Haemophilus influenzae is a cause of mild and severe invasive infections, especially among children under 5 years old. Serotype b (Hib) was very frequent before the introduction of the vaccine, which was introduced in Paraguay in 2004.
MethodsA total of 523 isolates of H. influenzae obtained from 1999 to 2017 and referred to the National Reference Laboratory in Paraguay were studied by conventional microbiological methods and molecular techniques.
ResultsThe most frequent serotype was non-typeable (HiNT) (51.8%; 271/523), followed by Hib (43%; 225/523), Hia and Hif (1.5%; 8/523, respectively), Hic (1%; 5/523), Hie (0.8%; 4/523), and Hid (0.4%; 2/523). A total of 48.4% invasive infections were caused by HiNT, and 46.1% by Hib; 88.6% of isolates corresponded to meningitis, 70.8% to sepsis and 50.9% to pneumonia in children under 5 years. A total of 84% (181/217) of isolates corresponded to invasive infections caused by Hib in children under 5 years, with the highest proportion observed between 2001 and 2003. The most prevalent biotypes were biotype I (29%), biotype II (12%), biotype III (24%), and biotype IV (13%). Among the total of isolates, 13% (68/523) of isolates were resistant to ampicillin.
ConclusionAfter the introduction of the Hib vaccine in Paraguay, the number of invasive Hib cases decreased in children under 5 years old, although we observed an increase of HiNT in children over 5 years. Continuous surveillance is necessary in order to monitor the effectiveness of the vaccine and for the development of preventive interventions.
Haemophilus influenzae (Hi) causa infecciones leves e invasivas graves, especialmente en niños menores de 5 años. El serotipo b (Hib) era muy frecuente antes de la introducción de la vacuna, que en Paraguay se introdujo en 2004.
MétodosSe estudiaron por métodos microbiológicos convencionales y técnicas moleculares 523 aislados de H. influenzae obtenidos desde 1999 a 2017 y remitidos al Laboratorio Nacional de Referencia de Paraguay.
ResultadosEl serotipo más frecuente fue el no-tipable (HiNT), en un 518% (271/523), seguido de Hib (43%; 225/523), Hia e Hif (1,5%; 8/523, respectivamente), Hic (1%; 5/523), Hie (0,8%; 4/523) y Hid (0,4%; 2/523). El 48,4% de las infecciones invasivas estaban causadas por HiNT y el 46,1% por Hib. El 88,6% de las cepas procedían de casos de meningitis, el 70,8% de sepsis y el 50,9% de neumonías en niños menores de 5 años. El 84% (181/217) de las cepas correspondían a enfermedades invasivas causadas por Hib en menores de 5 años, registrándose la mayor proporción entre 2001−2003. Los biotipos más prevalentes fueron el biotipo I (29%), biotipo II (12%), biotipo III (24%) y biotipo IV (13%). El 13% (68/523) de los aislados fueron resistentes a la ampicilina.
ConclusiónTras de la introducción de la vacuna frente a Hib en Paraguay, los casos de enfermedad invasiva por Hib disminuyeron en niños menores de 5 años y hubo un aumento de HiNT en los mayores de 5 años. Es necesaria una vigilancia continua para monitorizar la eficacia de la vacuna y desarrollar intervenciones preventivas.
Haemophilus influenzae (H. influenzae) is a commensal Gram-negative micro-organism in the nasopharynx that can cause serious invasive diseases, such as meningitis, pneumonia and sepsis, in susceptible individuals. The invasive disease primarily affects children under 2 years of age, as well as elderly and immunocompromised individuals.1,2
One of the most important factors in H. influenzae virulence is the polysaccharide capsule, which is the antigen involved in isolate serotyping and in vaccine immunity. This microorganism is divided into 6 different serotypes, based on their capsules, called H. influenzae a (Hia), b (Hib), c (Hic), d (Hid), (Hie) and f (Hif).3 Some capsule-free isolates are less virulent and are referred to as unencapsulated or non-typeable (NTHi).4,5H. influenzae is also divided into 8 different biotypes (I, II, III, IV, V, VI, VII and VIII) based on their biochemistry and enzyme properties.
