has been questioned. Yahav et al. recently performed an analysis of pooled data from previously published studies, and observed a significant increase of mortality in the group treated with colistin compared to other antibiotics.166 Although this effect could be explained to some extent by differences in the baseline features of patients with MDR organisms, the hypothesis of a reduced effectiveness of colistin was also supported by in vivo studies showing a poor tissue penetration167,168 and by the previously scarce available information available on its PK/PD properties169 which impaired the use of optimal dosing schedules. Moreover, since the exposure to colistin has been shown to be the main factor to induce the resistance to polymyxins,170,171 it seems reasonable to preserve this last-resource antibiotic for infections with scarce available alternative options, such as those caused by CPE.
Because of the importance of early initiation of active treatment in severe CPE infections135 colistin may be considered as part of the empirical regimen of patients with severe infections potentially caused by CPE, e.g. in the setting of a nosocomial outbreak or in patients with severe infections and risk factors in areas with a high prevalence of these organisms.
Colistin is usually available for endovenous administration as colistimethate (colistin methanesulfonate), a less-toxic prodrug which spontaneously hydrolyses to colistin (the active form).172 There are two commercial presentations. Colomycin injection® (Xellia) is commercialised in vials containing 1MU; 1MU is equivalent to 80mg of colistimethate. Coly-Mycin M Parenteral® (Parkdade Pharmaceuticals) is commercialised in vials with 150mg of “colistin base” which is equivalent to 360mg of colistimethate or 4.5MU. Of note, dosing regimens recommended in the product labelling have been found to be too low in terms of PK/PD data, efficacy and selection of resistant strains.173,174
Recent well performed studies in critically ill patients provided new useful information about the PK/PD properties of colistin, which are being applied to dosing regimens. Colistin plasma concentrations are below the breakpoint (2mg/L) for the first 48h unless a loading dose is administered.173–175 Therefore, administration of a 6–12MU loading dose (or a body weight-based individualised dose) was suggested in critically ill patients.173 Also, a longer half-life of colistin (up to 18h) was shown in these studies, compared to the short half-life observed for colistimethate, and therefore, the suggested maintenance dose was 4.5MU every 12h.173–175 However, caution is needed because the use of higher doses of colistin might increase the risk of nephrotoxicity.176–178 Dalfino et al. reported data on 28 infections caused by colistin-only susceptible Gram negative bacilli treated with a 9MU loading dose of colistin followed by 4.5MU each 12h.179 The clinical cure was high (82.1%), and the frequency of acute kidney injury was low (17.8%), all of which subsided rapidly. The study was uncontrolled, but it may be argued that these results improve the best favourable outcomes reported hitherto in similar ICU settings.180–183 There is a lack of data for non-critical subjects, in whom the volume of distribution may be lower, and thus the risk of nephrotoxicity when using higher doses of colistin could be increased. Also, there are no available data for patients with extreme body weights.
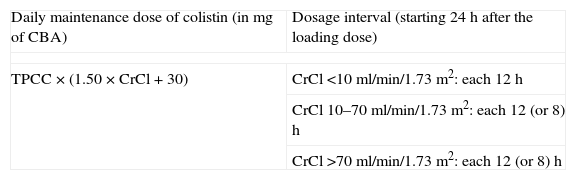
As regards dosing in patients with renal impairment, the information is scarce and based on PK/PD studies that have not been validated in clinical studies hitherto. For patients not receiving renal replacement therapies we found only one recent population study that provided dosing recommendations according to creatinine clearance, based on a nomogram elaborated with the PK population analysis of 101 critically ill patients (Table 8).184 Two studies including limited number of patients observed an efficient clearance of colistin in patients undergoing intermittent haemodialysis.174,184 The results of both studies suggest the traditional dosing of 1MU each 48h is inappropriate, and recommend 1–2MU each 12h instead. A supplemental dose after the haemodialysis session (50% to the daily maintenance dose if administered during the last hour of dialysis, 30% if administered after the session) was recommended if the session cannot wait until the end of the dosing interval,174 but no clinical studies have assessed these recommendations. Finally, a significant clearance of colistin has been observed in patients undergoing continuous venovenous haemodiafiltration, but heterogenous results were obtained in different studes.174,185,186 Probably, other factors (renal residual function, differences in the renal replacement mode, the haemofilter employed, etc.) are also important to explain these differences.
