The Human Papillomavirus (HPV) is a double-stranded DNA virus, with more than 200 different genotypes having been identified. This infection is considered the most common sexually transmitted infection (STI), and it is the cause of a significant number of diseases, both benign lesions (anogenital condylomas) and pre-malignant lesions and different cancers. The diagnosis of the infection is performed by molecular techniques based on the detection of viral DNA, the mRNA of oncogenic proteins and cellular alteration caused by the infection. Although there is no consensus regarding the best treatment, this should be individualised, and there are different options with ablative treatments being more effective but with greater recurrences, and immunomodulatory treatments being less effective in the short term but with fewer recurrences. Among the preventive strategies, vaccination against HPV is the best strategy against anogenital neoplasms and warts, its maximum effectiveness being when it is administered prior to exposure to HPV.
El virus del papiloma humano (VPH) es un virus ADN bicatenario, habiéndose identificado más de 200 genotipos. Su infección es considerada la infección de transmisión sexual (ITS) más frecuente, siendo causa de gran cantidad de enfermedad, tanto lesiones benignas (condilomas anogenitales) como lesiones premalignas y diferentes cánceres. El diagnóstico de la infección se realiza por técnicas moleculares basadas en la detección del ADN vírico, el ARNm de las proteínas oncogénicas y la alteración celular provocada por la infección. Aunque no existe un consenso respecto al mejor tratamiento, debiendo este individualizarse, hay diferentes opciones, siendo los tratamientos ablativos más eficaces, pero con recidivas, y los tratamientos inmunomoduladores menos eficaces a corto plazo, pero con menos recidivas. Entre las estrategias preventivas, la vacunación contra el VPH constituye la mejor frente a las neoplasias y verrugas anogenitales, siendo su eficacia máxima cuando se administra antes de la exposición al VPH.
Human papillomavirus (HPV) infection is considered to be the most common sexually transmitted infection (STI).1 It is particularly prevalent among young women and men and its incidence is directly associated with sexual activity.1,2 There are more than 200 different genotypes of HPV, which are clinically categorised as being of low or high oncogenic risk.2 Low oncogenic risk genotypes (HPV 6 and HPV 11) cause anogenital warts, which are very common benign lesions. High oncogenic risk genotypes (HPV 16 and HPV 18) cause dysplastic lesions, considered to be the direct precursor of a large number of malignancies, particularly of the cervix, anus and oropharynx. HPV infection, as well as the lesions that it causes, can be detected by a range of PCR, cytology and colposcopy techniques.3 The high incidence of cervical cancer and the existence of a clearly established precursor lesion led to the implementation of screening programmes more than four decades ago, which have resulted in a significant drop in cervical cancer rates. The introduction over the last decade of the systematic vaccination of girls with vaccines comprising the most common genotypes has reduced the risk of HPV infection and the onset of warts or malignant lesions even further.3 Nevertheless, the fact that evidence points to the persistence of high-risk groups for these infections (particularly immunosuppressed patients and highly sexually active subjects), as well as a rise in other HPV-related malignancies, is forcing us to prepare new prevention strategies.4 The following chapters will cover everything from virology to the treatment and prevention of genital HPV infection and its associated pathology, with the aim of encouraging the most practical application of the information presented.
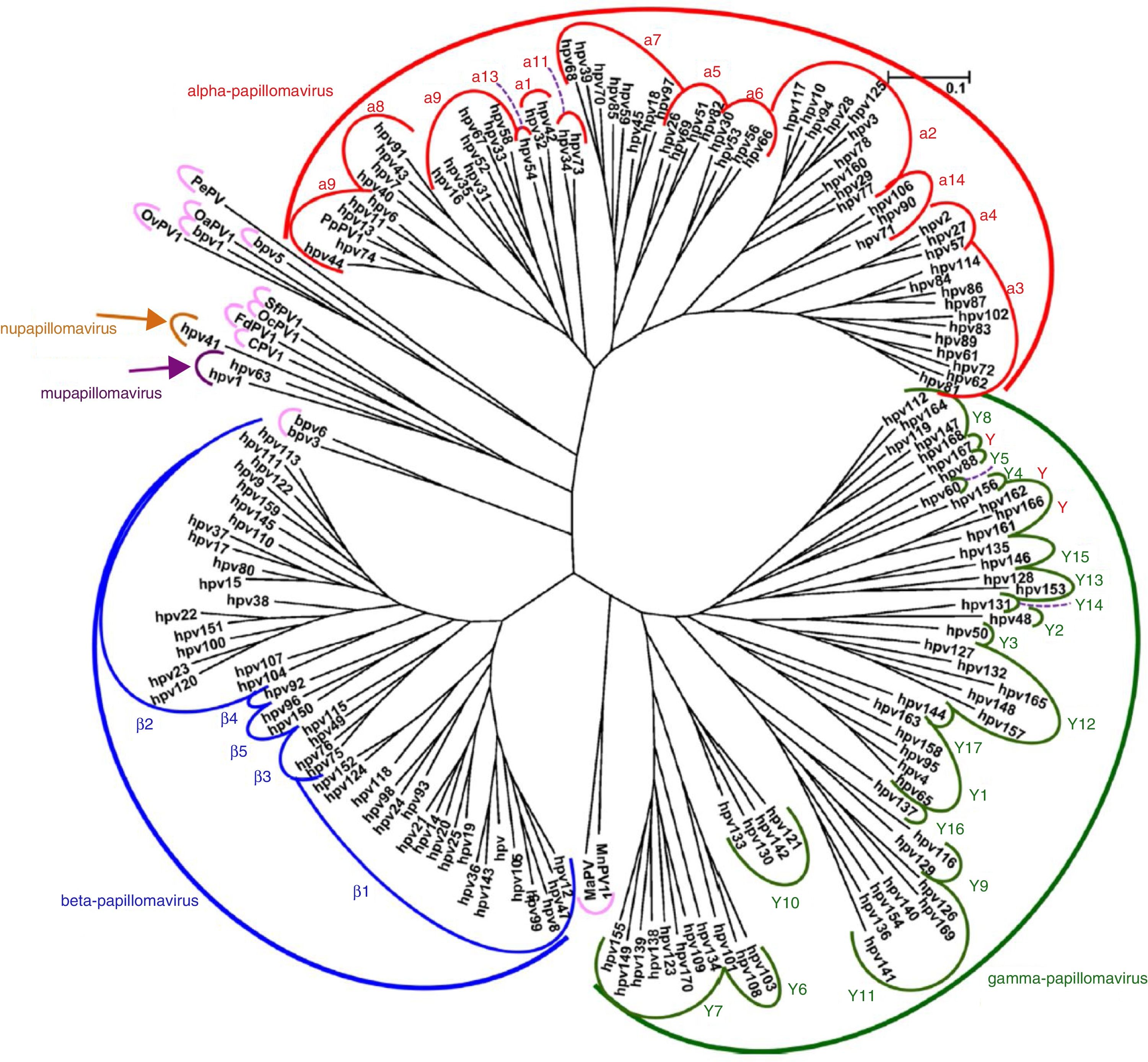
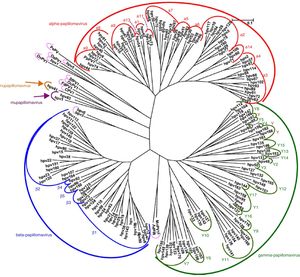
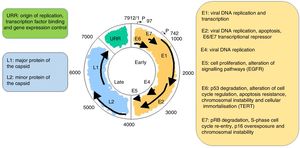
VirologyHPV is part of the family Papillomaviridae, composed of various genera (Alpha-, Beta-, Gamma-, Nu- and Mupapillomavirus) in which species named with correlative numbers are grouped (for example, Alphapapillomavirus has 15 species). Each species is further subdivided into genotypes (commonly known as HPV) (Fig. 1), and more than 200 different genotypes have been identified to date. They are classified according to the structure of the viral genome and human epithelial tissue tropism.5 The genus Alphapapillomavirus includes genotypes that have been reported to cause cancer, while Betapapillomavirus and Gammapapillomavirus generally cause asymptomatic infections. However, these genotypes may give rise to skin papilloma or increase predisposition to skin cancer in immunosuppressed patients (e.g. transplantation, HIV infection, epidermodysplasia verruciformis).6 Twelve genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59), also known as high-risk genotypes, have been classified as carcinogenic for humans by the International Agency for Research on Cancer (Table 1). The low-risk genotypes, including HPV 6 and HPV 11, generally cause benign diseases,7 while other genotypes classified as probably or possibly carcinogenic are rarely found in studies with sufficient frequency to establish a clear correlation.8
Papillomavirus phylogenetic tree based on the L1 region (adapted from De Villiers5).
