The aim of this study was to determine the antimicrobial resistance rates and their evolution in clinical isolates of Pseudomonas aeruginosa (P. aeruginosa) causing invasive infections in the south of Spain between 2012 and 2017.
MethodsRetrospective study consisting of the collection of microbiological data from 20 hospitals (14 from Andalucía, 5 from Extremadura and 1 from Ceuta) between 2012 and 2017. The main variables studied were the antimicrobial susceptibility testing system used, interpretation criteria (CLSI or EUCAST) and the rate or percentage of resistant isolates.
ResultsThe most widely used antimicrobial susceptibility testing system was MicroScan (58%). The global resistance rates varied between 25% (ciprofloxacin) and 4% (colistin) using EUCAST, and between 19% (ciprofloxacin and imipenem) and 3% (amikacin) using CLSI. The antimicrobial resistance rates were relatively stable throughout the period 2012–2017. 14% of isolates were MDR and 7% were XDR. Respiratory isolates were more resistant, particularly to ciprofloxacin and colistin, than isolates from urine or blood.
ConclusionsThe antimicrobial resistance rates in P. aeruginosa are not particularly high in the south of Spain. The highest resistance rates were observed with ciprofloxacin, piperacillin/tazobactam and meropenem, whereas the more active antimicrobials were colistin, tobramycin and amikacin. The highest resistance rates were seen in respiratory isolates. In general, the resistance rates remained stable during the study period for most of the antimicrobials studied.
El objetivo de este estudio es conocer las tasas de resistencia antimicrobiana y la evolución de las mismas durante 2012-2017 en aislados de Pseudomonas aeruginosa (P. aeruginosa) causante de infección invasiva en el sur de España.
MétodosEstudio retrospectivo de recogida de datos microbiológicos en 20 centros hospitalarios (14 de Andalucía, 5 de Extremadura y 1 de Ceuta) durante 2012-2017. Las principales variables de estudio fueron: sistema de antibiograma usado, interpretación (CLSI o EUCAST) y tasa o porcentaje de aislados resistentes.
ResultadosEl sistema de antibiograma más utilizado fue MicroScan (58%). Los porcentajes de resistencia globales variaron entre el 25% (ciprofloxacino) y el 4% (colistina) usando EUCAST, y entre el 19% (ciprofloxacino e imipenem) y el 3% (amicacina) usando CLSI. Las tasas de resistencia se mantuvieron relativamente estables durante 2012-2017. El 14% de los aislados fueron MDR y el 7% XDR. Los aislados de muestras respiratorias presentaron mayores tasas de resistencia antimicrobiana, particularmente a ciprofloxacino y colistina, que los aislados de orina o sangre.
ConclusionesLas tasas de resistencia antimicrobiana en P. aeruginosa no son demasiado elevadas en el sur de España. Las tasas de resistencia más elevadas se observan para ciprofloxacino, piperacilina/tazobactam y meropenem, siendo los antimicrobianos de mayor actividad colistina, tobramicina y amicacina. Las mayores tasas de resistencia se observan en aislados de muestras respiratorias. En general las tasas de resistencia se mantienen estables durante el periodo de estudio para la mayoría de los antimicrobianos evaluados.
Nosocomial infections constitute a serious global health problem with important economic and social repercussions.1,2Pseudomonas aeruginosa is an opportunistic pathogen capable of causing a wide spectrum of infections, with invasive infections being the most clinically relevant because they are associated with high morbidity and mortality rates.3,4 This pathogen is characterized by its high intrinsic resistance to many antimicrobials and its ability to acquire resistance even to broad-spectrum agents such as carbapenems and polymyxins.5–8
Among the therapeutic options in infections caused by non-multi-resistant P. aeruginosa are beta-lactams (e.g. piperacillin/tazobactam, ceftazidime, cefepime, imipenem, meropenem, aztreronam), fluoroquinolones and aminoglycosides. However, in infections produced by multi-resistant P. aeruginosa the therapeutic options can be considerably reduced, the only option being to use antimicrobials that can present toxicity problems, such as colistin, or newer antimicrobials with less experience, such as ceftolozane/tazobactam and ceftazidime/avibactam.9–13
The choice of an empirical antimicrobial treatment requires knowing, among other factors, the local rates of susceptibility or resistance. The rates of antimicrobial resistance in this species are variable and depend on geographic factors (e.g. local resistance rates) and microbiological factors (e.g. clone and resistance mechanisms). In Spain there are very few studies that provide knowledge of the rates of antimicrobial resistance in isolates of P. aeruginosa that cause invasive infection.14–18 The objective of this study is to find out (i) the rates of antimicrobial resistance globally and by type of sample in isolates of P. aeruginosa causing invasive infection in southern Spain, and (ii) how antimicrobial resistance rates have evolved during the 2012–2017 period.