Hib is the most invasive serotype and is recognised as a significant cause of pneumonia and meningitis.6 Prior to the introduction of the Hib conjugate vaccine, there were approximately 2.2 million cases of serious Hib disease due to Hib alone every year, most in low-income areas,7 and an estimated 386,000 deaths per year. Moreover, in the pre-vaccine period, Hib caused at least 95% of invasive diseases in children. The conjugate vaccine has been highly effective, reducing rates of disease in both developed and developing countries, with decreases in invasive disease due to Hib of 90%–98%.8 In 2004, Paraguay added the pentavalent vaccine, including Hib, to its national vaccination schedule for children aged 2, 4 and 6 months, free of charge. According to data from the country's extended immunisation schedule, this vaccine has a level of coverage in Paraguay of 73% (2019).
Over the past decade, an increased prevalence of infections caused by NTHi has been reported all around the world, suggesting the replacement of serotype b in the post-vaccine period, as a new ecological niche1; a trend towards an increased incidence of serious diseases caused by NTHi has also been reported.
Given that early treatment is required in cases of serious invasive disease, it is necessary to start empirical treatment while awaiting culture and antibiogram results, and so it is important to know the local antibiotic sensitivity of this pathogen. Ampicillin resistance led to the use of third-generation cephalosporins as empirical antimicrobial agents.9 Resistance is due to production of beta-lactamases and/or alteration of penicillin-binding proteins (PBPs).10
The objective of this study was to report the phenotypic characteristics, genotypic characteristics and sensitivity to antimicrobial agents of invasive and non-invasive H. influenzae isolates received at the Laboratorio Nacional de Referencia de Paraguay between 1999 and 2017.
MethodsThis was an observational, descriptive, retrospective, cross-sectional study. It included both invasive and non-invasive isolates in cerebrospinal fluid, blood, pleural fluid, eye secretions and ear secretions from different patients of all ages who were referred to the Laboratorio Central de Salud Pública (LCSP) by various sentinel sites and collaborating centres in Paraguay from 1999 to 2017. No isolates of other respiratory origin were included in this study.
Isolation, identification and serotypingIsolation and identification were performed by conventional microbiological methods: Gram staining, colony morphology on chocolate agar, and requirement for V and X factors (Difco, United States). Serotyping of isolates was performed using the plate agglutination technique, with polyvalent and monovalent antisera (Difco, United States), and confirmed by polymerase chain reaction based on detection of serotype-specific capsular genes, as previously reported.11 Biotypes were identified using urease, ornithine decarboxylase and indole production tests.
Sensitivity to antimicrobial agentsThe minimum inhibitory concentration of ampicillin and ceftriaxone was determined using the epsilometric Etest method (bioMérieux, France) and by means of disc diffusion (Kirby Bauer) for trimethoprim/sulfamethoxazole, chloramphenicol, cefuroxime and rifampicin in Haemophilus test medium agar (Oxoid, United Kingdom), supplemented with haematin and nicotinamide adenine dinucleotide (Oxoid, United Kingdom). Beta-lactamase production was determined by the chromogenic cephalosporin method, using nitrocefin discs (BBL, United States).
H. influenzae ATCC 49247 and H. influenzae ATCC 49766 were used as quality control strains.
The techniques were performed and interpreted according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI).12
Statistical analysisThis study was limited to data reporting and did not include any inferential statistical analysis. Absolute frequencies and percentages were calculated along with their corresponding 95% confidence intervals. The variables analysed were age group (under 2, 2–9, 10–19, 20–29, 30–39, 40–49, 50–59 and over 60 years of age); sample identification code; sample type; sample acquisition date; diagnosis; micro-organism (confirmed to be phenotypically or genotypically consistent with H. influenzae); H. influenzae serotype (a, b, c, d, e, f or NTHi); biotype (I, II, III, IV, V, VI, VII or VIII); sensitivity to ampicillin, ceftriaxone, cefuroxime, trimethoprim/sulfamethoxazole, chloramphenicol and rifampicin; and beta-lactamase production. Data from the pre-vaccine period (1999–2003) and the post-vaccine period (2004–2017) were grouped.
