Hospital-wide SARS-CoV-2 seroprevalence is rarely explored and can identify areas of unexpected risk. We determined the seroprevalence against SARS-CoV-2 in all health care workers (HCW) at a hospital.
MethodsCross-sectional study (14-27/04/2020). We determined SARS-CoV-2 IgG by ELISA in all HCW including external workers of a teaching hospital in Madrid. They were classified by professional category, working area, and risk for SARS-CoV-2 exposure.
ResultsAmong 2919 HCW, 2590 (88,7%) were evaluated. The mean age was 43.8 years (SD 11.1), and 73.9% were females. Globally, 818 (31.6%) workers were IgG positive with no differences for age, sex or previous diseases. Of these, 48.5% did not report previous symptoms. Seropositivity was more frequent in high- (33.1%) and medium- (33.8%) than in low-risk areas (25.8%, p=0.007), but not for hospitalization areas attending COVID-19 and non-COVID-19 patients (35.5 vs 38.3% p>0.05). HWC with a previous SARS-CoV2 PCR-positive test were IgG seropositive in 90.8%. By multivariate logistic regression analysis seropositivity was significantly associated with being physicians (OR 2.37, CI95% 1.61–3.49), nurses (OR 1.67, CI95% 1.14–2.46), nurse assistants (OR 1.84, CI95% 1.24–2.73), HCW working at COVID-19 hospitalization areas (OR 1.71, CI95% 1.22–2.40), non-COVID-19 hospitalization areas (OR 1.88, CI95% 1.30–2.73), and at the Emergency Room (OR 1.51, CI95% 1.01–2.27).
ConclusionsSeroprevalence uncovered a high rate of infection previously unnoticed among HCW. Patients not suspected of having COVID-19 as well as asymptomatic HCW may be a relevant source for nosocomial SARS-CoV-2 transmission.
Los estudios de seroprevalencia frente a SARS-CoV-2 en los trabajadores sanitarios (TS) permiten identificar áreas de riesgo inesperado en los hospitales.
MétodosEstudio transversal (14-27/04/2020). Se determinó IgG frente a SARS-CoV-2 mediante ELISA en todos los TS, incluidos los externos, de un hospital universitario de Madrid. Se clasificaron por categoría profesional, área de trabajo y riesgo de exposición al SARS-CoV-2.
ResultadosEntre 2.919 TS, se evaluaron 2.590 (88,7%); edad media 43,8años (DE11,1) y 73,9% mujeres. Globalmente, 818 (31,6%) trabajadores tuvieron IgG positiva, sin diferencias por edad, sexo o enfermedades previas. De estos, el 48,5% no comunicaron síntomas previos. La seropositividad fue más frecuente en las áreas de alto (33,1%) y medio (33,8%) que en las de bajo riesgo (25,8%, p=0,007), pero similar en las áreas de hospitalización que atendían a pacientes con y sin COVID-19 (35,5 vs 38,3%, p>0,05). El 90,8% de los TS con PCR previa positiva frente a SARS-CoV-2 tuvieron IgG positiva. Por análisis multivariante, la seropositividad se asoció con ser médico (OR2,37, IC95%: 1,61-3,49), enfermero (OR1,67, IC95%: 1,14-2,46), auxiliar de enfermería (OR1,84, IC95%: 1,24-2,73), trabajar en áreas de hospitalización COVID-19 (OR1,71, IC95%: 1,22-2,40) y no COVID-19 (OR1,88, IC95%: 1,30-2,73) y en Urgencias (OR1,51, IC95%: 1,01-2,27).
ConclusionesEl estudio de seroprevalencia desveló una alta tasa de infección que pasó desapercibida entre los trabajadores sanitarios. Los pacientes sin sospecha clínica de COVID-19 y los trabajadores sanitarios asintomáticos pueden ser una fuente importante de transmisión nosocomial del SARS-CoV-2.
Spain is one of most affected countries in the world by SARS-CoV-2.1 A sharp increase in the number of cases during March/2020 pushed the capacity of healthcare system in Madrid beyond the limit.2,3 As the pandemic accelerated, access to personal protective equipment (PPE) for health workers was a key concern due to shortages.4 Health-care workers (HCW) are at increased risk for infection, and specific requirements for their protection are advisable to ensure proper healthcare system functioning.5 Indeed, more than 50,000 HCW have been infected and at least 63 have died in Spain.2 Alongside concerns for the healthcare workers personal safety, anxiety about transmitting the infection to their relatives and patients adds another stress to HCW. At this time, it is known that SARS-CoV-2 human-to-human transmission occurs during the asymptomatic stage through droplets or direct contact. This possibility increases the challenges of containment measures.6 Moreover, presumed hospital-related transmission of SARS-CoV-2 has been suspected up to 35% of HCW.7 Nosocomial transmission may originate from patients (where protective measures are usually strict) but also from asymptomatic HCW (where protective measures may be more relaxed or simply non-existing).