Materials and methodsA retrospective descriptive study of demographic and microbiological data collected during 2012–2017 in 20 hospitals: 14 in Andalusia, 5 in Extremadura and 1 in Ceuta. Data were collected on the first 3 isolates for each year and type of infection (bacteraemia, intra-abdominal infection, nosocomial UTI and nosocomial pneumonia). In patients in whom more than one isolate was obtained per type of infection and year only the first isolate was included. The study variables were: department, origin of the samples, antibiogram system used, interpretation (CLSI or EUCAST) and rate or percentage of resistant isolates. Both bacterial identification and susceptibility tests for these antimicrobials were performed at each site. The antimicrobials analyzed were piperacillin/tazobactam, ceftazidime, cefepime, imipenem, meropenem, aztreonam, tobramycin, amikacin, ciprofloxacin and colistin. For each antimicrobial the corresponding clinical category was determined according to the EUCAST 2019 and CLSI 2019 breakpoints.19,20
Statistical analysis of the discrepancies between the qualitative variables was performed using the Chi squared test, or Fisher's exact test, if applicable. Differences with values of p<=0.05 were considered statistically significant.
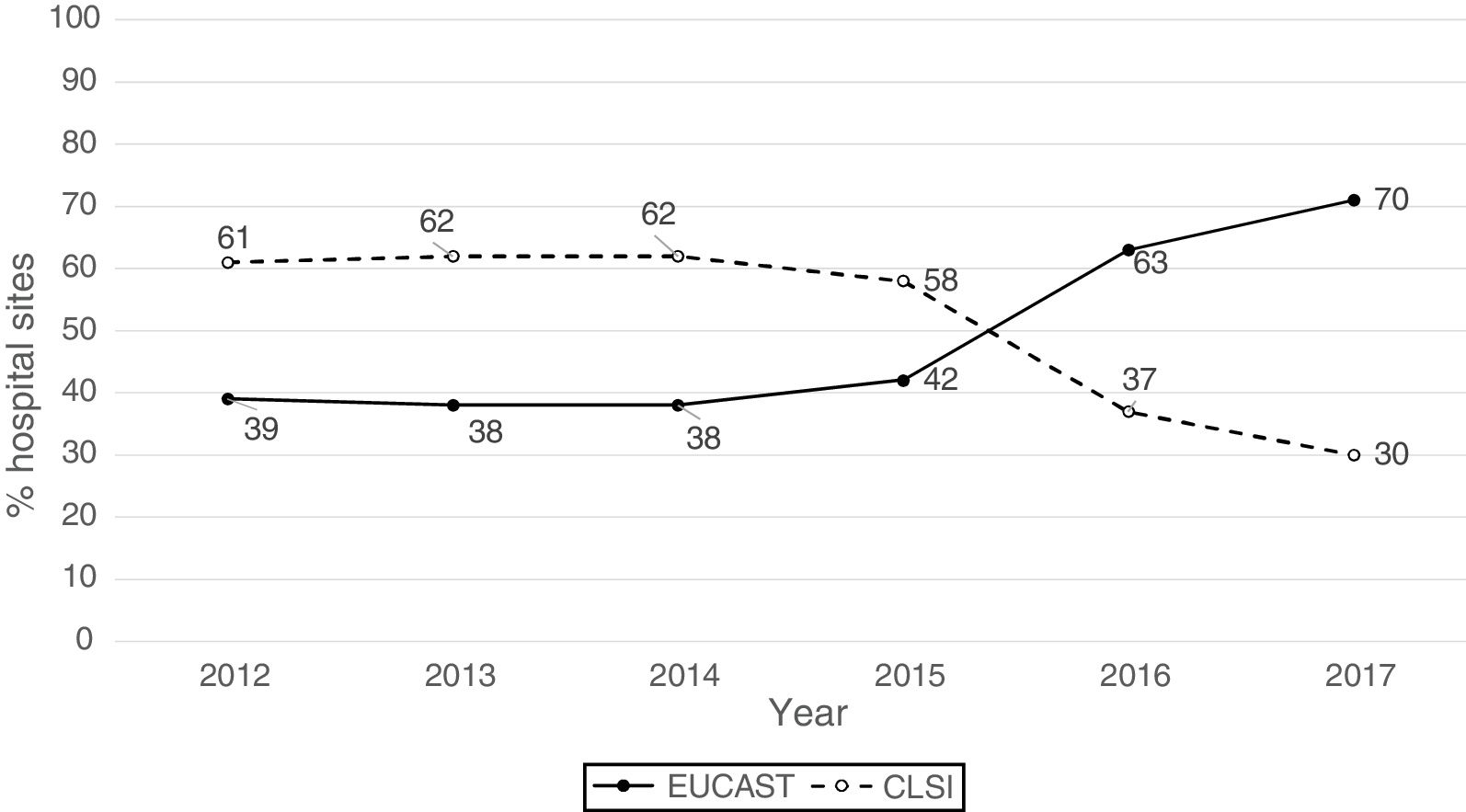
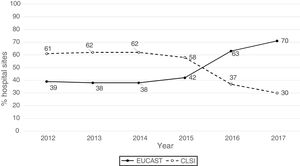