Classification of HPV genotypes by oncogenic risk and associated diseases, as per the proposal of the International Agency for Research on Cancer.
| Human papillomavirus | HPV genotypes | Associated disease |
|---|---|---|
| Oncogenic or high risk | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 | Cervical, anal, vaginal, vulvar, penile and oropharyngeal cancer and associated precursor lesions |
| Low-risk genotypes | 6, 11 | Condylomata acuminata, recurrent laryngeal papillomatosis |
| Probably carcinogenic | 68 | Cervical cancer |
| Possibly carcinogenic | 5, 8 | Squamous cell skin carcinoma in epidermodysplasia verruciformis |
| Possibly carcinogenic | 26, 30, 34, 53, 66, 67, 69, 70, 73, 82, 85, 97 | Association with cancer and precancerous lesions not confirmed |
Source: IARC.8
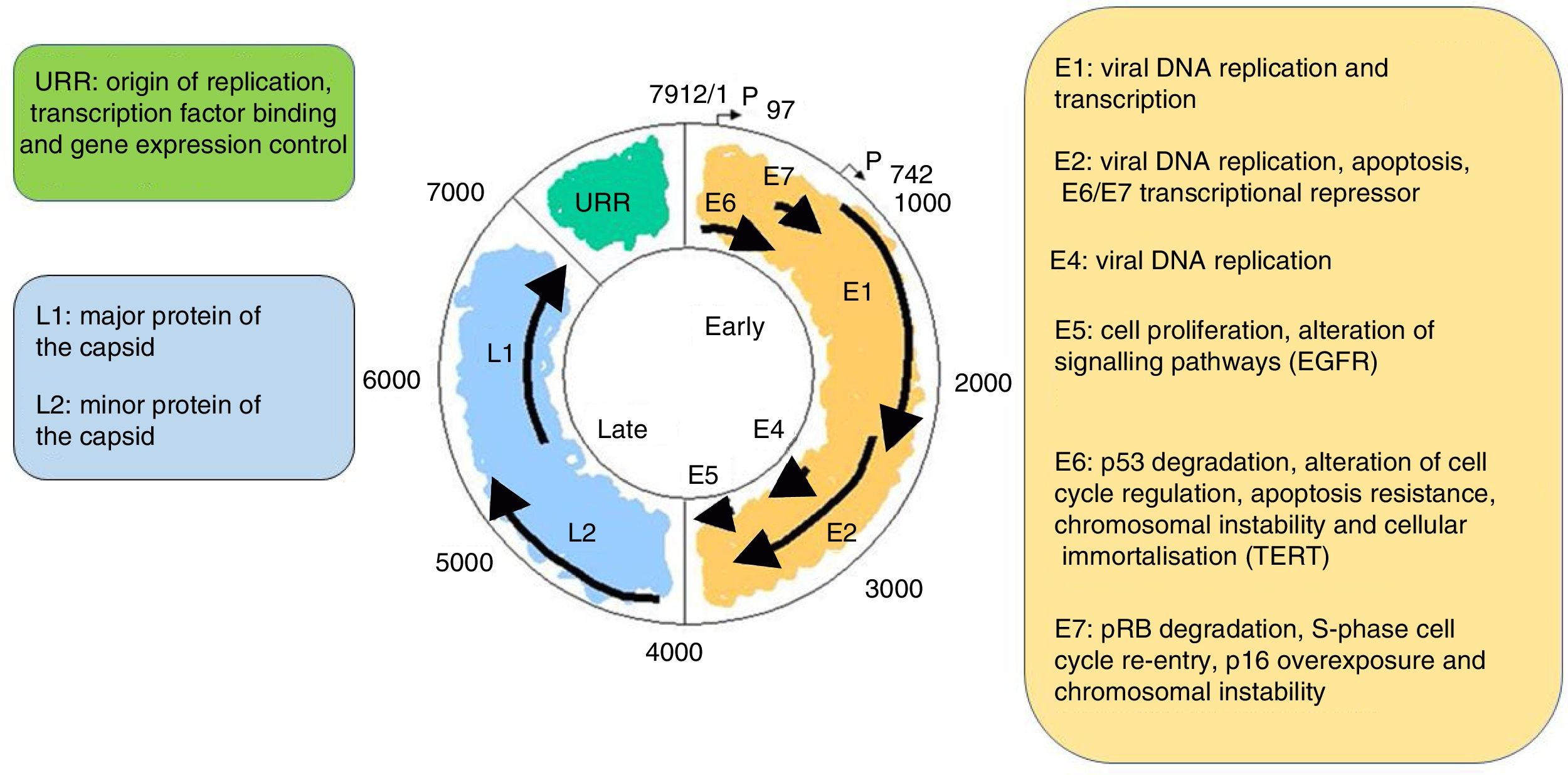
It is a non-enveloped virus measuring 55nm in diameter. The capsid is icosahedral and composed of 72 capsomeres. Filamentous and tubular forms can occur as a result of aberrant maturation. The virions are resistant to ether, acid and heat (50°C, 1h). Despite the fact that the family Papillomavirus is categorised by a largely heterogeneous group of viruses, they share the same structure and organisation of the genome (Fig. 2). The circular, double-stranded DNA genome is approximately 8kb in length and comprises three main regions: (a) The early (E) region encodes for genes responsible for virus replication and which play an important role in cell transformation (E1, E2, E4, E5, E6 and E7); (b) The late (L) region encodes for capsid proteins L1 and L2; (c) The non-encoding long control region (LCR), which contains the source of replication and the transcription factor binding sites, helps to regulate DNA replication by controlling the transcription of the viral gene. The expression of E6 and E7, together with E1, E2, E4 and E5, are essential for viral genome replication and the synthesis and release of virions, but they also play a key role in cell transformation.6
EpidemiologyHPV infection is considered to be the most common STI. It is estimated that more than 80% of sexually active people will contract HPV infection at some time in their life.1 Peak incidence of this infection occurs in the first decade after first sexual intercourse, generally between the ages of 15 and 25, and is closely associated with the number of sexual partners and contacts. Its primary focus is the anogenital region, although it can also manifest in other areas like the oral cavity. Recent studies conducted in the USA in 2013–20142 found that the prevalence of anogenital HPV infection was 42.5% in adults aged 18–59 (45.2% in men and 39.9% in women). When type of HPV was considered, the prevalence of high oncogenic risk viral infection was 22.7%. The prevalence of oral HPV infection was 7.3% (4% for high oncogenic risk infection).
The infection is generally temporary and tends to clear in less than one year.3 However, patients with a weakened immune system (patients with HIV infection or transplant recipients) have been found to have greater difficulty clearing the virus, making it the most persistent infection (median infection time of two years).4
The infection is poorly immunogenic, which means that infection by a particular genotype offers no protection against infections caused by other genotypes or from reinfections in the event of re-exposure to the virus. It therefore follows that in cases of multiple sexual contacts, there may be an overlap between viral clearance and reinfection, giving rise to chronic infection.