Recommendations for colistin maintenance dosing for patients with impaired renal function not receiving renal replacement therapies (data are adapted from Ref. 183). This algorithm is not recommended for patients with creatinine clearance >70ml/min, since calculated dose could be substantially be higher than 300mg of colistin base activity, whose safety has not been proved.
| Daily maintenance dose of colistin (in mg of CBA) | Dosage interval (starting 24h after the loading dose) |
| TPCC×(1.50×CrCl+30) | CrCl <10ml/min/1.73m2: each 12h |
| CrCl 10–70ml/min/1.73m2: each 12 (or 8)h | |
| CrCl >70ml/min/1.73m2: each 12 (or 8)h | |
CBA, colistin base activity (150mg of CBA=4.5MU of colistin methanesulfonate); TPCC, targeted plasmatic concentration of colistin, according to MIC of the strain, the site and the severity of the infection; CrCl, creatinine clearance.
Example: For a patient with a CrCl of 40ml/min/1.73m2 who needs colistin for treating a bloodstream infection produced by a K. pneumoniae strain with a colistin MIC=1mg/L, the minimum daily maintenance dose would be: 1mg/L×(1.50×40ml/min+30)=90mg of CBA=3MU of colistin methanesulfonate. Thus, 24h after the initial loading dose, a maintenance scheme of 1.5 MU each 12h would be started.
Fosfomycin is an “old” antibiotic that inhibits the first step of peptidoglycan synthesis and shows potent bactericidal action against many Gram-negative and Gram-positive pathogens.187 Fosfomycin tromethamine, an oral formulation, is approved in several countries for the treatment of cystitis. Also, fosfomycin disodium is also available for parenteral use in several countries. The drug shows little toxicity, achieves very high peak levels in serum and urine, and rapidly penetrates tissues.187 Unfortunately, resistance may develop when used in monotherapy; available evidence suggest that emergence of resistance occurs more frequently with P. aeruginosa than with E. coli (the data for other Enterobacteriaceae are scarce), and less frequently in UTI than in other types of infection.188
In vitro studies showed that fosfomycin frequently retains good activity against several MDR Enterobacteriaceae, including ESBL and CPE.2,189–193 Fosfomycin has also been shown to sometimes provide synergistic effect in combination with other antibiotics against different MDR Enterobacteriaceae; however, the grade of synergy depends on the strain and the accompanying antibiotics, and ranged from 55% to 79% with carbapenems, 7.1% to 36% with colistin, 21% to 30% with tigecycline, and 25% to 43% with gentamicin in one study.194 However, this is not always the case: in another study which analysed 16 isolates of Enterobacteriaceae with decreased susceptibility to carbapenems (non-KPC-producers), only an additive effect with carbapenem was observed in isolates with low carbapenem MIC195; and combinations were not bactericidal against MBL-producers in another study.196 Other potential benefit of using a combination regimen is the prevention of fosfomycin resistance development.197
In clinical practice, fosfomycin has been used in combination for the treatment of difficult-to-treat infections due to CPE.189,190 Published data are reduced to small case series, and detailed information of all cases is not always specified. When analysing the data, it is necessary to consider the possibility of publication bias, that many patients who received fosfomycin in combination were seriously ill, and that fosfomycin may have been administered as a last resort, rescue therapy. Because of the scarcity of data, these series will be reviewed.
One study investigated a case series of 8 severely ill patients with invasive infections due to carbapenem-resistant K. pneumoniae who were treated with fosfomycin in combination with colistin, gentamicin or piperacillin-tazobactam; all-cause in-hospital mortality was 18.2%.198 Secondary endpoints (such as clinical and microbiological outcomes, and the occurrence of any adverse effect) were favourable in all cases. In another study, fosfomycin was added after failure of initial therapy in 3 severely ill immunosuppressed patients with bactereamia due to carbapenem-resistant K. pneumoniae; in all cases, bactereamia relapsed after a short time period, in parallel with the development of antimicrobial resistance to fosfomycin and some of the other antibiotics.199 Five patients within a series of 40 episodes of bacteraemia due to OXA-48-producing K. pneumoniae were treated with fosfomycin in combination with colistin (4 cases, one of primary BSI and 3 UTI) or tigecycline (soft tissue infection); 2 patients died because of the infection.138 Finally, a series of 48 patients with invasive infections caused by carbapenem-resistant K. pneumoniae or P. aeruginosa treated with fosfomycin in combination with colistin or tigecycline were analysed.200 The most frequent infections were primary bactereamias and VAP. Mean fosfomycin daily dose was 24g per day. 54.2% of patients were cured at day 14; mortality at day 30 was 37.5%. Nine of 15 patients with pandrug-resistant K. pneumoniae survived. Overall, 3 patients developed fosfomycin resistance.