ResultsThe origin by type of Unit or Department of the 1341 analysable data points was as follows: medical units (47%), surgical units (26%), ICU (22%), Emergency departments (4%) and other departments (1%). The isolates were obtained from respiratory tract (27%), urine (25%), blood (24%), abdominal (23%), and other samples (1%). The antibiogram systems used were: MicroScan (58%), Vitek 2 (24%), Wider I (15%), Phoenix (2%), diffusion with discs (0.7%) and diffusion with gradient strips (0.3%). Fifty-two percent of the susceptibility results were obtained using the CLSI 2019 breakpoints and 48% using the breakpoints proposed by EUCAST in 2019. Fig. 1 shows the percentages of susceptibility results by year and by breakpoint used (CLSI or EUCAST of 2019) and as can be seen, as of 2014 there is a progressive increase in the number of sites that use the EUCAST breakpoints at the expense of a decrease in sites using the CLSI breakpoints.
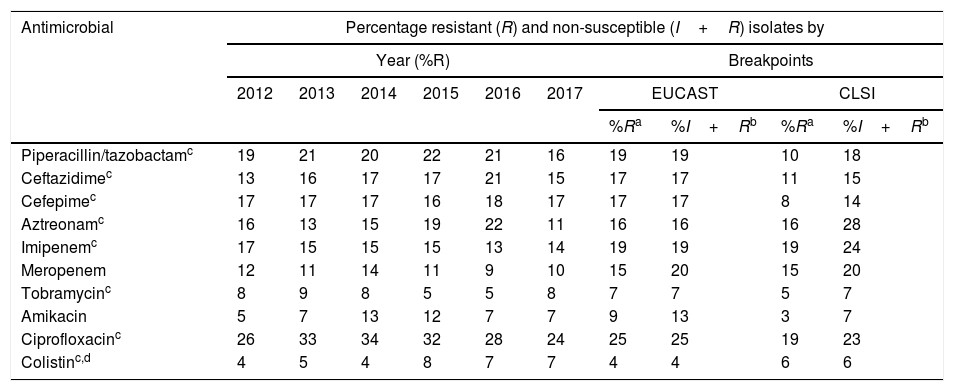
To analyze the resistance rates, two groups of isolates were considered: non-susceptible isolates (includes resistant isolates and isolates with intermediate susceptibility) and resistant isolates (includes resistant but non-isolated isolates with intermediate susceptibility). As shown in Table 1, the overall resistance rates of resistant isolates varied between 25% (ciprofloxacin) and 4% (colistin), using the breakpoints proposed by EUCAST in 2019, and between 19% (ciprofloxacin and imipenem) and 3% (amikacin), using the 2019 CLSI breakpoints (Table 1). In non-susceptible isolates, overall resistance rates varied between 25% (ciprofloxacin) and 4% (colistin) using the 2019 EUCAST breakpoints, and between 28% (aztreonam) and 6% (colistin) using the 2019 CLSI breakpoints (Table 1).