The main risk groups for HPV infection are immunosuppressed individuals and people with multiple sexual partners and a greater number of sexual contacts. The estimated prevalence of anogenital HPV infection in men who have sex with men (MSM) is 63.9% (37.2% for high-risk genotypes), while the prevalence in HIV-infected MSM is 92.6% (73.5% for high-risk genotypes).9 The prevalence of oral HPV infection in MSM is 17.3% (9.2% for high-risk genotypes), which rises to 27.8% (11.1% for high-risk genotypes) in HIV-infected MSM.10
The onset of lesions requires persistent infection, which explains why the prevalence of lesions is lower than the prevalence of infection. Although few population studies have been conducted, the prevalence of anogenital warts in the general population is estimated to be 0.13–0.56% in studies based on medical reports, and between 0.2% and 5.1% based on genital examinations.11 The prevalence of these lesions is greater in high-risk groups, reaching 4.6% in HIV-infected women, 7.2% in HIV-infected men and 18.5% in HIV-infected MSM.12
Even fewer studies have been conducted on the prevalence of precancerous lesions (high-grade squamous intraepithelial lesions [HGSIL]). In the USA, the estimated annual incidence of cervical HGSIL in women who have undergone cervical screening is 4–5%.13 Although there are no population data for anal HGSIL as screening is not systematically performed, in MSM the estimated prevalence of anal HGSIL is 15%, which rises to 24% in HIV-infected MSM.9
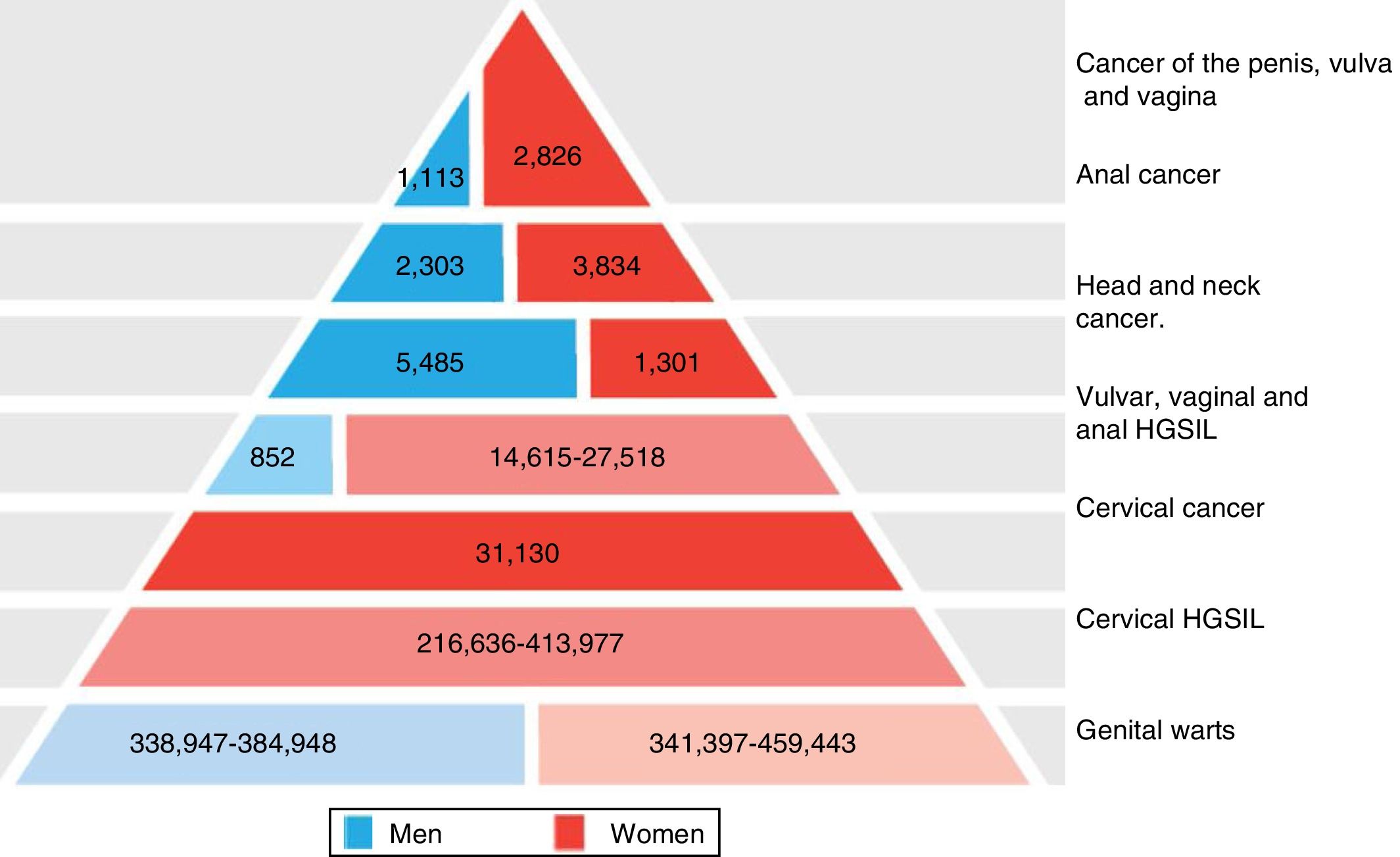
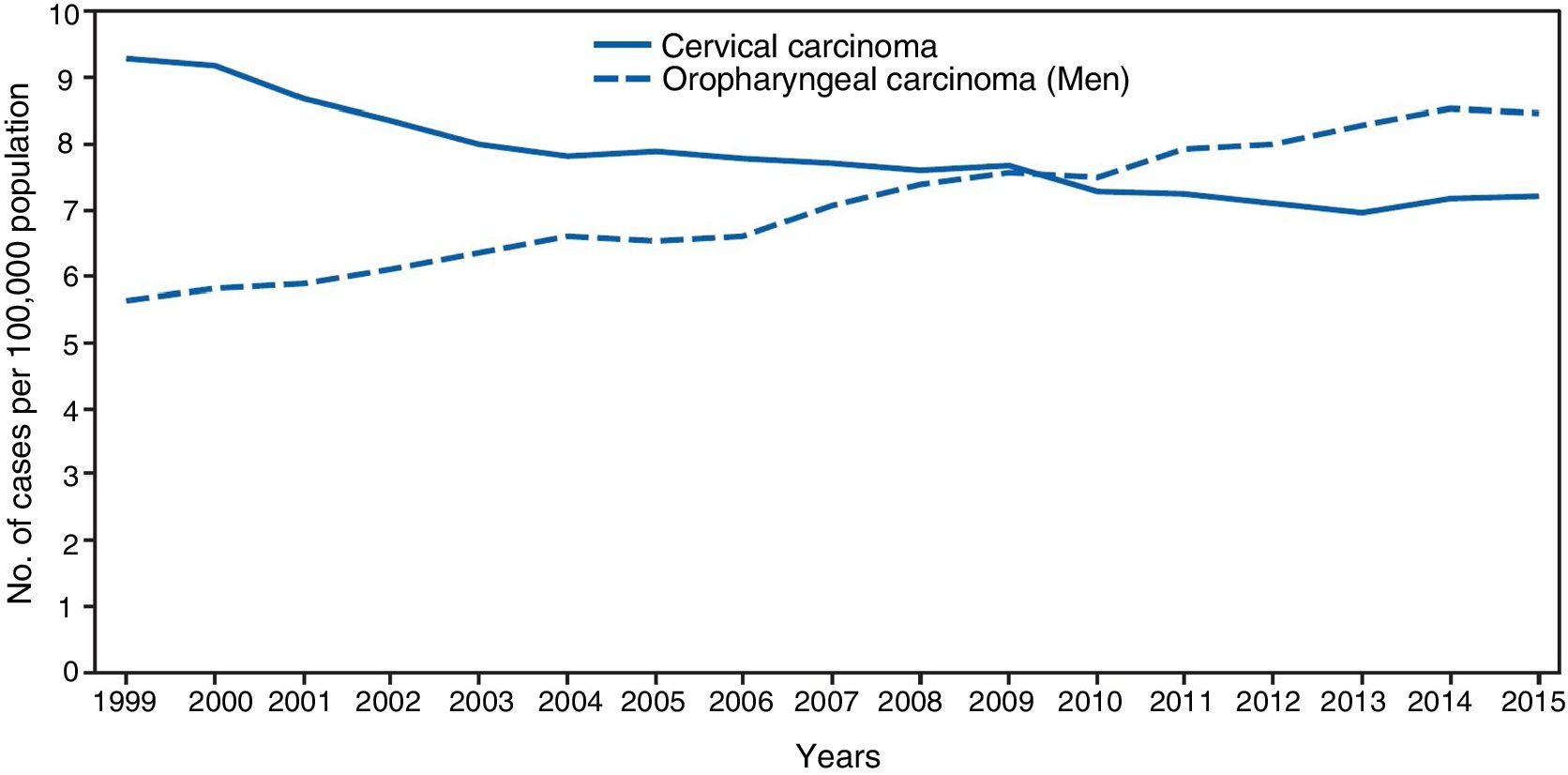
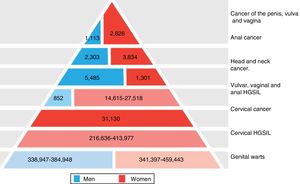
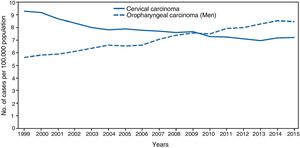
The significance of HPV infection can be attributed to the fact that it is the aetiological agent for a large number of malignancies (Fig. 3), being responsible for 100% of cervical cancers, 87% of anal cancer, 20% of oropharyngeal cancer and 10–30% of other squamous carcinomas (of the vagina, vulva and penis).14 Until recently, the most prevalent malignancy was cervical cancer, with a rate of 7cases/100,000women/year in the general population, which rose to 40cases/100,000women/year in HIV-infected women.14 However, a changing trend has been observed in the last few years, with a 1.6% annual fall in cervical cancer but a 2–3% increase in oropharyngeal cancer (Fig. 4) and anal cancer.14 In 2015, the most prevalent HPV-associated malignancy was oropharyngeal cancer, with a rate of 8.5cases/100,000people/year in men. The rate of anal cancer has remained relatively constant at around 2cases/100,000people/year in the general population, but peaks at 35cases/100,000people/year in MSM and 70–128cases/100,000people/year in HIV-infected MSM. Worldwide, HPV infection is responsible for more than 6% of all malignancies that occur each year.