As regards fosfomycin doses, 24g per day (usually 6g every 6h or 8g every 8h) were administered in most of recent case series; a recent systematic review concluded that probably higher and more frequent doses may be needed in critically ill patients at least for the first 24–48h, although there are no data to support any specific dosing scheme.201
When and how should aminoglycosides be used in the treatment of invasive infections caused by MDR Enterobacteriaceae?Aminoglycosides are often one of the few antibiotics to which MDR and XDR Enterobacteriaceae are susceptible. However, the susceptibility rates are heterogeneous according to the specific microorganism and the local epidemiology.2 In a meta-analysis and an evidence-based review for all types of pathogens and infections, monotherapy with aminoglycosides was found as efficacious as comparators (β-lactams and quinolones) for UTIs, although may be lower for other infections202,203; also, combinations of β-lactams and aminoglycosides did not seem to produce any benefit in comparison to monotherapy with a fully active β-lactam.204 However, the situation may be different in case of MDR and XDR Enterobacteriaceae, in which the activity of first line drugs is seriously compromised.
Results of combinations in in vivo studies have shown heterogeneous results. A synergistic effect was observed with the combination of fosfomycin and netilmicin against several carbapenemase-producing K. pneumoniae, ESBL-producing K. pneumoniae and ESBL-producing E. coli strains194; however, the combination of gentamicin and fosfomycin was indifferent against KPC-2 producing-K. pneumoniae.197 Combinations of carbapenems and aminoglycosides have frequently shown in vitro synergy against some KPC-producing K. pneumoniae isolates,204,205 which was also shown in an animal model.204 In a endocarditis model of infection caused by an ESBL-producing K. pneumoniae (TEM-3), the combination of piperacillin-tazobactam and gentamicin also showed a synergistic effect.206 However, no synergistic effect has been observed in vitro in other studies with ESBL-producers.207,208 To the best of our knowledge, studies with carbapenemases other than KPC are lacking.
In the clinical setting aminoglycosides have been used alone or in combination for the treatment of several infections caused by carbapenemase producers. During an outbreak due to KPC-2-producing K. pneumoniae in Greece involving 22 patients, 5 patients with pneumonia received aminoglycoside plus colistin (plus tigecycline in 2 patients) and all of them presented a favourable outcome; an additional patient with bactereamia achieved clinical cure with gentamycin alone.209 Daikos et al., in their cohort study of bactereamic patients with KPC or VIM producing K. pneumoniae, found 8 patients treated in monotherapy with aminoglycosides, of which one died, and 57 treated in combinations, of which 28% died.139 Zarkotou found no infection-related mortality in 2 patients treated in monotherapy with aminoglycosides and 8 treated in combination.135 However, the types of the infection were not detailed in these reports. Navarro-San Francisco et al. found that, among patients with bactereamia due to OXA-48-producing K. pneumoniae, 8 out of 12 patients who received an aminoglycoside in combination with other antibiotics (6 with tigecycline, 3 with carbapenems, 2 with colistin and 1 with ciprofloxacin) died; on the other hand, 2 patients with catheter-related bacteraemia who received monotherapy with an aminoglycoside survived, whereas a third patient with bacteraemia from the urinary tract died from other causes.138 A case report described a patient with endocarditis due to KPC-3-producing K. pneumoniae who fully recovered after antibiotic treatment with gentamicin plus colistin, without the need of surgical intervention.210 Finally, Alexander et al., in a series of UTI due to KPC-producing K. pneumoniae, found that all 7 patients receiving aminoglycoside therapy (gentamycin monotherapy in 5 of them) had successful clinical and microbiologic responses.211
The experience is also limited in infections due to ESBL-producing organisms. In a retrospective study involving 44 patients with bacteraemia due to ESBL-producing K. pneumoniae, 2 out of 4 patients who received an active aminoglycoside in monotherapy died.212 Two more patients received aminoglycosides in monotherapy in a retrospective series of 35 episodes of bacteraemia caused by TEM-52-producing K. pneumoniae (the source was not specified); even though the isolates were not susceptible, both patients responded.213 Gudiol et al. observed that aminoglycosides plus BLBLI showed similar mortality rates than carbapenems as empiric therapy of BSI due to ESBL-producing E. coli among cancer patients; a better outcome was observed when the source of BSI was the urinary tract.214
As regards dosing, general recommendations for aminoglycoside use apply. High dose have been recommended in critically ill patients, and for severe infections caused by isolates with borderline MIC (Table 5).215–217 Very high dose of amikacin (25–30mg/kg/day) with continuous venovenous haemodiafiltration was used in 2 critically ill patients with pandrug-resistant P. aeruginosa with good results.217
Is aztreonam useful for the treatment of invasive infections caused by MBL-producing Enterobacteriaceae?MBLs are characterised by the ability to hydrolyse carbapenems and all the available β-lactams with the exception of aztreonam. Thus, the usefulness of aztreonam for the treatment of infections due to microorganisms carrying this type of enzymes is to be considered.