Distribution of resistance rates per year using 2019 EUCAST breakpoints and overall resistance rates based on the breakpoints used (EUCAST or CLSI) and of the inclusion or otherwise of isolates with intermediate susceptibility.
| Antimicrobial | Percentage resistant (R) and non-susceptible (I+R) isolates by | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year (%R) | Breakpoints | |||||||||
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | EUCAST | CLSI | |||
| %Ra | %I+Rb | %Ra | %I+Rb | |||||||
| Piperacillin/tazobactamc | 19 | 21 | 20 | 22 | 21 | 16 | 19 | 19 | 10 | 18 |
| Ceftazidimec | 13 | 16 | 17 | 17 | 21 | 15 | 17 | 17 | 11 | 15 |
| Cefepimec | 17 | 17 | 17 | 16 | 18 | 17 | 17 | 17 | 8 | 14 |
| Aztreonamc | 16 | 13 | 15 | 19 | 22 | 11 | 16 | 16 | 16 | 28 |
| Imipenemc | 17 | 15 | 15 | 15 | 13 | 14 | 19 | 19 | 19 | 24 |
| Meropenem | 12 | 11 | 14 | 11 | 9 | 10 | 15 | 20 | 15 | 20 |
| Tobramycinc | 8 | 9 | 8 | 5 | 5 | 8 | 7 | 7 | 5 | 7 |
| Amikacin | 5 | 7 | 13 | 12 | 7 | 7 | 9 | 13 | 3 | 7 |
| Ciprofloxacinc | 26 | 33 | 34 | 32 | 28 | 24 | 25 | 25 | 19 | 23 |
| Colistinc,d | 4 | 5 | 4 | 8 | 7 | 7 | 4 | 4 | 6 | 6 |
Of the antimicrobials evaluated, the only ones with which statistically significant differences were observed when comparing the overall percentages of resistant isolates and the overall percentages of non-susceptible isolates, using EUCAST breakpoints, were amikacin (9% resistant isolates and 13% non-susceptible isolates; p=0.004) and meropenem (15% resistant isolates and 20% non-susceptible isolates, p=0.004). Using the CLSI breakpoints, these percentages were 3% (resistant isolates) and 7% (non-susceptible isolates) for amikacin (p=0.004) and 15% (resistant isolates) and 20% (non-susceptible isolates) for meropenem (p=0.004).
As can be seen in Table 2, during the 2012–2017 period the resistance rates remained relatively stable, except for aztreonam, which decreased from 22% in 2016 to 11% in 2017 (p=0.013), and to a lesser extent piperacillin/tazobactam (21% in 2016 and 16% in 2017; p=0.20) and ceftazidime (21% in 2016 and 15% in 2017; p=0.17).
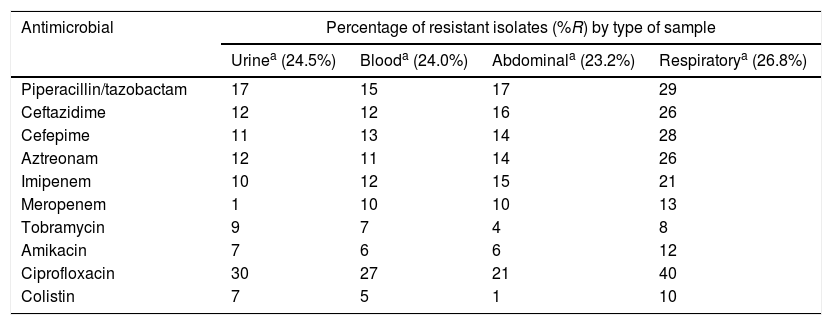
Distribution of antimicrobial resistance rates by sample type.