Disease burden attributed to genotypes 6, 11, 16, 18, 31, 33, 45, 52 and 58 in men and women in Europe (adapted from Hartwig et al.13).
Incidence of cervical and oropharyngeal cancer in men in the USA from 1999 to 2015 (adapted from Van Dyne et al.65).
HPV enters the basal layer through the microtraumas that compromise the epithelial barrier, triggering the onset of infection. The HPV genome maintains a low number of copies in the host's infected basal cells. After differentiation of the epithelial cells, the virus replicates to a high number of copies and expresses the capsid genes (L1 and L2), which results in the production of new progeny virions that are released from the epithelial surface. Most infections are temporary and clear within an average time of eight months, but they may become chronic if infection persists for more than two years. To become persistent, HPV must infect basal cells that exhibit similar characteristics to stem cells and that are still capable of proliferating.15 This phenomenon is far less common in low-risk HPV genotypes. Epithelial transition zones, such as the endo/ectocervix and anorectal junctions, are the most susceptible regions to carcinogenesis by high-risk HPV types. The high-risk genotypes are more likely to activate cell proliferation in basal and differentiated layers, which promote the transition from a productive infection to an infection that cannot complete the viral replication cycle, but which can activate several essential pathways for epithelial transformation. The increased oncogenicity of the high-risk genotypes, particularly HPV 16, can be explained by the activity of oncoproteins E6 and E7. Although E6 and E7 are active in high-risk and low-risk genotypes, their role in low-risk types is limited to increasing viral production capacity and is generally not sufficient to trigger the onset of precancerous lesions and cancer.16
The high-risk HPV genotypes have developed several mechanisms to avoid the immune response of the host, which is important for viral persistence and progression to HPV-associated neoplastic diseases. One of the primary strategies to avoid detection is to maintain a very low profile. The HPV cycle is exclusively intraepithelial and non-lytic so it avoids the associated proinflammatory signal. As a result, the recruitment of antigen-presenting cells, such as Langerhans cells, and the release of cytokines that mediate the immune response are very low or non-existent after HPV infection. Other mechanisms to evade the immune response include the regulation of interferon signalling, the inhibition of Langerhans cells by the activity of E6 and E7, the inhibition of adhesion molecules, such as CDH1, and the modulation of the intracellular signalling pathways.
Carcinogenesis processThe early proteins E6 and E7 play a key role in the carcinogenic process by inhibiting the tumour suppressors p53 and pRB. E6 is also responsible for activating telomerase activity and deregulating the pathways involved in immune system response, epithelial differentiation, cell proliferation and apoptosis signalling. As well as deregulation and proliferation of the cell cycle, E7 increases genomic instability and promotes the accumulation of chromosomal abnormalities. The deregulation of the cell cycle, the activation of telomerase activity and genomic instability create a favourable environment for the transformation of epithelial cells. The integration of HPV can also boost the carcinogenic process by inactivating E2 expression, the main inhibitor of E6 and E7, and by disrupting host genes due to the insertion of the viral sequence into the chromosome of the cell.17 In order to lead to the invasive phenotype of cancer, the carcinogenic process, which starts with the activation of E6 and E7, must be complemented by the accumulation of additional abnormalities in the host gene. The mutations identified may exhibit a pattern consistent with the activity of APOBEC, an innate immune system that can bind to and change viral DNA, limiting the viral infection. In turn, APOBEC3B may be activated by oncoproteins E6 and E7. As such, APOBEC could be an important source of mutagenesis in HPV-related cancers, as has been reported in multiple human cancers.18
Viral genetic variation, beyond the HPV genotype, could partly explain the differences in clearance, persistence and the risk of developing cancer between positive infections from the same genotype. HPV isolates of the same genotype are classified into lineages and sub-lineages that are associated with the risk of cancer.
Epigenetic alterations, which do not involve gene mutation, such as methylation of tumour suppressor genes, related directly or indirectly to E6 and E7 activity, are common events during the first stages of epithelial malignancy and have been described as potential biomarkers for cervical cancer.19
Clinical manifestationsThe clinical manifestations of genital HPV infection are variable and can range from no symptoms with spontaneous resolution, to the onset of carcinogenic processes.
One of the most common clinical manifestations is condylomata acuminata, also known as genital or anogenital warts. They are benign, proliferative, pink or whitish-grey lesions that generally occur in multiples and are sometimes pigmented, whose surface is covered in filiform or papillomatous projections.20 They are usually exophytic, sessile or pedunculated lesions, but they could also be flat. They tend to manifest in the anogenital region at sites exposed to increased trauma during sexual intercourse. They can also appear on the pubis, in inguinal, perineal and perianal regions and even in the anal canal, the urethral meatus, the vagina, the cervix and the oral cavity. Perianal condyloma acuminata are particularly common in MSM, and around two-thirds of patients with perianal warts also have intra-anal warts.21,22 Their size can vary significantly, from a few millimetres to several centimetres. Their clinical course is unpredictable. They may grow rapidly to a considerable size or remain stable and spontaneously shrink before disappearing altogether. Data from randomised, placebo-controlled studies suggest that the spontaneous remission of untreated lesions occurs in 10–20% of cases after 3–4 months.20 These lesions tend to be asymptomatic, although multiple large lesions can sometimes bleed and cause pruritus and exudation. They are clinically significant because they promote the transmission of HPV and other infections (including HIV infection) and can give rise to significant emotional stress for the patient. These types of lesion primarily affect young adults (20–40 years), with immunosuppressed patients and people with multiple sexual contacts at greater risk. The mechanism of acquisition is by sexual contact, with the site of their manifestation varying depending on the sexual activity. In 90% of cases, these lesions are caused by HPV 6 and HPV 11, which are considered to be of low oncogenic risk. However, coinfection by other HPV genotypes (16, 18, 31, 33 and 35) of high oncogenic risk and onset of HGSIL is not uncommon in immunosuppressed patients and particularly in HIV-infected patients. Diagnosis is fundamentally clinical, although some authors defend the application of acetic acid, which causes the lesions to turn a whitish colour, to differentiate them from other diseases. Use of PCR techniques to detect HPV, or biopsy, is generally not recommended.
One of the most significant manifestations of HPV infections are HGSIL.20 They are malignant proliferative lesions believed to be the direct precursors of a large number of squamous carcinomas. They are primarily found in the cervix and anus, although they can also manifest on the vulva, penis, perineum and in the oral cavity. These lesions are not generally visible to the naked eye but require microscopic examination and application of specific dyes (acetic acid and Lugol's solution) by experienced clinicians for their diagnosis. They are completely asymptomatic. They are particularly prevalent in middle-aged adults (30–50 years) and HIV-infected and other immunosuppressed patients are especially susceptible. They are also acquired by sexual contact. These lesions are primarily caused by oncogenic HPV genotypes like 16, 18, 31, 33 and 35.