Aztreonam showed a bactericidal effect in vitro against a VIM-1-producing K. pneumoniae strain; however, its effect was slower than that of carbapenems, and resistance to aztreonam emerged in some isolates.218 In a rabbit intra-abdominal abscess model due to a carbapenem-susceptible VIM-1-producing E. coli strain, the efficacy of carbapenems and aztreonam was assessed; aztreonam showed the best bactericidal effect in sterilising the abscesses, although it did not reach statistical significance compared with carbapenems.219 Furthermore, aztreonam was the only antibiotic which prevented death of all treated animals, suggesting a possible clinical benefit over carbapenems. However, there are no clinical studies evaluating the use of aztreonam in infections caused by MBL-producing Enterobacteriaceae.
Are cephalosporins useful for the treatment of invasive infections caused by OXA-48-producing Enterobacteriaceae?OXA-48 β-lactamases hydrolyse penicillins, are resistant to β-lactamase inhibitors, and hydrolyse carbapenems at a moderate level. Weak (cefotaxime) or no (ceftazidime) hydrolysis activity of broad-spectrum cephalosporins by this enzyme has been reported. Consequently, many OXA-48 producers that do not coproduce any ESBL (which is unfortunately not frequent) may be categorised as susceptible in vitro to broad-spectrum cephalosporins.2,136 However, it is not known whether the results obtained in vitro can be translated to the in vivo setting. In an experimental peritonitis model in mice due to OXA-48-K. pneumoniae, ceftazidime exhibited the highest efficacy effect compared with the rest of broad-spectrum β-lactams tested (piperacillin-tazobactam, imipenem, ertapenem and cefotaxime).220 However, there are no clinical data evaluating the use of cephalosporins in infections caused by OXA-48-producing Enterobacteriaceae.
When and how should tigecycline be used in invasive infections caused by MDR Enterobacteriaceae?Tigecycline has been tested in randomised controlled trials for complicated skin and soft tissue infections (cSSTI), community-acquired pneumonia, HAP, cIAI, and diabetic food infections using different comparators.221–231 The tigecycline dose used was 100mg loading-dose followed by 50mg/12h. From the published clinical trials, information can be obtained about the clinical response in Enterobacteriaceae infections. Overall, 92% of potentially susceptible Enterobacteriaceae (excluding Proteeae tribe) were E. coli and Klebsiella spp. The typical MIC90 was 0.5mg/L for E. coli and 1–2mg/L for K. pneumoniae. In none of the studies the overall clinical response rate with tigecycline was significantly different in comparison with the comparators. However, in the group of patients with ventilator-associated pneumonia (VAP), the clinical cure rate was significantly lower with tigecycline than with imipenem (31% vs 82%).224 In one study of cIAI, the cure rate in the subgroup of patients with documented E. coli infection treated with tigecycline was 10% lower than that observed in the imipenem arm (88% vs 98%), in line with the lower clinical cure rate observed in the subgroup with complicated appendicitis (87% vs 100%).229 This difference was not found in other studies in patients with cIAI. Regarding the severity of the infections, most were moderate, and immunocompromised patients were excluded.221–231 Therefore, from the evidence provided by these studies it cannot be inferred that the efficacy of tigecycline in monotherapy would be the same in more severe infections.
We found 4 meta-analyses of randomised clinical trials exploring whether overall mortality and cure rates were different in patients assigned to tigecycline compared to comparators.232–235 Three agreed on the finding that patients assigned to tigecycline had a higher frequency of clinical failure than patients assigned to the comparators,233,234 and two of them also found higher mortality with tigecycline.234,235 Tigecycline was also found to be associated with higher rate of adverse events.230–232 No differences in microbiological eradication in E. coli222,233 or K. pneumoniae233 were observed. One of the studies also found a higher rate of clinical failure with tigecycline among patients with Gram-negative infections.234 In summary, the results from meta-analyses suggest that clinical failure, adverse effects and death were more frequent among patients treated with tigecycline.