| Antimicrobial | Percentage of resistant isolates (%R) by type of sample | |||
|---|---|---|---|---|
| Urinea (24.5%) | Blooda (24.0%) | Abdominala (23.2%) | Respiratorya (26.8%) | |
| Piperacillin/tazobactam | 17 | 15 | 17 | 29 |
| Ceftazidime | 12 | 12 | 16 | 26 |
| Cefepime | 11 | 13 | 14 | 28 |
| Aztreonam | 12 | 11 | 14 | 26 |
| Imipenem | 10 | 12 | 15 | 21 |
| Meropenem | 1 | 10 | 10 | 13 |
| Tobramycin | 9 | 7 | 4 | 8 |
| Amikacin | 7 | 6 | 6 | 12 |
| Ciprofloxacin | 30 | 27 | 21 | 40 |
| Colistin | 7 | 5 | 1 | 10 |
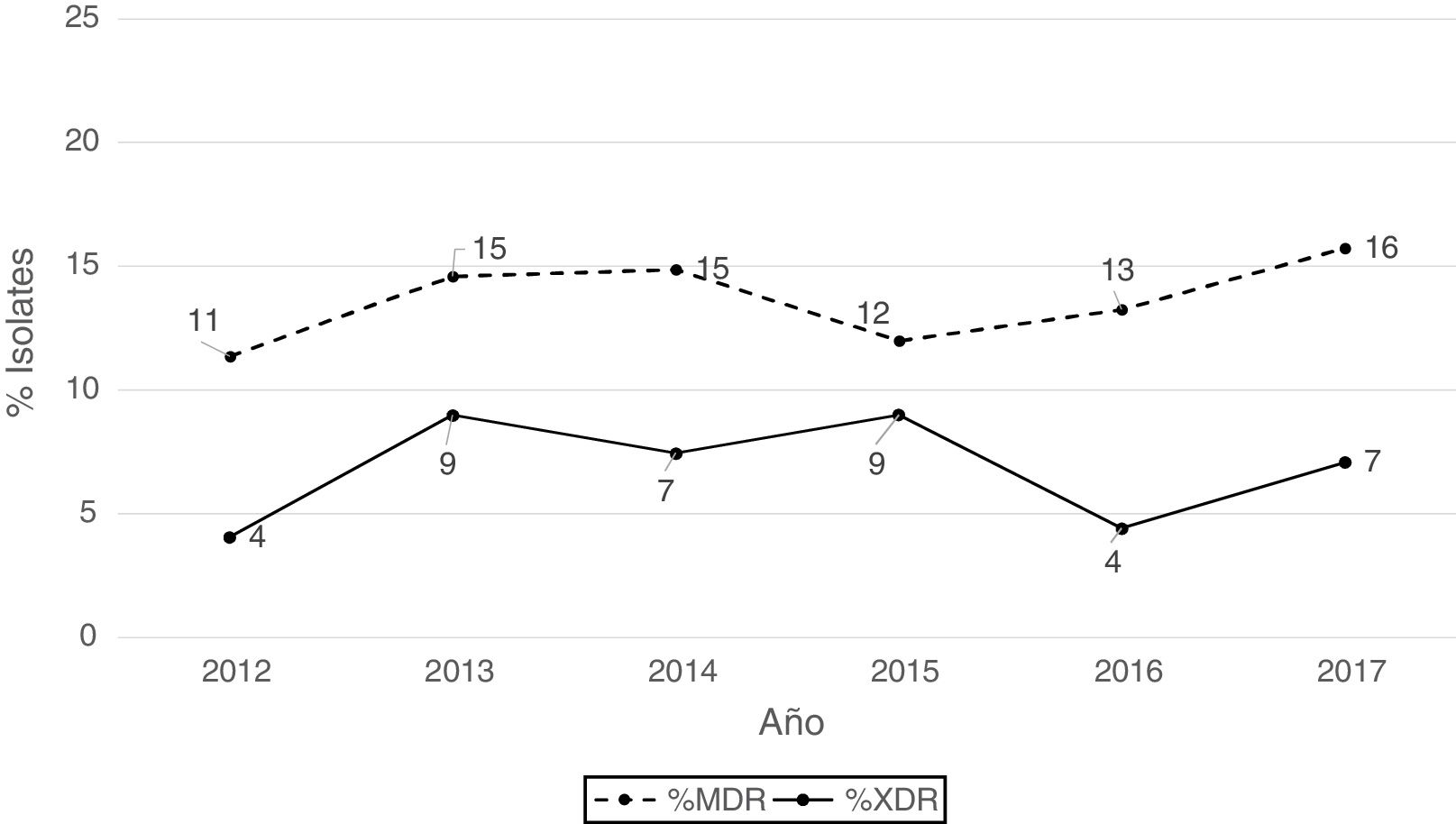
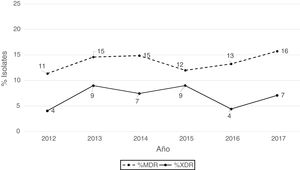
According to the criteria of Magiorakos et al.21 no panresistant isolates were observed, while 14% of the isolates were multidrug-resistant (MDR) and 7% extensively drug-resistant (XDR). As can be seen in Fig. 2, the percentage of MDR isolates increased slightly from 2012 onwards, going from 11% in 2012 to 16% in 2017. With respect to XDR isolates, there is also an increase, albeit less pronounced than in MDR isolates, from 4% in 2013 to 7% in 2017.