Data analysis was performed using the EPI-Info software program, version 7.2.
Ethical considerationsThis study was approved by the Laboratorio Central de Salud Pública Independent Ethics Committee (IEC) in its opinion no. 112/2019. No informed consent statement was obtained as the study was observational, with minimal or null risk for the subjects, and covered a very long period such that it was impossible to collect informed consent forms from all study subjects. Personal data confidentiality was respected at all times and only investigators were able to access the referenced data.
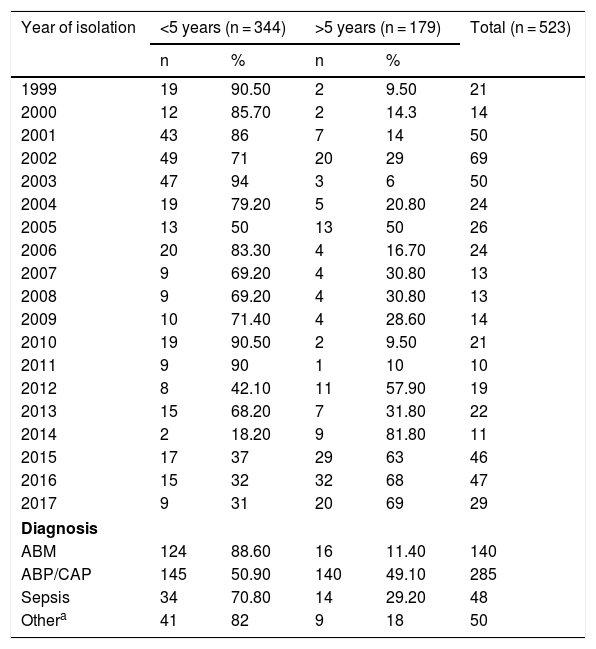
ResultsBetween 1999 and 2017, a total of 523 H. influenzae isolates from invasive disease (473/523) and non-invasive disease (50/523) in all age groups were studied by conventional microbiology techniques and polymerase chain reaction. The distribution by age group was as follows: 48.4% were under 2; 24.47% were 2–9; 3.63% were 10–19; 2.87% were 30–39; 2.1% were 40–49; 4.58% were 50–60 and 10.32% were over 60 years of age. In general, 65.77% of isolates (344/523) corresponded to children under 5 and 34.23% (179/523) corresponded to children over 5 years of age. Among them, 88.6% came from cases of meningitis, 70.8% came from cases of sepsis and 50.9% came from cases of pneumonia in children under 5 years of age. In the entire sample, 50/523 (9.56%) isolates corresponded to non-invasive disease (conjunctivitis and otitis) (see Table 1).
Distribution of Haemophilus influenzae isolates by year, diagnosis and age group in Paraguay (1999–2017) (n = 523).