There are also other extragenital manifestations of HPV infection that are not acquired by sexual contact, which will only be mentioned briefly here. Skin warts, which include verruca vulgaris, plantar warts and flat warts, are one of the most common lesions. They are small, benign and confined epithelial lesions that may manifest anywhere on the skin, although they tend to be found on the hands, soles of the feet, face or neck. They most commonly affect small children and young adults, and are transmitted by contact. They are generally asymptomatic and resolve spontaneously in more than two-thirds of cases within two months. They are primarily caused by HPV genotypes 1, 2, 3 and 4.23 Another even rarer manifestation of HPV infection not associated with sexual contact is recurrent respiratory papillomatosis. It only affects small children and is characterised by exophytic lesions in the trachea and respiratory tract, which may cause crying and stridor. The mechanism of acquisition is believed to be maternal–foetal at birth and is primarily caused by HPV genotypes 6 and 11. Finally, epidermodysplasia verruciformis is an autosomal-recessive genodermatosis characterised by the onset of multiple wart-like lesions similar in appearance to pityriasis versicolor, often on the torso and arms and primarily affecting children up to the age of 10. They develop into invasive squamous cell carcinomas in up to one-third of cases. This entity cannot be transmitted to healthy subjects. Although many different HPV genotypes could be involved, genotypes 5 and 8 present the highest risk of malignancy.20
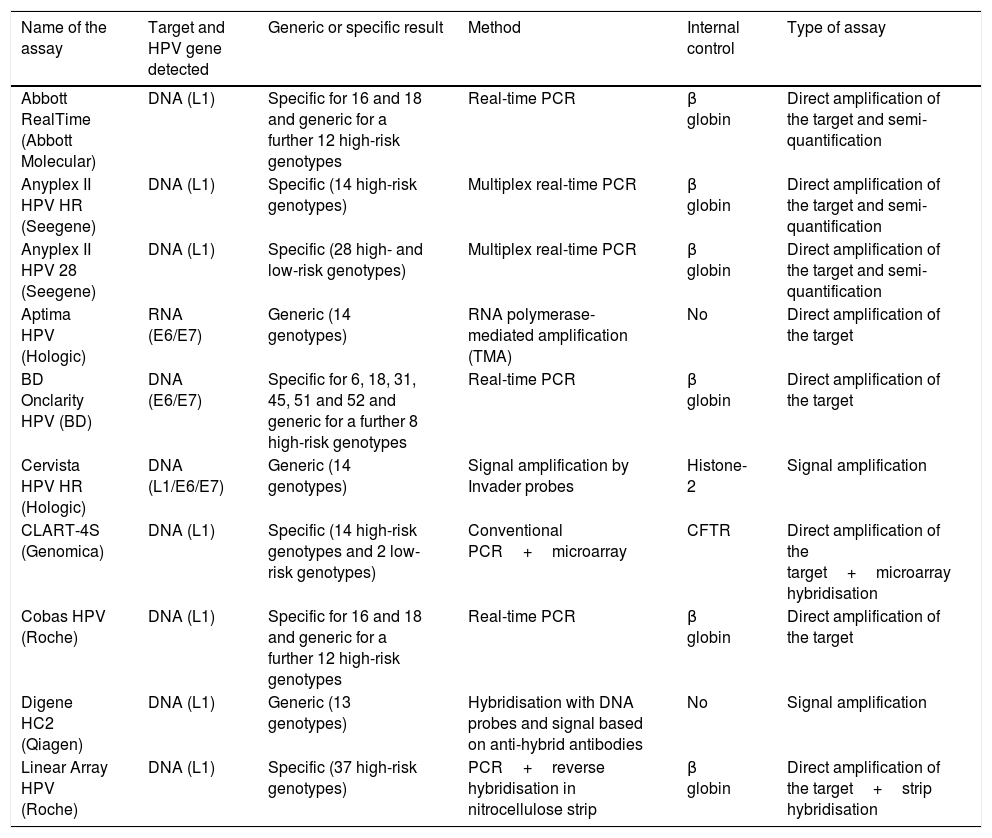
DiagnosisHPV infection is identified by detecting viral nucleic acids in clinical samples. The first commercial HPV tests were developed in the early 1980s in the light of strong evidence suggesting that the HPV test could play a significant role in the detection and treatment of cervical carcinoma in women with precancerous lesions. The last decade has seen a rapid rise in the number of new commercial tests available (Table 2). The association between HPV and other types of cancer, particularly anal and oropharyngeal cancer, necessitated the establishment of HPV diagnostic criteria at these sites using assays that were designed and validated for endocervical samples. A recent review24 analysed 193 commercial HPV kits available in 2015. Most targeted the detection of alpha genotypes. Twelve HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59) were considered carcinogenic (class I) or high-risk. HPV 68 was considered probably carcinogenic and 12 genotypes were considered possibly carcinogenic (26, 30, 34, 53, 66, 67, 69, 70, 73, 82, 85 and 97).
Description of some HPV detection assays available on the market.
| Name of the assay | Target and HPV gene detected | Generic or specific result | Method | Internal control | Type of assay |
|---|---|---|---|---|---|
| Abbott RealTime (Abbott Molecular) | DNA (L1) | Specific for 16 and 18 and generic for a further 12 high-risk genotypes | Real-time PCR | β globin | Direct amplification of the target and semi-quantification |
| Anyplex II HPV HR (Seegene) | DNA (L1) | Specific (14 high-risk genotypes) | Multiplex real-time PCR | β globin | Direct amplification of the target and semi-quantification |
| Anyplex II HPV 28 (Seegene) | DNA (L1) | Specific (28 high- and low-risk genotypes) | Multiplex real-time PCR | β globin | Direct amplification of the target and semi-quantification |
| Aptima HPV (Hologic) | RNA (E6/E7) | Generic (14 genotypes) | RNA polymerase-mediated amplification (TMA) | No | Direct amplification of the target |
| BD Onclarity HPV (BD) | DNA (E6/E7) | Specific for 6, 18, 31, 45, 51 and 52 and generic for a further 8 high-risk genotypes | Real-time PCR | β globin | Direct amplification of the target |
| Cervista HPV HR (Hologic) | DNA (L1/E6/E7) | Generic (14 genotypes) | Signal amplification by Invader probes | Histone-2 | Signal amplification |
| CLART-4S (Genomica) | DNA (L1) | Specific (14 high-risk genotypes and 2 low-risk genotypes) | Conventional PCR+microarray | CFTR | Direct amplification of the target+microarray hybridisation |
| Cobas HPV (Roche) | DNA (L1) | Specific for 16 and 18 and generic for a further 12 high-risk genotypes | Real-time PCR | β globin | Direct amplification of the target |
| Digene HC2 (Qiagen) | DNA (L1) | Generic (13 genotypes) | Hybridisation with DNA probes and signal based on anti-hybrid antibodies | No | Signal amplification |
| Linear Array HPV (Roche) | DNA (L1) | Specific (37 high-risk genotypes) | PCR+reverse hybridisation in nitrocellulose strip | β globin | Direct amplification of the target+strip hybridisation |
This consists of a group of qualitative or semiquantitative assays that use a range of technology to identify the presence or absence of carcinogenic HPV genotypes. The results are given as positive or negative for the individual HPV genotypes without determining the specific type of HPV. The most widely-used HPV tests with abundant clinical performance data in cervical cancer screening belong to the group of tests that target HPV 66 and/or HPV 68, defined as class I carcinogens.25
Screening with partial or reflex genotypingIn recent years, partial HPV genotyping has been studied for its clinical potential to improve detection accuracy and efficacy, and it has been shown to identify those patients at greatest risk of disease. These techniques can differentiate between the highest risk genotypes (HPV 16 and HPV 18) and generically identify the rest of the high-risk genotypes. This type of test lends itself to cancer screening programmes in which partial genotyping will be used as part of the decision-making process.