The isolates obtained from respiratory samples showed greater resistance to the evaluated antimicrobials, particularly ciprofloxacin (40%), amikacin (12%) and colistin (10%), than the isolates obtained from other samples, such as urine or blood.
DiscussionAccording to the results of the 2017 EPINE study, P. aeruginosa was the second most common nosocomial pathogen (9.6%), along with Staphylococcus aureus, after Escherichia coli (15.6%).22 In recent years there has been a significant, although variable, increase in the antimicrobial resistance rates of P. aeruginosa. This fact justifies the need to know the local rates of resistance to antimicrobials as well as their trends or evolution over time, in order to establish or design adequate therapeutic strategies, especially empirical ones.
The data obtained in this study indicate that the overall rates of antimicrobial resistance in P. aeruginosa in southern Spain (Andalusia, Extremadura and Ceuta) are not very high compared to what is described in other national studies,14–16 and that these vary depending on the clinical breakpoint used (EUCAST or CLSI), the inclusion criteria for isolates (resistant isolates or resistant isolates plus isolates with intermediate susceptibility) and the origin of the isolates.
In recent years, there is a clear trend in the growing use of EUCAST breakpoints, especially in Europe.23,24 The reason is clearly because they are the official breakpoints of the European Medicines Agency. Additionally, many countries in Europe have had an “order” from their ministries for this.
The breakpoint used (EUCAST or CLSI) has had an important effect on most global rates of antimicrobial resistance. For some antimicrobials, such as piperacillin/tazobactam, cefepime, amikacin and ciprofloxacin, higher resistance rates were obtained using EUCAST than with CLSI, so the criterion or breakpoints used to interpret the clinical categories is of special relevance due to the great significance or clinical impact it may have.
Regardless of the breakpoint used, the highest resistance rate was observed with ciprofloxacin (25% EUCAST and 19% CLSI), while the most active antimicrobials were colistin, tobramycin and amikacin, with resistance rates below 10%. These results are consistent with those obtained in previous studies, such as those obtained in a Spanish national study conducted with isolates obtained during 1998–2003 in which the highest resistance rates were observed with ciprofloxacin (28%), aztreonam (23%) and cefepime (20%), while the most active antimicrobials were tobramycin (11%), amikacin (8%) and piperacillin/tazobactam (7%).14 The data obtained in more recent studies conducted in Spain indicate a similar or very similar trend. In a study conducted in Córdoba with isolates from 2005 to 2010, it was observed that the highest resistance rates corresponded to ciprofloxacin (20%), gentamicin (16%) and cefepime (12%), and the antimicrobials with the highest activity were tobramycin (5%), meropenem (7%), piperacillin/tazobactam (7%) and ceftazidime (7%).16 Similar results were obtained in another study conducted in Castellón with isolates from 2004 to 2008, in which the highest resistance rate was obtained with ciprofloxacin (17%), while the most active antimicrobials were amikacin (2%), piperacillin/tazobactam (6%) and meropenem (6%).15
The calculation of antimicrobial resistance rates can be performed using non-susceptible isolates (isolates with intermediate susceptibility and resistant isolates). In our study this consideration had a great impact on the resistance rates of some antimicrobials, particularly when EUCAST breakpoints are applied, as seen above, such as meropenem (15% resistant isolates vs. 20% non-susceptible isolates) and amikacin (9% resistant isolates vs. 13% non-susceptible isolates). Recently, EUCAST has made some modifications or recommendations in relation to isolates with intermediate susceptibility in the sense that these isolates can be considered as susceptible depending on certain factors such as the dose of antimicrobial or the focus of the infection, among others.25 This change will imply a very significant decrease in the resistance rates of many antimicrobials.
