The incidence and morbidity and mortality rates of diseases caused by non-tuberculous mycobacteria (NTM) have been on the increase over the last twenty or thirty years.1 There are a number of predisposing factors for the development of NTM infections, including chronic obstructive pulmonary disease, having had tuberculosis, bronchiectasis, immunodeficiency, cancer and diabetes, although no risk factors are identified in a significant proportion of patients.2,3 Although the lung is the most commonly affected organ, NTM can also infect bones, lymph nodes, joints and the skin.4 Most NTM lung infections are diagnosed by sputum examination and culture.5. If NTM cannot be isolated from expectorated secretions, the next step is usually to perform bronchoalveolar lavage (BAL) or even a lung biopsy.1,5 We present the case of an immunocompetent patient with a multifocal infection (vertebral and lung) caused by Mycobacterium avium complex (MAC) in which the first microbiological confirmation was achieved after percutaneous instillation (with radiological control) of a cavitary lung lesion and provocation of "induced sputum".
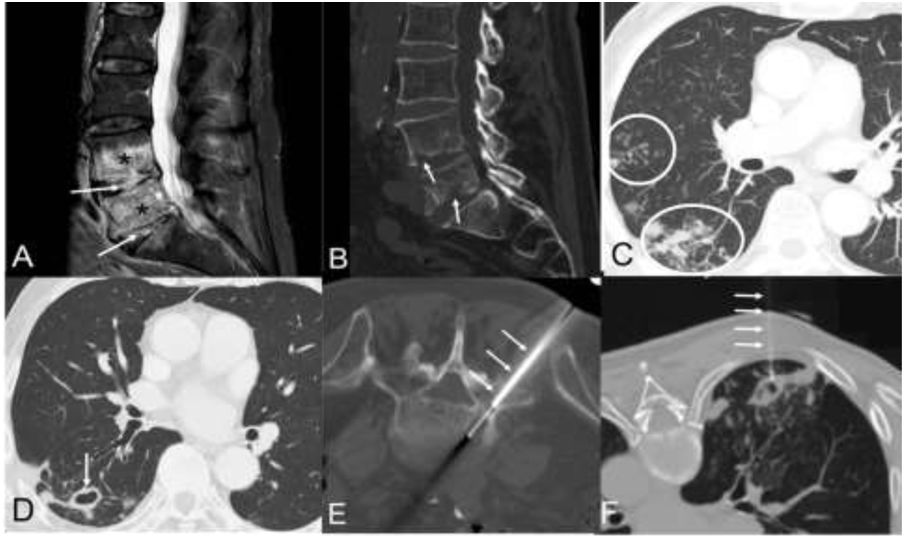
This was a 75-year-old man with a history of high blood pressure, dyslipidaemia and atrial fibrillation (but without any of the predisposing factors outlined in the previous paragraph), who consulted with a five-month history of asthenia, dry cough and progressive low-back pain, whose magnetic resonance imaging (MRI) and computed tomography (CT) scan of the lumbar spine showed signs of spondylodiscitis of the intervertebral spaces L4-L5 and L5-S1 (Figs. 1A and B). The chest X-ray showed opacities of infectious appearance in the right lung, with some cavitary lesions in the right lower lobe (RLL). The chest CT scan confirmed centrilobular nodules and signs of infectious bronchiolitis (Fig. 1C), as well as several cavitary lesions in the RLL, suggesting an active infection by Mycobacterium tuberculosis (M. tuberculosis) (Fig. 1D). BAL and several attempts at induced sputum (after inhalation of hypertonic saline solutions) did not demonstrate the presence of acid-fast bacteria (AFB) by auramine staining or of M. tuberculosis genetic material by nucleic acid amplification (Xpert® MTB/RIF Ultra, Cepheid). A percutaneous biopsy with radiological control of the L5-S1 intervertebral disc was performed (Fig. 1E), also with negative results. In the absence of microbiological confirmation, and pending the results of the specific cultures for mycobacteria (VersaTREK®, Thermo Fisher Scientific), it was decided to perform a percutaneous puncture-aspiration (with radiological control by CT) of one of the cavitary lesions in the RLL (Fig. 1F) after the instillation of about 10 ml of saline in one of the cavitary lesions. However, only 1 ml of blood-stained fluid could be aspirated. At the end of the procedure, the patient expectorated abundant thick sputum (15 ml) onto a sterile cloth (used to place the material used during the instillation procedure). There were no complications in the follow-up images taken immediately after the procedure. The expectorated sputum was aspirated with a sterile syringe and placed in a test tube for immediate microbiological processing, confirming the presence of 1,500 AFB/100 fields in the auramine stain. Both the staining of the small amount of fluid aspirated from the cavitary lesion and the test to detect M. tuberculosis genetic material in the two samples were negative. The growth of MAC in the sputum obtained after the above procedure after 16 days of incubation confirmed the aetiological agent. The microbiological diagnosis was confirmed after the subsequent late growth of MAC in one of the previously obtained respiratory samples (the BAL); the cultures of the vertebral biopsy and of the rest of the respiratory samples were negative. The patient was treated with clarithromycin, ethambutol and rifampin, and both the spondylodiscitis and his lung infection responded well to the treatment.