| Year of isolation | <5 years (n = 344) | >5 years (n = 179) | Total (n = 523) | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| 1999 | 19 | 90.50 | 2 | 9.50 | 21 |
| 2000 | 12 | 85.70 | 2 | 14.3 | 14 |
| 2001 | 43 | 86 | 7 | 14 | 50 |
| 2002 | 49 | 71 | 20 | 29 | 69 |
| 2003 | 47 | 94 | 3 | 6 | 50 |
| 2004 | 19 | 79.20 | 5 | 20.80 | 24 |
| 2005 | 13 | 50 | 13 | 50 | 26 |
| 2006 | 20 | 83.30 | 4 | 16.70 | 24 |
| 2007 | 9 | 69.20 | 4 | 30.80 | 13 |
| 2008 | 9 | 69.20 | 4 | 30.80 | 13 |
| 2009 | 10 | 71.40 | 4 | 28.60 | 14 |
| 2010 | 19 | 90.50 | 2 | 9.50 | 21 |
| 2011 | 9 | 90 | 1 | 10 | 10 |
| 2012 | 8 | 42.10 | 11 | 57.90 | 19 |
| 2013 | 15 | 68.20 | 7 | 31.80 | 22 |
| 2014 | 2 | 18.20 | 9 | 81.80 | 11 |
| 2015 | 17 | 37 | 29 | 63 | 46 |
| 2016 | 15 | 32 | 32 | 68 | 47 |
| 2017 | 9 | 31 | 20 | 69 | 29 |
| Diagnosis | |||||
| ABM | 124 | 88.60 | 16 | 11.40 | 140 |
| ABP/CAP | 145 | 50.90 | 140 | 49.10 | 285 |
| Sepsis | 34 | 70.80 | 14 | 29.20 | 48 |
| Othera | 41 | 82 | 9 | 18 | 50 |
ABP/CAP: acute bacterial pneumonia/community-acquired pneumonia; MBA: acute bacterial meningitis.
The most commonly isolated serotype was NTHi with 51.8% (271/523), followed by Hib with 43% (225/523), Hia and Hif each with 1.5% (8/523), Hic with 1% (5/523), Hie with 0.8% (4/523) and Hid with 0.4% (2/523).
Regarding invasive disease (meningitis, pneumonia and sepsis), 48.4% (229/473) of cases were caused by NTHi isolates, 46.1% (218/473) by Hib isolates, 1.7% (8/473) by Hif isolates, 1.1% (5/473) by Hic isolates, 0.6% (3/473) by Hie isolates and 0.4% (2/473) by Hid isolates. With regard to non-invasive disease, (conjunctivitis and otitis), NTHi was found in 84% (42/50) of cases, Hib in 14% (7/50), Hia in 1% (8/50) and Hie in 2% (1/50).
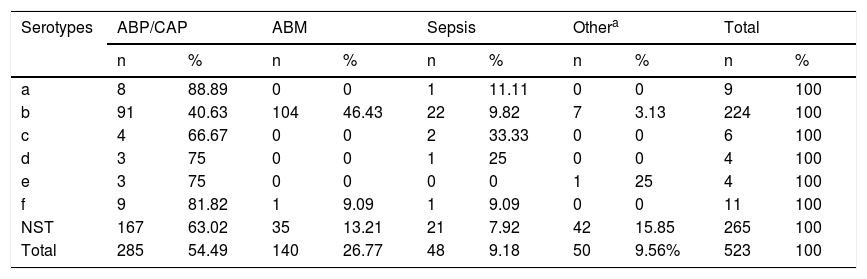
Serotype a was associated primarily with pneumonia, in 88.89% (8/9) of cases; Hib with meningitis, in 46.42% (104/224); and Hic, Hid, Hie and Hif with pneumonia, in 66.67% (4/6), 75% (3/4), 75% (3/4) and 81.82% (9/11) of cases, respectively. As for NTHi, 63.02% (167/265) of cases were isolated from patients with pneumonia, 15.84% (42/265) from patients with other diseases such as conjunctivitis and otitis, 13.2% (35/265) from patients with acute bacterial meningitis and 7.92% (21/265) from patients with sepsis (see Table 2).