Results from animal models with E. coli and K. pneumoniae suggest that the PK/PD parameter predicting the efficacy of tigecycline is the AUC/MIC ratio, regardless of production of ESBL or carbapenemases.236 An area under the curve (AUC)/MIC=1 was associated with 50% maximum antibacterial effect for E. coli (eradication was associated with a AUC/MIC=1.39 in clinical trials237,238) but was 1.6 for K. pneumoniae, suggesting that this microorganism requires a greater exposure. Monte Carlo simulations showed that standard tigecycline dose have a 90% probability of achieving an AUC/MIC ≥1.39 when the MIC is ≤0.5mg/L, but only 27% if the MIC is 1mg/L, and 0 if the MIC exceeds 1mg/L.239 It should be noted that more than 90% of E. coli but less than 50% of K. pneumoniae have a MIC ≤0.5mg/L regardless of the mechanism of resistance.240–243 Because of that, a phase II study explored the efficacy and safety of higher doses of tigecycline in patients with HAP.227 Although the number of patients was too low to draw conclusions (36 and 25 subjects received 150mg followed by 75mg every 12h, and 200mg followed by 100mg every 12h, respectively), the clinical response rate was numerically higher in the group receiving the highest dose; the 100mg/12h dose was associated with a higher incidence of gastrointestinal adverse effects (nausea, vomiting and diarrhoea).
There is little clinical experience on the efficacy of tigecycline in infections caused by ESBL-producing Enterobacteriaceae. Four studies including 118 patients with infections caused by ESBL-producing enterobacteria provided some additional data.244–247 The most frequent infections were cIAI, cSSTI and HAP. Clinical response rates ranged between 63% and 81%, but the percentage of patients receiving combination therapy was not always specified. The lower response rate among patients with VAP caused by Enterobacteriaceae found in randomised trials also occurred among those patients with VAP due to ESBL-producers (1/4 [25%] vs 6/6 [100%]; P=0.03 by the test Fisher's exact test).226 Fifteen patients from 2 double-blind trials on cIAI had an infection caused by an ESBL-producer, and 12 (80%) showed a favourable clinical response.228,229
As regards the potential utility of tigecycline in the treatment of infections caused by CPE, available clinical data come from observational studies and case series in which patients were usually treated in combinations, in which some of them received tigecycline; most reported infections were caused by KPC-, VIM- or OXA-48-producing K. pneumoniae.133,138,139,164,211,248–254 Overall, crude mortality among patients treated with tigecycline in monotherapy tended to be higher than in those treated with tigecycline in combination (range of mortality in studies in which data for this comparison was provided, 40–80% vs 0–33%133–135,138,139,251,252), but these data are not controlled for confounders. Because the combinations and clinical situations in which tigecycline was used are heterogenous, it is not possible to raise clear conclusions from these data.
Two additional recent retrospective studies have assessed the efficacy of different tigecycline doses for therapy of MDR Gram-negative bacteria. In one study, combination therapy with tigecycline was used at standard or high doses in 100 episodes of infection due to carbapenem-resistant A. baumannii or K. pneumoniae.255 In patients with VAP, the use of high tigecycline dose (100mg every 12h) was the only independent predictor of clinical cure. In another study, combined therapy with tigecycline was used in 16 episodes of infection due to carbapenemase-producing K. pneumoniae in critically ill patients.256 A crude univariate analysis showed that mortality was significantly associated with the presence of immunosuppression and mean APACHE II and SOFA scores, but not with the use of standard or high (100mg every 12h) tigecycline dose.
Priority areas for future researchThe panel selected the following areas as priorities for future research:
- -
Randomised trials comparing the clinical impact of the use of rapid diagnostic techniques in antibiotic use and clinical outcomes of patients with invasive infections caused by ESBL and carbapenemase-producing Enterobacteriaceae.
- -
Randomised controlled trials comparing the efficacy and safety of BLBLI, temocillin, and cephamycins with that of carbapenems in the treatment of invasive infections caused by ESBL-producing Enterobacteriaceae; ideally, the studies should be performed for specific sources of infection.
- -
Randomised controlled trials comparing the efficacy, safety and ecologic impact of ertapenem and group 2 carbapenems (imipenem, meropenem, doripenem) in the treatment of invasive infections caused by AmpC- and ESBL-producing Enterobacteriaceae.
- -
Randomised controlled trials comparing the efficacy and safety of fosfomycin and aminoglycosides with that of carbapenems in the treatment of cUTI caused by AmpC-, ESBL- and carbapenenase-producing Enterobacteriaceae.
- -
Randomised controlled trials comparing the efficacy of colistin monotherapy with the combinations of colistin plus carbapenem in invasive infections caused by CPE isolates with low carbapenem MIC, and colistin plus tigecycline or aminoglycosides or fosfomycin in invasive infections caused by CPE isolates with high carbapenem MIC.