A) Sagittal MR image of the lumbar spine (STIR sequence) showing a marked alteration in the signal intensity of the intervertebral discs L4-L5 and L5-S1 (arrows) and the adjacent vertebral endplates (asterisks). B) Sagittal reconstruction of lumbar spine CT (bone window) which shows the bone erosions of the vertebral endplates (arrows). C) and D) Axial CT images of the chest (lung window) showing centrilobular lung nodules and “tree-in-bud” images (C, circles) and a dominant thin-walled cavitary lesion (D, arrow). E) Axial CT image of the lumbar spine (bone window, patient placed in the prone position) showing the percutaneous biopsy (arrows) of disc infection foci. F) Axial CT image of the chest (lung window, patient placed in the prone position) showing saline instillation into a cavitary lung lesion (arrows). After the instillation of 10 ml of saline, only 1 ml of fluid could be aspirated, although the patient expectorated abundant sputum (15 ml) at the end of the procedure.
Demonstrating NTM lung infections microbiologically is not easy, and even when they are detected, patients will not necessarily be started on treatment.6 Among the most common diagnostic techniques are the study of sputum (spontaneous or induced), BAL, lung biopsy (transbronchial or surgical) and percutaneous aspiration of a cavitary lung lesion.6–8 Percutaneous aspiration with radiological control of cavitary lung lesions is a cost-effective diagnostic technique for the detection and culture of multiple microbiological agents and makes it possible to differentiate between fungal infections, bacterial abscesses and mycobacterial infections.9–11 In our case, 10 ml of saline solution was instilled into a cavitary lung lesion, but only 1 ml of the instilled fluid was aspirated, as the fluid we introduced was drained by the bronchi communicating with the lung cavity. Fortunately, the patient expectorated the percutaneously instilled saline at the end of the procedure, enabling direct detection of AFB by staining and subsequent confirmation of MAC infection by culture. Several days later, MAC growth was confirmed in one of the respiratory samples obtained initially. We were unable to find any instance in the scientific literature of microbiological demonstration of an NTM infection in "induced" sputum occurring after percutaneous instillation (with radiological control) of saline in a cavitary lung lesion. There is one published report in which saline was instilled in a cavitary lung lesion and then aspirated back through the same needle followed by BAL, with the causal microorganism being confirmed in both samples (the fluid aspirated directly from the cavitary lesion and from the BAL).12 We believe that this "percutaneous" sputum induction technique may be useful in certain patients with cavitary lung lesions in whom microbiological confirmation has not been achieved using other more conventional diagnostic methods (induced sputum, BAL).
Please cite this article as: Gorospe-Sarasúa L, Alarcón-Rodríguez J, Tato-Díez M, Dronda F. Infección diseminada por Mycobacterium avium complex: confirmación microbiológica mediante inducción «percutánea» de esputo tras instilación intracavitaria de suero salino. Enferm Infecc Microbiol Clin. 2022;40:456–458.