Specific genotypingThere are a large number of complete HPV DNA genotyping tests available. The vast majority are of limited use in clinical management but are valuable tools for monitoring the efficacy and development of vaccines and for conducting epidemiological studies. Unlike the two HPV test groups mentioned above, there is no consensus on how to evaluate the performance characteristics of the HPV genotyping tests. Nevertheless, a procedure to ascertain clinical and analytical performance26 has been proposed, as well as a validation and comparison protocol.27 The WHO quality control panels (HPV LabNet) found that the diagnostic capacity of laboratories to correctly identify samples with standardised quantities of DNA from the most common genotypes increased in a panel of samples from 26% in 2008 to 59% in 2014.28 Real-time PCR complete genotyping tests are assays designed to amplify and detect individual genotypes. Other tests use the principle of reverse hybridisation by which, after PCR amplification, the amplified product is denatured and hybridised with specific probes of each HPV genotype immobilised in a microtitre strip, filter or wells. Commercial reverse hybridisation tests are available for typing HPV genera other than Alphapapillomavirus. The medium- or low-density microarray tests are based on a similar principle, detecting the amplified product by fluorescence or chromogenic precipitation. Tests based on beads marked with fluorophores (Luminex assay) are suspension arrays and are very specific and sensitive. Other conventional PCR-based methods are also available, as well as RFLP, LAMP and nucleic acid sequencing.
Detection of high-risk human papillomavirus E6/E7 mRNAAlthough most HPV tests currently on the market are DNA-based, several studies have shown that mRNA tests could be clinically useful thanks to their greater clinical specificity. HPV is detected by transcription assay (TMA/NASBA), which quantifies the expression of viral oncoproteins E6 and E7. It may also be considered clinically validated for use in the primary detection of cervical cancer once longitudinal data is available.25
In situ human papillomavirus detection techniquesFluorescence in situ hybridisation (FISH) enables HPV DNA to be detected and visualised in a morphological context. This format has also been adapted to an in situ PCR reaction and the detection of E6 and E7 mRNA transcripts, with cells infected by the virus identified by flow cytometry.
Techniques based on the detection of cellular biomarkersOne of the diagnostic limitations of viral detection in HPV infection is its modest clinical specificity. As such, cellular biomarkers that have a relevant prognostic factor and provide a positive predictive value are required in order to monitor those patients in whom they are detected. There are already morphological biomarkers that are detected by immunohistochemical techniques like p16 expression in isolation or together with Ki-67 in cytological preparations.29,30 Molecular biomarkers based on DNA methylation are of enormous interest because its association with cervical cancer is well established and it can be detected in cytological and histological samples.31
With such a large number of commercial tests available, HPV detection is currently one of the most attractive options for molecular diagnostics companies. However, HPV tests are one of the most confusing, least regulated and most diverse diagnostic products on the market. It is safe to assume that the number and diversity of commercial HPV tests will continue to increase in the coming years due to the favourable commercial opportunities open to manufacturers all over the world. Other more established molecular diagnostic areas, like hepatitis B and C diagnosis, see annual growth rates of 3% and 5%, respectively, while it is estimated that HPV diagnosis could grow by 7.2% between 2017 and 2024, in line with the growth of other STI diagnostic tests (http://www.transparencymarketresearch.com). That is why mechanisms must be introduced that guarantee their rigorous assessment and ensure that their marketing is not based on purely descriptive studies. The European conformity mark for in vitro diagnostic medical devices (CE-IVD) essentially stipulates technical criteria and is considerably less stringent than the equivalent US FDA regulations or the evaluation procedure of the Australian Therapeutic Goods Administration.
The decision-making process for selecting an HPV diagnostic test is complex and depends on the purpose of the test. The two most important parameters are the set of HPV genotypes detected and the level of analytical sensitivity, and the diagnostic purpose of the HPV test must be defined. There are currently three types of HPV test available, each designed for a different purpose: (a) studies that provide utility results in clinical practice; (b) epidemiological and vaccine-related studies; and (c) research trials. The first HPV tests are indicated for the management and classification of dubious cytological findings, post-treatment follow-up, cancer screening and the resolution of diagnostic uncertainties. Trials capable of exceeding quality controls that mimic clinical samples as far as possible are required, including all the steps of the test, which will enable the clinical sensitivity and specificity to be determined with greater precision. In this regard, trials must be conducted that control the sample collection process, its cellularity and the nucleic acid extraction process. In contrast, a test with high sensitivity and analytical specificity, and with low cross-reactivity, shall be required if an HPV test is needed for the development and evaluation of vaccines or epidemiological studies.
Prevention of human papillomavirus infectionGeneral measuresUsing a condom could reduce the risk of HPV infection and its associated lesions.32,33 However, as the condom does not cover the entire genital area, transmission is not completely prevented and it therefore only offers partial protection. Circumcision seems to reduce the prevalence of infection in men, shortening the viral clearance time and reducing the risk of infecting the female partner.34 Limiting the number of sexual partners may reduce the risk of HPV infection. Sexual abstinence is the only sure way to prevent HPV infection.32
Human papillomavirus vaccinesBy preventing persistent infection, prophylactic HPV vaccination is the best preventive strategy against malignancy and anogenital warts.35 The HPV vaccines that are currently available are composed of virus-like particles (VLPs) obtained by genetic recombination. The structure of the VLPs is similar to the virus capsid, which induces the production of protective neutralising antibodies against the actual virus. As the VLPs do not contain viral DNA, they cannot cause infection or associated lesions.36
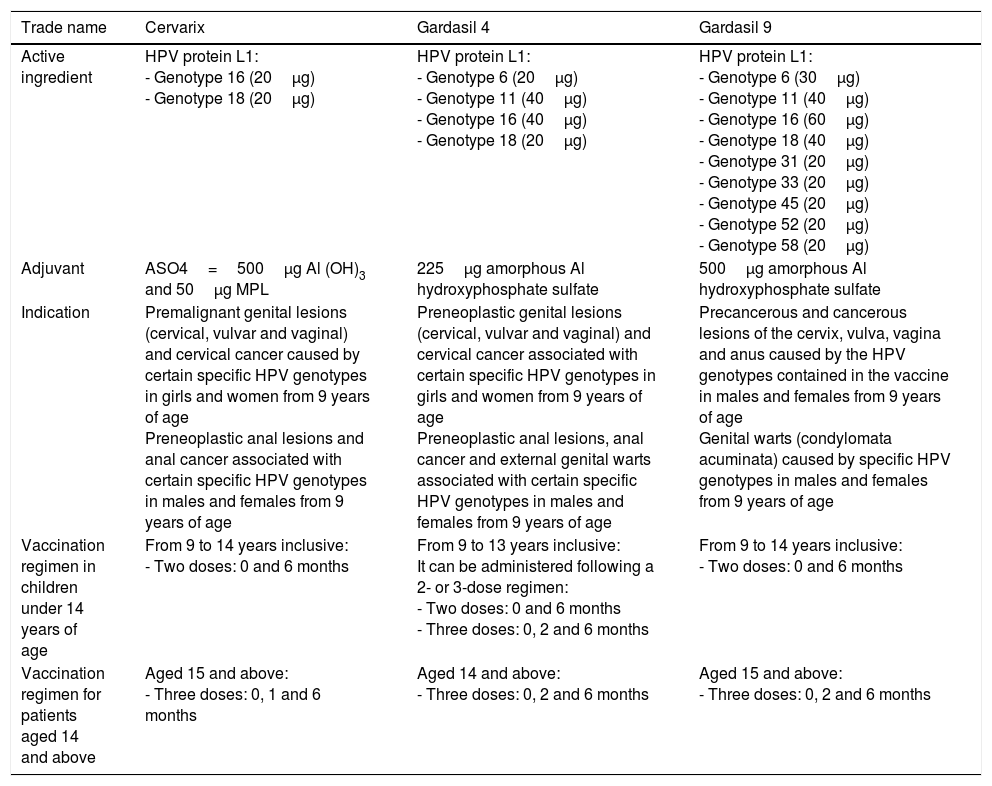
There are three prophylactic vaccines registered in Spain and authorised by the European Medicines Agency (EMA): bivalent Cervarix® (HPV-2), tetravalent Gardasil® (HPV-4) and nonavalent Gardasil® (HPV-9). The fundamental difference between the three vaccines is the number of VLPs each contains. They also differ by adjuvant used. It has been published that the anti-VLP antibody titres double when VLPs are formulated with AS04 compared to with aluminium hydroxide.37 The three vaccines are administered intramuscularly. Table 3 summarises the most important characteristics of each vaccine.38 All the formulations tested exhibit high immunogenicity, with seroconversion rates of around 100% for all three vaccines.