Distribution of Haemophilus influenzae serotypes by diagnosis in Paraguay (1999–2017) (n = 523).
| Serotypes | ABP/CAP | ABM | Sepsis | Othera | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| a | 8 | 88.89 | 0 | 0 | 1 | 11.11 | 0 | 0 | 9 | 100 |
| b | 91 | 40.63 | 104 | 46.43 | 22 | 9.82 | 7 | 3.13 | 224 | 100 |
| c | 4 | 66.67 | 0 | 0 | 2 | 33.33 | 0 | 0 | 6 | 100 |
| d | 3 | 75 | 0 | 0 | 1 | 25 | 0 | 0 | 4 | 100 |
| e | 3 | 75 | 0 | 0 | 0 | 0 | 1 | 25 | 4 | 100 |
| f | 9 | 81.82 | 1 | 9.09 | 1 | 9.09 | 0 | 0 | 11 | 100 |
| NST | 167 | 63.02 | 35 | 13.21 | 21 | 7.92 | 42 | 15.85 | 265 | 100 |
| Total | 285 | 54.49 | 140 | 26.77 | 48 | 9.18 | 50 | 9.56% | 523 | 100 |
ABP/CAP: acute bacterial pneumonia/community-acquired pneumonia; MBA: acute bacterial meningitis; NST: non-serotypeable.
The biotypes of 384 H. influenzae strains were determined. Of these, 350/584 were from invasive disease and 34/584 were from non-invasive disease. Biotype I was identified in 29%, biotype II in 12%, biotype III in 24%, biotype IV in 13%, biotype V in 4%, biotype VI in 7%, biotype VII in 2% and biotype VIII in 9%. Invasive diseases were most often associated with biotype I (39%), and non-invasive diseases were most often associated with biotype III (32%). Biotype I was prevalent in serotype b in 70% of cases of invasive disease, and biotype III was prevalent in 96% of cases in non-typeable isolates (NTHi) from non-invasive disease.
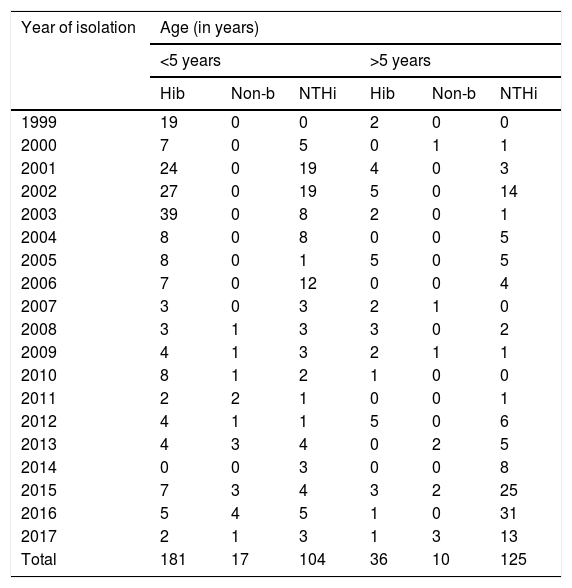
Some 84% (181/217) of the strains included between 1999 and 2017 corresponded to invasive diseases caused by serotype b in children under 5 years of age, with the largest proportion recorded between 2001 and 2003. No variations were observed in isolation of serotype b in children over 5 years of age.
In those under 5, non-typeable strains were primarily isolated in 2001, 2002 and 2006. However, in those aged over 5, the largest number of isolates was detected in the last few years of the study (2015, 2016 and 2017) (see Table 3).
Distribution of Haemophilus influenzae serotypes in invasive disease by age group in Paraguay (1999–2017) (n = 473).