- -
Trials with high/optimised doses of tigecycline, carbapenems and colistin in patients with invasive CPE infections. Appropriate, prospectively collected historical controls may be used as comparators.
- -
Clinical studies assessing the efficacy of alternative antibiotics such as aztreonam, ceftazidime/avibactam and temocillin for therapy of infections due to susceptible MDR Enterobacteriaceae.
- -
For detection of ESBL or carbapenemase-producing Enterobacteriaceae in surveillance samples, specific chromogenic media are recommended (BII); alternatively, molecular-based methods toward a specific target may be used (CIII).
- -
Confirmation of ESBL-producers should be performed in isolates showing after screening increased MIC or reduced inhibition zone to third generation cephalosporins according to either EUCAST or CLSI criteria by microdilution (Etest is to be also considered) or disk diffusion (BII).
- -
The recommended phenotypic confirmation tests for ESBL production are those methods which use clavulanic acid as ESBL inhibitor. Either double-disk synergy test (DDST), combined-DDST (CDDST) or microdilution can be used; the best option is probably the use of CDDST with cefotaxime, ceftazidime, and cefepime (BII).
- -
Confirmation of carbapenemase-producers should be performed in isolates showing after screening increased carbapenem MIC (>0.12μg/ml or <25mm for ertapenem and/or meropenem and/or >1μg/ml or <23mm for imipenem) (BII). Meropenem is preferred for this purpose; imipenem is not recommended as a stand-alone screening test compound (CIII). In areas with high prevalence of class D enzymes (OXA-48-like), with isolates showing reduced susceptibility to carbapenems, screening with temocillin (either 30μg disk diffusion or MIC, ≤10mm and >64mg/L, respectively) in the absence of synergy of other inhibitors may be used as a first step method to identify an OXA-48 producer (BII).
- -
For phenotypic confirmation for carbapenemase production, the best option is the use of DDST or CDDST (which is commercially available and has been validated) using a carbapenem (usually meropenem) combined with specific class A, B, and C enzyme inhibitor (BII). However, since currently there are no available inhibitors for class D carbapenemases and temocillin as a marker is not specific for OXA-48-type carbapenemase, these enzymes must be confirmed by using a genotypic method (CIII).
- -
In the case rapid tests are needed to confirm the presence of ESBL or carbapenemase producers, molecular methods are recommended, according to local resources (BIII).
- -
In case of sepsis potentially caused by Enterobacteriaceae, clinicians should evaluate the risk of ESBL-producers considering both the epidemiological setting (e.g. rate of ESBL-producing microorganisms in a given institution) and individual risk factors (BII).
- -
The following individual risk factors should be assessed in all community-onset sepsis potentially caused by Enterobacteriaceae in order to evaluate the risk for ESBL-producers: recent use of fluoroquinolones or cephalosporins; recent hospitalisation; transfer from another healthcare facility, including long term care facilities; Charlson index >3; and age >70 years (BII). Recent travel to high endemic areas must also be considered (BIII). The same risk factors should be considered for community-onset AmpC-producers (BIII).
- -
ESBL-producers should be considered for empirical therapy in patients with community-onset severe sepsis or septic shock potentially caused by Enterobacteriaceae if presenting at least one of the previous risk factors, and in patients with non-severe sepsis if more than 2 risk factors are present (CIII).
- -
If ESBL-coverage is decided for a community-onset infection, a carbapenem is of choice (BII); however, in case of complicated urinary tract infection (cUTI) a β-lactam/β-lactamase inhibitor (BLBLI) plus an aminoglycoside (BII) or a third generation cephalosporin (probably ceftazidime) plus an aminoglycoside (CIII) are alternatives, according to local prevalence of susceptibility to these drugs among ESBL-producers.
- -
In nosocomial sepsis potentially caused by Enterobacteriaceae, individual risk factors (longer hospital stay, exposure to mechanical ventilation, and previous receipt of cephalosporins, fluoroquinolones or carbapenems) should be considered according to local epidemiology (e.g., local prevalence, outbreak) for decision about empirical therapy in order to cover for ESBL, AmpC and/or carbapenemase-producers (BIII). Because of the current rates of ESBL-producers in Spanish centres, empirical therapy against ESBL-producers is recommended in all patients with nosocomial infections potentially caused by Enterobacteriaceae and presenting with severe sepsis or septic shock (CIII).