Overall, during the study period (2012–2017) there have been no significant changes or trends in the resistance rates of most of the antimicrobials evaluated, except for aztreonam, whose resistance rate in 2017 was halved (11%) compared to 2016 (22%). These results suggest that important changes are not taking place in hospitals in southern Spain at the level of dissemination of resistance determinants or resistant clones. This trend seems to have been maintained since the beginning of 2000 as shown by the results of some previous studies conducted in Spain.14
In our study, no panresistant isolates were observed, while the overall prevalence of MDR (14%) and XDR (7%) isolates was relatively low. These combined resistance percentages are relatively similar to those described in some countries in central and southern Europe, according to ECDC data from 201726; however, these percentages were well below the rates recently described in a multicentre study conducted in Spain, Greece and Italy in which these percentages were 4% for panresistant isolates, 30% for MDR isolates and 36% for XDR isolates.27
The isolates obtained from respiratory samples showed greater resistance to the evaluated antimicrobials, particularly ciprofloxacin and colistin, than the isolates obtained from other samples, such as urine or blood. These results are similar to those observed in other studies and could be related to an increased use of antimicrobials for the treatment of respiratory infections due to P. aeruginosa.15
This work has some limitations. The susceptibility data are those reported by the sites using different methodology, without the data having been verified in a reference laboratory, but we consider that the number of participating sites and the years and types of sample included give us a fairly accurate picture of the situation of P. aeruginosa susceptibility in the south of our country. As long as there are no national standardized databases, this type of study is necessary to acquire this type of information. Another limitation presented by this work is the absence of molecular epidemiology studies (e.g. PFGE, MLST) that could help to understand whether the prevalence of the observed resistance can be affected (or not) in part by certain endemic situations (e.g. resistant clones or dissemination of horizontal resistance determinants) in any of the participating sites.
In conclusion, antimicrobial resistance in P. aeruginosa in southern Spain is not too high compared to what is described in other studies.14–16,26 The highest resistance rates are observed with ciprofloxacin, piperacillin/tazobactam and meropenem, with the most active antimicrobials being colistin, tobramycin and amikacin. The highest resistance rates are observed in respiratory sample isolates. In general, resistance rates remained stable during the study period for most of the antimicrobials evaluated.
FundingThe meetings of the GRAM Group were partially financed by MSD Laboratories.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors of this work express their gratitude to all the sites that have participated selflessly in this study.
Eugenio Garduño (H. Infanta Cristina, Badajoz), Purificación Hernández Pérez (H. San Pedro de Alcántara, Cáceres), José Román Muñoz Sanz (H. Virgen del Puerto, Plasencia, Cáceres), Carmen González Velasco (H. General de Mérida, Mérida, Badajoz), Saray Rodríguez (H. Don Benito, Badajoz), José M. Navarro Marí and María Dolores Rojo Martín (H. Virgen de las Nieves, Granada), Marta Álvarez Estévez and Alejandro Peña Monje (H. Universitario San Cecilio, Granada), Waldo Sánchez Yebra (H. Torrecárdenas, Almería), Carolina Roldán Fontana and Lina Martín Hita (Complejo Hospitalario Jaén, Jaén), Encarnación Clavijo Frutos and María Gracia Ortega (H. Virgen de la Victoria, Málaga), Begoña Palop Borrás and Pilar Bermúdez Ruiz (H. Regional de Málaga, Málaga), José Antonio Lepe and Javier Aznar (H. Virgen del Rocío, Seville), M. Carmen Domínguez (H. La Merced, Osuna, Seville), Irene Gracia Ahufinger and Fernando Rodríguez (H. Reina Sofía, Córdoba), Ana Domínguez Castaño (H. Juan Ramón Jiménez (Huelva), Manuel Rodríguez Iglesias and Fátima Galán Sánchez (H. Puerta del Mar, Cádiz), M. Dolores López Prieto and Juan Manuel Sánchez Calvo (H. de Jerez, Cádiz), José Luis López Barba (H. de Ceuta, Ceuta), Carmen Martínez Rubio (H. Puerto Real, Cádiz).
The members of the GRAM Group are listed in Appendix A.
Please cite this article as: Fernández-Cuenca F, Martínez-Martínez L, Pascual Á, Grupo GRAM. Evolución de la resistencia antimicrobiana en aislados clínicos de Pseudomonas aeruginosa productores de infecciones invasivas en el sur de España. Enferm Infecc Microbiol Clin. 2020;38:150–154.