| Year of isolation | Age (in years) | |||||
|---|---|---|---|---|---|---|
| <5 years | >5 years | |||||
| Hib | Non-b | NTHi | Hib | Non-b | NTHi | |
| 1999 | 19 | 0 | 0 | 2 | 0 | 0 |
| 2000 | 7 | 0 | 5 | 0 | 1 | 1 |
| 2001 | 24 | 0 | 19 | 4 | 0 | 3 |
| 2002 | 27 | 0 | 19 | 5 | 0 | 14 |
| 2003 | 39 | 0 | 8 | 2 | 0 | 1 |
| 2004 | 8 | 0 | 8 | 0 | 0 | 5 |
| 2005 | 8 | 0 | 1 | 5 | 0 | 5 |
| 2006 | 7 | 0 | 12 | 0 | 0 | 4 |
| 2007 | 3 | 0 | 3 | 2 | 1 | 0 |
| 2008 | 3 | 1 | 3 | 3 | 0 | 2 |
| 2009 | 4 | 1 | 3 | 2 | 1 | 1 |
| 2010 | 8 | 1 | 2 | 1 | 0 | 0 |
| 2011 | 2 | 2 | 1 | 0 | 0 | 1 |
| 2012 | 4 | 1 | 1 | 5 | 0 | 6 |
| 2013 | 4 | 3 | 4 | 0 | 2 | 5 |
| 2014 | 0 | 0 | 3 | 0 | 0 | 8 |
| 2015 | 7 | 3 | 4 | 3 | 2 | 25 |
| 2016 | 5 | 4 | 5 | 1 | 0 | 31 |
| 2017 | 2 | 1 | 3 | 1 | 3 | 13 |
| Total | 181 | 17 | 104 | 36 | 10 | 125 |
Hib: Haemophilus influenzae serotype b; Non-b: Haemophilus influenzae serotype a, c, d, e or f; NTHi: non-typeable Haemophilus influenzae.
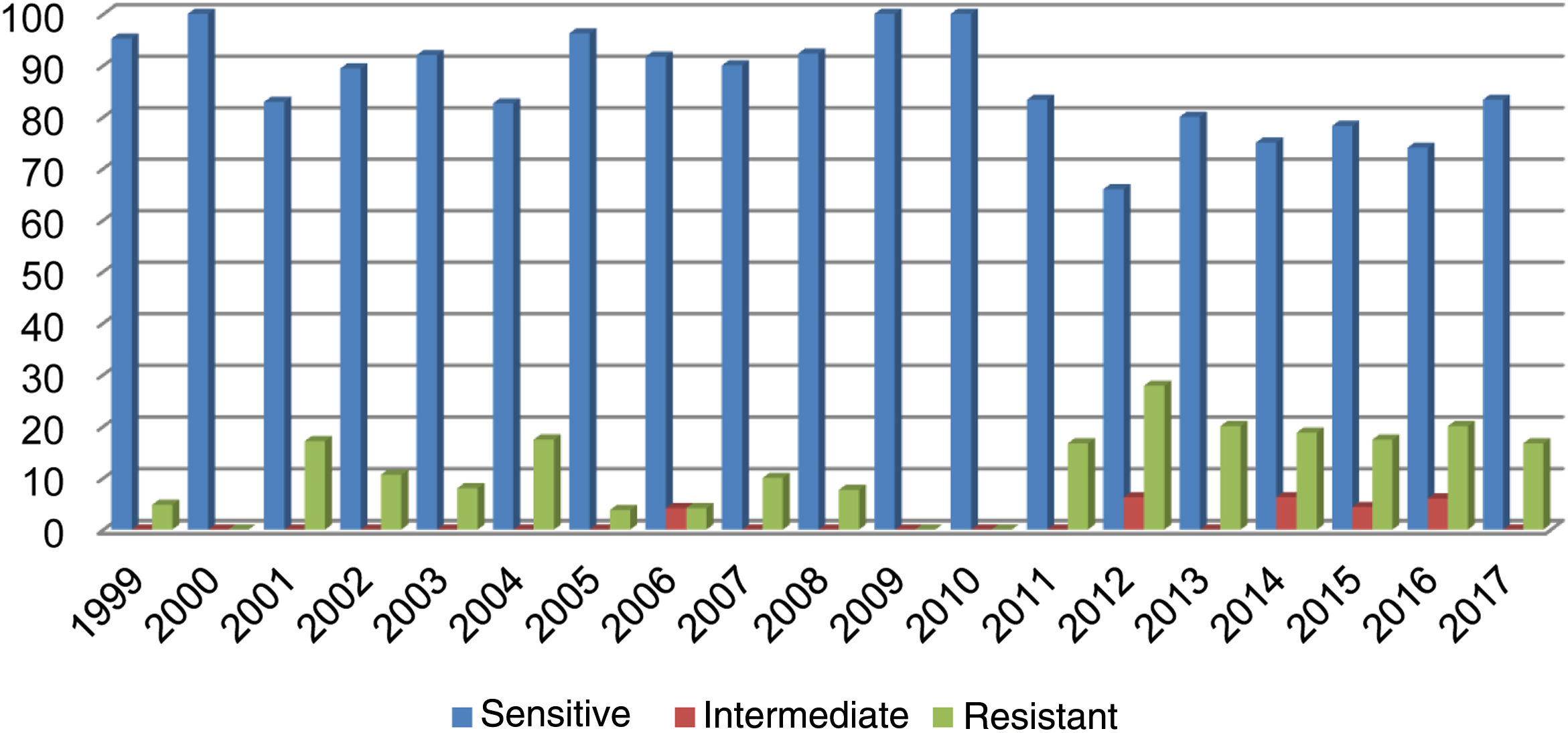
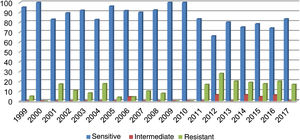
Out of all the isolates studied, 13% (68/523) were beta-lactamase-positive and ampicillin-resistant, and of those, 36/68 corresponded to pneumonia, 16/68 to meningitis, 9/68 to sepsis and 7/68 to other cases (conjunctivitis or otitis) and one case of non-beta-lactamase-producing, ampicillin-resistant H. influenzae. The highest resistance (27.80%) was recorded in 2012 (Fig. 1). Of the isolates, 17% were resistant to trimethoprim/sulfamethoxazole and 7.83% were resistant to chloramphenicol, while all isolates were sensitive to ceftriaxone, cefuroxime, and rifampicin.
DiscussionThis study characterised 523 H. influenzae isolates from invasive and non-invasive disease. Regarding diagnosis, pneumonia and meningitis were most common in children under 5 years of age and were predominantly caused by serotype b.
The incidence of Hib in invasive diseases and in healthy carriers has drastically decreased in countries where a national immunisation schedule has been implemented.13 Very little is known about the epidemiology and clinical significance of invasive H. influenzae infection following the introduction of conjugate vaccines against Hib. The conjugate Hib vaccine was introduced in the United States in 1987, and from 1999 to 2000 the annual incidence of invasive disease due to Hib in children under 5 years of age decreased by 99%, corresponding to less than one case per 100,000 children.14 Therefore, the increase in disease caused by serotypes a, c, d, e and f is infinitesimal compared to the reduction in disease caused by Hib. Various studies conducted in the United States, Canada, England and Wales have shown an increasing incidence of Hif over time. Hie has also increased in England and Wales, being the third most common capsular subtype after Hif and Hib in those countries.15