Marketed HPV vaccines.
| Trade name | Cervarix | Gardasil 4 | Gardasil 9 |
|---|---|---|---|
| Active ingredient | HPV protein L1: - Genotype 16 (20μg) - Genotype 18 (20μg) | HPV protein L1: - Genotype 6 (20μg) - Genotype 11 (40μg) - Genotype 16 (40μg) - Genotype 18 (20μg) | HPV protein L1: - Genotype 6 (30μg) - Genotype 11 (40μg) - Genotype 16 (60μg) - Genotype 18 (40μg) - Genotype 31 (20μg) - Genotype 33 (20μg) - Genotype 45 (20μg) - Genotype 52 (20μg) - Genotype 58 (20μg) |
| Adjuvant | ASO4=500μg Al (OH)3 and 50μg MPL | 225μg amorphous Al hydroxyphosphate sulfate | 500μg amorphous Al hydroxyphosphate sulfate |
| Indication | Premalignant genital lesions (cervical, vulvar and vaginal) and cervical cancer caused by certain specific HPV genotypes in girls and women from 9 years of age Preneoplastic anal lesions and anal cancer associated with certain specific HPV genotypes in males and females from 9 years of age | Preneoplastic genital lesions (cervical, vulvar and vaginal) and cervical cancer associated with certain specific HPV genotypes in girls and women from 9 years of age Preneoplastic anal lesions, anal cancer and external genital warts associated with certain specific HPV genotypes in males and females from 9 years of age | Precancerous and cancerous lesions of the cervix, vulva, vagina and anus caused by the HPV genotypes contained in the vaccine in males and females from 9 years of age Genital warts (condylomata acuminata) caused by specific HPV genotypes in males and females from 9 years of age |
| Vaccination regimen in children under 14 years of age | From 9 to 14 years inclusive: - Two doses: 0 and 6 months | From 9 to 13 years inclusive: It can be administered following a 2- or 3-dose regimen: - Two doses: 0 and 6 months - Three doses: 0, 2 and 6 months | From 9 to 14 years inclusive: - Two doses: 0 and 6 months |
| Vaccination regimen for patients aged 14 and above | Aged 15 and above: - Three doses: 0, 1 and 6 months | Aged 14 and above: - Three doses: 0, 2 and 6 months | Aged 15 and above: - Three doses: 0, 2 and 6 months |
Source: Adapted from the vaccination document of the Asociación Española de Pediatría [Spanish Association of Paediatrics]. Vaccines Advisory Committee (CAV-AEP).38
According to the results of clinical trials performed with the three vaccines, HPV vaccination effectively prevents high-grade cervical lesions.39 In addition, Gardasil® HPV-4 and HPV-9 have shown more than 98% efficacy against genital warts in women and more than 90% in men. There are also data suggesting more than 95% protection against other high-grade genital lesions (VIN/VAIN 2/3).40,41 In April 2014, after demonstrating efficacy in preventing anal HPV infection and associated lesions,42 the HPV-4 vaccine was authorised for the prevention of HGSIL and anal cancer in MSM aged from 16 to 26.
The safety findings of the clinical trials show that the three vaccines are well tolerated and have an adequate safety profile.43 The most common local adverse effects associated with the vaccines are pain, erythema and local inflammation at the injection site. Although less common, fever, fatigue, headache and muscle pain have also been reported. Since their market launch in 2006, more than 200 million doses have been distributed worldwide and the WHO concluded in 2017 that all the evidence accumulated to date endorses the HPV vaccines’ good safety profile.44Table 4 details the recommendations of some scientific societies.
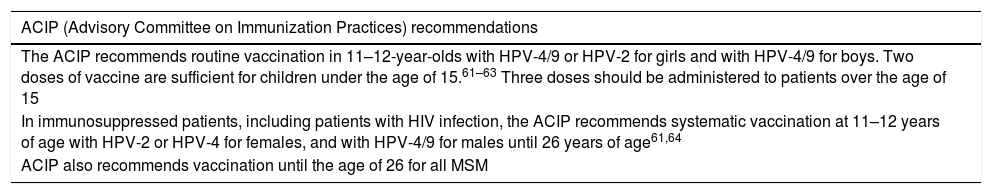
HPV vaccine recommendations.
| ACIP (Advisory Committee on Immunization Practices) recommendations |
|---|
| The ACIP recommends routine vaccination in 11–12-year-olds with HPV-4/9 or HPV-2 for girls and with HPV-4/9 for boys. Two doses of vaccine are sufficient for children under the age of 15.61–63 Three doses should be administered to patients over the age of 15 |
| In immunosuppressed patients, including patients with HIV infection, the ACIP recommends systematic vaccination at 11–12 years of age with HPV-2 or HPV-4 for females, and with HPV-4/9 for males until 26 years of age61,64 |
| ACIP also recommends vaccination until the age of 26 for all MSM |
| Consensus of the Spanish scientific societies in 2011 |
|---|
| The following recommendations were put forward by the HPV vaccination consensus statement signed by nine Spanish scientific societies: |
| - For females: |
| • Systematic vaccination of all preadolescent or adolescent girls aged 9–14, regardless of their sexual activity |
| • Recommended vaccination of all women until the age of 26, regardless of their sexual activity |
| • Personalised vaccination assessment for women aged 26 and above |
| • Recommended vaccination for women after treatment of intraepithelial lesions of the cervix, vagina or vulva, or genital warts |
| - For males: |
| • Personalised vaccination assessment with HPV-4/9 for males aged 9–26 for the prevention of genital warts |
| • Recommended vaccination with HPV-4/9 for males aged 9–26 for the prevention of anal neoplasia |
| Vaccines Advisory Committee of the AEP (Asociación Española de Pediatría [Spanish Association of Paediatrics]) 2016 |
|---|
| The 2018 vaccination schedule maintained the systematic vaccination recommendation for all girls and boys aged 11–1238 |
As there is no single treatment of choice for anogenital warts, each case must be assessed on its own merits before deciding on the appropriate treatment. Several factors must be considered, including the psychological impact, the extent and type of lesions, the cost that the patient can afford and even the doctor's experience.45 The patient should understand that the aim of treatment is not to eradicate the HPV infection, but rather to remove the warts and improve any other symptoms. The disease has a significant psychological impact in some patients and treatment of the lesions could help to reduce it. The genital warts could resolve spontaneously even without treatment, which means that watchful waiting is a valid alternative, although this is not acceptable to most patients.46,47
Treatments have traditionally been classified as follows:
- 1.
If they can be applied by the patient themselves or by a healthcare professional at the clinic.
- 2.
If they are ablative or topical treatments.
- 3.
By pharmacological group or mechanism of action: antimitotic agents (5FU, podophyllotoxin), antivirals (interferons, cidofovir), immunomodulators (imiquimod, sinecatechins), caustic agents (dichloroacetic acid and trichloroacetic acid) and photosensitisers (5-aminolevulinic acid).
This review uses the first classification, differentiating between treatments applied by the patient and those applied by the doctor at the clinic or by a surgeon.
Agents applied by the patientImmunomodulators (imiquimod and sinecatechins)Imiquimod is an immune response modifier that stimulates the production of interferon alpha, TNF, IL-1, IL-6, IL-8 and other cytokines.48 To treat genital warts it is applied three times a week (Monday, Wednesday and Friday) for up to 16 weeks. In Spain, imiquimod is currently only available as a 5% cream (Imunocare®5%). The patient should apply it to clean skin at night. There is a second 3.75% formulation (Zyclara®) that is applied once daily for eight weeks, but in Spain only the indication for the treatment of actinic keratosis is currently approved in the summary of product characteristics. However, consistent with other countries, the indication is expected to be broadened to include genital warts. Several clinical trials have found imiquimod 5% cream to be an effective treatment in clearing genital warts. Between 72 and 84% of patients have some form of treatment response, 40–70% have complete response and 13–19% experience relapse.45 6–26% of patients experience recurrence.45 With regard to imiquimod 3.75% cream, two clinical trials demonstrated its efficacy versus placebo, with complete clearance in 36.6% of patients.49 Some patients experienced adverse effects during treatment, the most common of which was local inflammation (reddening, erythema, erosions, ulcers and blisters). Sequelae such as excessive scarring, phimosis, pigmentation disorders and lichen sclerosus have also been reported. Local reactions can be managed with periods of rest or by reducing the frequency of application. Despite the fact that systemic absorption is minimal, flu-like symptoms, including headache, asthenia, myalgia and nausea can manifest in some cases.50 Although animal studies found no teratogenicity and there are isolated cases of imiquimod being administered during pregnancy to treat condyloma, its use during pregnancy or breastfeeding is not recommended.