- -
Carbapenems are the drugs of choice for invasive infections caused by ESBL- and AmpC-producing Enterobacteriaceae (BII). Ertapenem is suggested for patients without septic shock and isolates with MIC ≤0.25mg/L to avoid the selective pressure on P. aeruginosa posed by group-2 carbapenems (CII). For other infections, imipenem or meropenem are recommended (BII); the experience with doripenem is scarce but it is probably as useful (CIII).
- -
In vitro active BLBLI (specifically, amoxicillin/clavulanic acid and piperacillin/tazobactam) are reasonable alternatives for bacteraemic UTI or biliary tract infections caused by ESBL-producing E. coli, and may be used as carbapenem-sparing regimens; the recommended doses for patients with normal renal function are: 2/0.2g/8h in 30min for amoxicillin/clavulanic acid and 4/0.5g/6h in 30min or 4/0.5g in extended infusion/8h (or/6h in critically ill patients) for piperacillin/tazobactam (CII). Data for other infection or Enterobacteriaceae are scarce.
- -
There are insufficient data to recommend the use of active cephalosporins for invasive infections caused by ESBL-producers according to EUCAST or CLSI breakpoints; however, until more data are available, we recommend caution when considering the use of these drugs; their use is only recommended as an alternative option for the treatment of non-severe UTI sepsis in low risk patients (CIII).
- -
There are insufficient data to recommend the use of cephamycins, fluoroquinolones, aminoglycosides, trimethoprim-sulfamethoxazole, colistin or fosfomycin. For fully susceptible isolates there is no reason to expect different efficacy than in non-ESBL-producers (CIII).
- -
Therapy must be individualised according to susceptibility results, source of infection and severity of disease (CII). According to general knowledge in management of infections, source control and support therapy are key aspects in the management of these infections (BII).
- -
Combination therapy is recommended for severe infections caused by KPC-producing K. pneumoniae (CII), and possibly for other carbapenemase-producing Enterobacteriaceae until more data are available (CIII). There are no data to support combination therapy for patients with mild infections for which fully active drugs, useful for the specific type of infection, are available; therefore, we recommend monotherapy for such infections, and particularly for non-severe UTIs (CIII).
- -
Monotherapy with a carbapenem is not recommended for patients with invasive infections caused by CPE but may be considered in cases of mild invasive infections if adequate source control is readily achieved and the isolate is susceptible according to EUCAST or CLSI breakpoints; the typical example would be sepsis from the urinary tract, without urinary tract obstruction nor severe sepsis or septic shock (CIII).
- -
For patients in which combination therapy is indicated, a regimen with a carbapenem (see preferred drug and recommended dose below) plus one or two fully active drugs (including colistin, tigecycline, an aminoglycoside or fosfomycin, the latter preferably as a third drug) is recommended if the carbapenem MIC is ≤8mg/L; this applies mainly to patients with severe infections caused by KPC-producing K. pneumoniae (BII). We suggest a similar approach for other carbapenemases until more data are available (CIII). No recommendation can be given for using the combination of ertapenem plus doripenem or meropenem for KPC-producers (unresolved issue).
- -
There are not enough data to recommend including a carbapenem in combination regimens if minimal inhibitory concentration (MIC) is >8mg/L; if this is the case, carbapenems are probably useless, particularly if MIC is >16mg/L; we recommend including at least two fully active drugs in the combination regimen according to susceptibility testing and source of infection (drugs to be considered: colistin, aminoglycosides, fosfomycin and tigecycline) (CIII).
- -
Patients with less severe invasive infections and cUTIs might be treated with carbapenem-sparing combinations (drugs to be considered: colistin, aminoglycosides, fosfomycin, tigecycline – the latter not for UTI) or even monotherapy (see above) (CIII).
- -
Dosing of all administered drugs should be optimised to increase the probability of reaching the appropriate pharmacodynamics target (BII); in the case of carbapenems, we recommend using meropenem at 2g every 8h in extended infusion (BII).
- -
Colistin should be preserved for treating infections produced by Enterobacteriaceae strains showing resistance to all β-lactam antibiotics (CIII).
- -
Colistin is suggested as part of the empirical treatment of patients with severe infections if participation of CPE is suspected, such as in outbreaks or colonised patients (CIII).
- -
When using colistin and until more data are available, we recommend the use of a loading dose of 9MU and subsequent high, extended-interval maintenance doses (4.5MU/12h) in critically ill patients and patients with severe sepsis or septic shock with creatinine clearance above 50ml/min; maintenance dose should be individually adjusted according to creatinine clearance according to published nomograms (CIII).
- -
Data to recommend a loading dose in non-critically ill patients with non-severe infections are unavailable (unresolved issue); maintenance dosing with 4.5MU/12h (or alternatively 3MU/8h) is suggested, but renal function should be closely monitored (CIII).