In Paraguay, with the introduction of the Hib vaccine in 2004, a decrease in Hib was seen in cases of invasive disease in children under 5 years of age; in contrast, an increase in relative frequency of NTHi was found in those over 5 years of age in 2016. Other countries in the Americas have reported similar changes.16,17
In the pre-vaccination period, NTHi was not a common known cause of invasive infection; however, it is currently the principal cause of invasive disease in all age groups.18 Although NTHi is a well known cause of pneumonia in adults, data on childhood pneumonia are limited.19 A study conducted in England and Wales found, between 2000 and 2013, 1585 cases of invasive infection due to H. influenzae in children 1 month to 10 years old, with NTHi causing 31–51 cases per year (0.53−0.92/100,000).20 NTHi is gaining recognition as an emerging pathogen. Routine immunisation of infants with conjugate Hib vaccines has brought about a marked change in the predominant invasive serotype, from Hib to NTHi.21
The other H. influenzae serotypes (a, c, d, e and f) were isolated less often. Serotype f was the third most common, and serotype e proved the least common. According to some studies, serotypes e and f did not increase following vaccination campaigns, rather they appeared as opportunistic infections in adults with underlying disease.22 Serotype a was isolated with a frequency of 1.72%. As most cases of Hia correspond to sporadic diseases, published reports are not always consistent in terms of findings. The highest rates of incidence of invasive diseases due to Hia have been found in certain indigenous populations, such as Native Americans/First Nations including the Inuit in Alaska and northern Canada, reaching the order of magnitude of the rates of incidence of Hib in the pre-vaccine period.1 In Brazil, passive surveillance showed that Hib accounted for 59% of isolates from patients with meningitis, Hia 14% and Hif 2.90%, and that NTHi increased from 2% to 22% in the post-vaccine period.23
During this study period, a prevalence of biotype i was found in serotype b. In children, biotype I and serotype b are commonly associated with meningitis, biotypes II and III are commonly associated with respiratory tract infections, and NTHi isolates are commonly associated with biotype IV.4 Previous studies conducted in Denmark, Norway, Eastern Europe and the United States had shown biotype i to account for the majority of cases of meningitis due to H. influenzae (96%) with a predominance in children under 10 years of age.24 In the United States, 95% of invasive H. influenzae