Sinecatechins or polyphenon E (Veregen®) is the other self-applied topical immune response modifier currently marketed in Spain. It is obtained from a green tea leaf extract (Camellia sinensis) and the most important catechin is epigallocatechin gallate. Its mechanism of action is not well understood but it is believed to block the cell cycle and HPV transcription, activating apoptosis of the HPV-infected cells.45 It is marketed in Europe as a 10% ointment and in the USA as a 15% ointment, to be applied three times a day for 16 weeks. Two placebo-controlled trials and one pool analysis of 10,005 patients have been published, showing total clearance rates of prevalent anogenital warts and incidences of 47–59%.51,52 More than 50% of warts disappeared after 12–16 weeks in 76% of the patients treated. This figure increased to 64.5% in patients who completed 16 weeks of treatment. The published recurrence rates are around 7–11% after 12 weeks of follow-up. The pivotal clinical trials report that complete clearance is associated with the onset of local adverse effects like erythema and erosion, and their manifestation could constitute a prognostic factor for good response. These adverse effects are pronounced and occur in up to 80% of patients, particularly between the third and sixth weeks.53 As with imiquimod, sequelae such as phimosis, pigmentation changes and urethral meatal stenosis, etc. have been reported.
No head-to-head studies comparing the efficacy of both immunomodulators have been published. An analysis of pooled data from the clinical trials that compared the efficacy, clearance and recurrence of sinecatechins, imiquimod 5%, imiquimod 3.75% and podophyllotoxin 0.5% daily found similar clearance rates for all treatments.
Cytotoxic agentsPodophyllotoxin (Wartec®) is a cytotoxic agent available in two formulations: 0.15% cream and 5% cutaneous solution, the latter being more effective but also more irritating.45 It is applied to external palpable warts twice a day for three days, followed by a four-day period of rest, repeating this regimen for up to four cycles for the cream and two cycles for the solution. The cream is preferred for the anal and vulva regions and the solution for the penis, where self-application is easier. This treatment has greater efficacy and is more cost-effective than podophyllin, which is no longer used in clinics. A systematic review of published randomised trials found clearance rates of 45–83% for podophyllotoxin solution applied for three to six weeks, and 43–70% for podophyllotoxin cream applied for four weeks.45 A comparative trial of podophyllotoxin solution versus imiquimod found a clearance rate of 72%, with no significant difference between the two drugs. However, the relapse rate seems to be higher with podophyllotoxin than with immunomodulators.45 It is embryotoxic, which means that its use during pregnancy or breastfeeding, as well as in children, is completely contraindicated. Podophyllotoxin may also reduce the efficacy of condoms. Common adverse effects include local reactions, particularly within two weeks of application.
Treatments administered in the clinicCryotherapyThis is an ablation therapy that destroys tissue by liquid nitrogen-induced necrosis at −196°C. The technique involves the application of complete freeze–thaw cycles that vary depending on the type of lesion, but there is no evidence from clinical trials comparing the most effective number of applications.
Small blisters tend to manifest after treatment, which re-epithelialise in one to two weeks.54 Cycles usually need to be repeated every two weeks for up to 10 weeks. Scarring, sensitivity alterations or changes in pigment are rare sequelae.45 It benefits from being an effective, inexpensive, simple and safe treatment during pregnancy and breastfeeding, with complete remission achieved in 44–75% of cases. However, at 21–42%, its recurrence rates are quite high.45 It is particularly useful in hairy areas and on large isolated or papillomatous lesions.54 A recent meta-analysis concluded that the efficacy of cryotherapy is equivalent to trichloroacetic acid, podophyllin or imiquimod.55
Trichloroacetic and dichloroacetic acidTrichloroacetic acid is a very inexpensive caustic agent that destroys warts by chemical coagulation of proteins. It is particularly useful on small warts located on mucosa and semi-mucosa. It is applied at concentrations of 80–90%, achieving clearance rates of 56–81% in published clinical trials after two to six sessions,45,54 with recurrences in 36% of patients. It is not unusual to experience burning pain and scarring abnormalities as side effects after its application. Its indications, mode of application and side effects are the same as for dichloroacetic acid.54
Surgical excision and electrocoagulationGenital warts of the superficial dermis can be destroyed by excision with a scalpel or electrocautery, which are always performed under anaesthesia.54 A surgical approach should be considered in large pedunculated lesions, as well as in lesions located in complicated areas, like the urethral meatus, the intra-anal or intra-vaginal region or in the cervix.56 Although the cure rates of these techniques are high (89–100%), as with all ablation therapies, so too are the relapse rates, with one in three patients suffering relapse.45,54
Laser ablation therapy, particularly CO2 laser, has also been used.
Combination therapyProactive sequential therapy (PST) is defined as the sequential use of rapid ablation therapy in the clinic followed by administration of a topical immunomodulator. The concept is considered to reflect routine clinical practice and to be a strategy that removes warts quickly while also reducing recurrences, which are particularly common within the first six months. It is recommended to use ablation therapy until clearance of all lesions, and to apply an immunomodulator (sinecatechins or imiquimod) to the affected area three to five days after re-epithelialisation for 12–16 weeks.57 The efficacy of the sequential treatments should be confirmed by prospective clinical trials.
Uncommon treatmentsPhotodynamic therapyThere is insufficient evidence to recommend the first-line use of photodynamic therapy to treat anogenital warts. However, it could play a role in refractory cases.58
CidofovirIt is a nucleotide analogue of deoxycytidine monophosphate that has a broad spectrum of action against several DNA viruses. It has been used for compassionate use (outside the summary of product characteristics) either topically or intralesionally. The most widely-used formulation for genital warts is a 1–2% cream. Two randomised placebo-controlled clinical trials evaluating its efficacy have been published, both finding it to be superior to placebo.59
Cidofovir is not currently marketed in Spain, although it can be ordered as a foreign medicinal product (Cidofovir Mylan Institutional®: 75mg/ml, 5-ml vial) to be formulated as a cream, gel or solution from 5-ml vials.
InterferonInterferon has been proven to be effective, particularly when applied intralesionally, but also intramuscularly. However, its high cost and adverse effects limit its use to refractory cases and it should not be considered a first-line therapy.
Nitric-zinc complex (Verrutop®)This topical solution contains nitric acid, zinc, copper and organic acids that have a caustic effect on warts. Further evidence to establish the efficacy of this new treatment is required.60
Choosing treatmentThe most appropriate treatment should be chosen in accordance with the type of lesions, their location, comorbidities, cost and prior treatments administered. When choosing a treatment, these factors, together with severity and patient preferences, should be taken into account. Monotherapy should be appropriate in mild cases of fewer than three lesions, while sequential therapy should be more effective in more serious cases.57 Immunomodulators in monotherapy should preferably be chosen for fast-growing lesions of recent onset. For older lesions located on keratinised skin, cryotherapy could be the treatment of choice.45 The best treatment for large, pedunculated lesions would probably be some form of surgical approach. For more serious lesions, proactive sequential therapy could be the treatment of choice to reduce recurrences after achieving more rapid clearance.57
Special situationsWatchful waiting or liquid nitrogen therapy are recommended during pregnancy or breastfeeding.45 Ablation therapy is preferred in children under the age of 12 as use of topical agents is not approved in this age group. Although imiquimod is not approved by the FDA in children under the age of 12, numerous publications have found it to be safe and effective in children from the age of six months. Visible warts in the urethral meatus can be treated with cryotherapy, while inaccessible lesions must be referred to a urologist.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sendagorta-Cudós E, Burgos-Cibrián J, Rodríguez-Iglesias M. Infecciones genitales por el virus del papiloma humano. Enferm Infecc Microbiol Clin. 2019;37:324–334.