Initial studies have provided very different estimates of hospital HCW seroprevalence for SARS-CoV-2 (ranging form 3.0 to 21.3%).8–10 Nosocomial transmission may be an important amplifier of infection in epidemics. Serological surveillance of exposed individuals allows one to identify areas of unexpected risk.
This approach is essential since the safety of health-care workers must be ensured. Screening all health-care workers for SARS-CoV-2 in the hospital would be helpful to maintain the welfare of the staff, to enable identification of infected health-care workers, and to identify unrecognized areas at risk of transmission. Here, our objective was to evaluate the prevalence of immunoglobulin G (IgG) against SARS-CoV-2 among all employees of a second level teaching hospital in southern Madrid.
MethodsDesignThis was a cross sectional study of all hospital workers, direct hospital employees (clinical and not clinical), as well as workers for external contractors who perform their regular tasks inside the hospital.
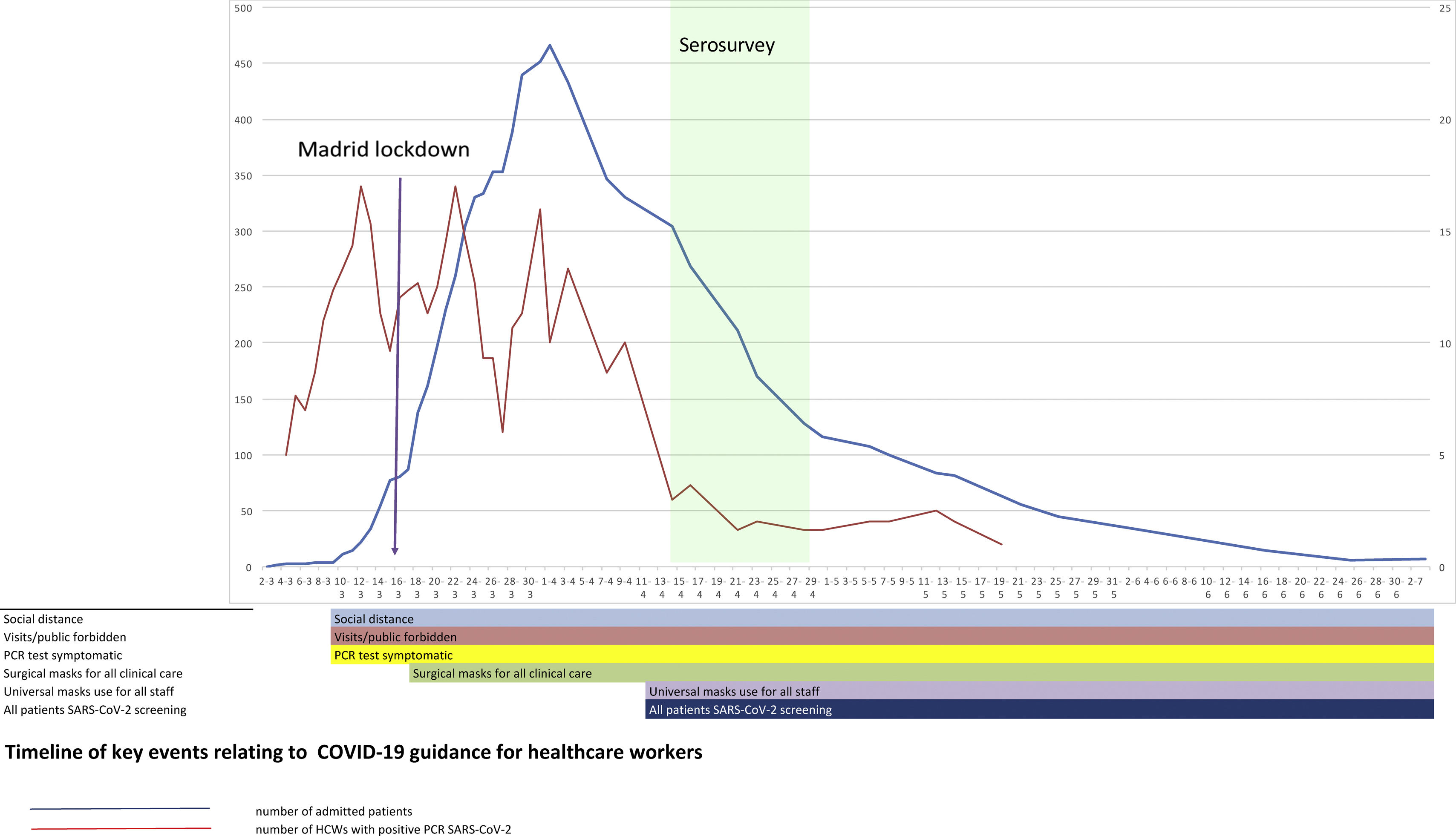
SettingHospital Universitario Fundación Alcorcón (HUFA) is a 400-bed general hospital it covers a population of 1,70,000 inhabitants. The total number of COVID-19 patients admitted by April 14th was 1638 patients including 236 deaths (Fig. 1); the peak incidence in Alcorcón was 1133.63 cases/1,00,000 inhabitants.2 A quick structural and functional reorganization was required ultimately reaching a peak occupancy of 370% of internal medicine hospitalization, 293% in Intensive Care Unit (ICU), and 320% in Emergency Room (ER). The number of PPE providers increased 10-fold. Despite this, occasional shortages of appropriate PPE occurred several times. Infection prevention measures were instituted in the hospital beginning March 1st (Fig. 1).