- -
No recommendations can be given for patients with extreme weights (unresolved issue).
- -
We recommend 1–2MU of colistin every 12h for patients undergoing intermittent haemodialysis. Whenever possible, haemodialysis should be conducted at the end of the dosing interval to minimise the clearance of colistin. If this is not possible, a supplemental dose at the end of the dialysis should be considered (CIII). For patients undergoing continuous venovenous haemodiafiltration, even though the data are not consistent, a dose of 9MU/day is suggested (CIII).
- -
Experience with fosfomycin for the treatment of invasive infections caused by MDR and XDR Enterobacteriaceae is limited; however, in patients with limited options, fosfomycin (4–6g every 6h to 8g every 8h) may be considered as part of a combination regimen including at least one more active agent (CIII).
- -
Based on the experience of non MDR Enterobacteriaceae infections, monotherapy with an active aminoglycoside may be considered for the treatment of cUTI caused by MDR and XDR Enterobacteriaceae. Toxicity should be closely monitored (BII).
- -
Monotherapy with aminoglycosides for other invasive infections is not recommended. For such infections, combination therapy with other drug is suggested (BII). However, aminoglycosides are to be considered as an accompanying agent in all combination regimens according to the susceptibility results with close monitorisation of toxicity (CIII).
- -
There is no clinical experience with aztreonam or cephalosporins for the treatment of invasive infections due to susceptible MBL- or OXA-48-producing Enterobateriaceae, respectively. Very scarce in vitro and animal model data suggest that they may be useful; if considered, we recommend using these drugs in combination except in cUTI (CIII).
- -
Tigecycline monotherapy should be avoided whenever possible (AI); exceptions may be selected patients with mild cIAI and cSSSI infections caused by XDR Enterobacteriaceae with other few adequate alternative options (CIII).
- -
Tigecycline should be considered as part of a combination regimen in patients with infections other than UTI caused by Enterobacteriaceae producing either ESBLs (if a carbapenem-spare regimen is to be used) or carbapenemases when tigecycline MIC is ≤1mg/L (CIII).
- -
Higher dose of tigecycline (150mg loading dose followed by 75mg/12h, or 200mg loading dose followed by 100mg/12h) should be considered for patients in septic shock, VAP or Enterobacteriaceae with MIC ≥1mg/L, but adverse events should be carefully monitored (BIII).
JRB was consultant for MSD, AstraZeneca, Pfizer, Roche, Novartis, Astellas and Anchaogen; speaker for MSD, AstraZeneca, Pfizer, Novartis and Astellas; and received research grants from Novartis and Gilead. CG was speaker for Novartis, Pfizer and Astellas; and received a research grant from Astellas. JPH was speaker and consultant for MSD, Astellas, Astra-Zeneca, Novartis, Pfizer and Basilea. GB was speaker for Pfizer, Novartis, Astellas and Janssen; and received research grants from Pfizer. All other authors have no conflict of interest.
Supported by the Spanish Society of Clinical Microbiology and Infectious Diseases, and Ministerio de Economía y Competitividad, Instituto de Salud Carlos III – co-financed by European Development Regional Fund “A way to achieve Europe” ERDF, Spanish Network for the Research in Infectious Diseases (REIPI RD12/0015).
We are grateful to Belén Padilla, Jose María Gutiérrez and Juan E. Losa for their valuable comments on the manuscript.
- -
BLBLI: β-lactam/β-lactam inhibitor combinations
- -
BSI: bloodstream infections
- -
cIAI: complicated intraabdominal infection
- -
cUTI: complicated urinary tract infection
- -
CLSI: Clinical and Laboratory Standards Institute
- -
CPE: carbapenemase-producing Enterobacteriaceae
- -
CRE: carbapenem-resistant Enterobacteriaceae
- -
ESBL: extended-spectrum β-lactamase
- -
EUCAST: European Committee for Antimicrobial Susceptibility Testing
- -
GNB: Gram negative bacilli
- -
HAP: hospital-acquired pneumonia
- -
IAI: intraabdominal infection
- -
ICU: intensive care unit
- -
KPC: Klebsiella pneumoniae carbapenemase
- -
MDR: multi-drug resistant
- -
MIC: minimum inhibitory concentration
- -
MBL: metallo-β-lactamase
- -
PK/PD: pharmacokinetic/pharmacodynamic
- -
UTI: urinary tract infections
- -
VAP: ventilator-associated pneumonia
- -
XDR: extensively drug resistant