isolates belong to serotype b and biotype I. In Eastern Europe, only biotypes I and II are prevalent in most countries, though biotypes III, IV and V have also been detected. Serotypes a and c are associated with biotypes I and IV, respectively, whereas NTHi isolates feature a predominance of biotypes II and III. In Brazil, it was demonstrated that 51% of strains belonged to biotype I, 2% belonged to biotype III, 31% belonged to biotype IV, 2% belonged to biotype V, 7% belonged to biotype VI and 7% belonged to biotype VIII.25 In Argentina, the biotypes of 306 isolates were determined; all Hia isolates corresponded to biotype II and 66.7% of Hib isolates belonged to biotype I.26 A study in Cuba found no change in biotypes I and II in the pre-vaccine and post-vaccine periods and the appearance of Hib isolates belonging to biotype VIII, which had not been reported in the pre-vaccine period.27
The rate of ampicillin resistance found in this study was 13%. Similar data have been observed in Latin America and the Caribbean.28 Resistance in Europe and North American has ranged from 8% to 30% and been as high as 50% in some East Asian countries.29 Beta-lactamase-negative, ampicillin-resistant isolates with modifications in PBP3 may show decreased sensitivity both to aminopenicillins and to some cephalosporins.30 This study found no resistance to the cephalosporins evaluated.
One limitation of this study was that certain data were incomplete owing to the study's retrospective nature. However, its strength lies in the fact that these data were collected over a long period of time at the Laboratorio de Referencia Nacional [Paraguay National Reference Laboratory], where these isolates are frequently received for analysis and confirmation.
It can be concluded that ongoing surveillance is needed to monitor the efficacy of the Hib vaccine and to detect any emerging invasive capsular type in all age groups and clinical presentations so that preventive interventions may be developed.
FundingThis study has received no specific funding from public, private or non-profit organisations.
Conflicts of interestNone.
The conduct of this study was supported by grants from the Fondo para la convergencia estructural del Mercosur [Mercosur Structural Convergence Fund], (FOCEM)-Mercosur, FOCEM agreement no. 03/11, project "Investigación, Educación y Biotecnologías Aplicadas a la Salud" ["Research, Education and Biotechnologies Applied to Health"] (COF 03/11).
Please cite this article as: León ME, Kawabata A, Nagai M, Rojas L, Chamorro G, Zárate N, et al. Estudio epidemiológico de Haemophilus influenzae causante de enfermedad invasiva y no invasiva en Paraguay (1999–2017). Enferm Infecc Microbiol Clin. 2021;39:59–64.