For the purpose of this analysis, workers were classified into categories according to the estimated risks for nosocomial exposure to SARS-CoV2. Those at high risk included professionals with direct contact with COVID-19 patients: critical care & anaesthesiology, ER, COVID-hospitalization ward, and external workers (such as cleaners) who worked in the COVID-hospitalization areas. Medium risk was attributed to professionals attending patients not suspected of having COVID-19 both in hospital wards and outpatient clinics as well as central units (pharmacy, laboratory, radiology, pathology). Finally, low risk was attributed to administrative and management units and external workers (cooks, food service, ambulance drivers, store sellers, and security guards) with no direct contact with COVID-19 patients.11 PPE was distributed according to the estimated risk in different areas with a priority for critical care units and ER in the case of a shortage. A total of 1561 workers most likely to attend COVID-19 patients received intensive training in the use of PPE at the hospital‘s Centre for Medical Simulation (IDEhA).
Selection and participation of individualsAll HCW were invited by the institutional email to attend an interview conducted by the staff of the Occupational Health Unit (OHU) and additional clinical assistants with blood sample extraction for serologic studies from April 14th to April 27th.
Throughout the study period, professionals with symptoms suggestive of COVID-19 were encouraged to attend the OHU where a nasopharyngeal swab was obtained for SARS-CoV-2 RT-PCR-exam. Patients with a positive test were sent home for quarantine or to the ER for further clinical evaluation.
Results of IgG status were informed to all HCW one to two weeks after blood extraction. HCW with a positive IgG and COVID-19 compatible symptoms in the previous 14 days that had not previously requested OHU attention were identified and tested by RT-PCR.
VariablesWe collected clinical and epidemiological variables (Table 1),
Demographics. General description of the participants in the study.
| Variable | Total no=2590 |
|---|---|
| Women | 1915 (73.9%) |
| Age (mean±SD)ayears | 43.8±11.1 |
| Clinical conditions | |
| Tobacco use | 545 (21%) |
| Chronic lung disease | 214 (8.3%) |
| High blood pressure | 180 (6.9%) |
| Obesity | 151 (5.8%) |
| Other cardiovascular disease | 68 (2.6%) |
| Diabetes mellitus | 54 (2.1%) |
| Immunodeficiency | 26 (1.0%) |
| Cancer | 9 (0.3%) |
| Liver disease | 7 (0.3%) |
| Chronic kidney disease | 8 (0.3%) |
| Pregnancy | 8 (0.3%) |
| Occupational SARS-CoV-2 exposure | 1946 (75.1%) |
| Previous PCR test | 727 (28.1%) |
| Professional category | |
| Technicians | 192 (7.4%) |
| Administrative and management | 170 (6.6%) |
| External workers | 337 (13%) |
| Patient carriers | 168 (6.5%) |
| Nurse | 687 (26.5%) |
| Physician | 564 (21.8%) |
| Nurse assistant | 472 (18.2%) |
| Work area | |
| Critical care unit | 226 (8.7%) |
| External workers | 204 (7.9%) |
| Hospitalized COVID area | 887 (34.2%) |
| Hospitalized non COVID area | 373 (14.4%) |
| Management | 226 (8.7%) |
| Non-hospitalized non COVID area | 122 (4.7%) |
| Central units | 298 (11.5%) |
| Emergency room | 253 (9.8%) |
We measured serum IgG antibodies via an enzyme-linked immunosorbent assay (ELISA) IgG using a SARS-CoV-2 S spike and nucleocapsid recombinant antigens (Diapro [Palex], Italy), to screen for the presence of human anti-SARS-CoV-2 IgG. The ELISA test simultaneously detects both antigens in the same assay. This assay (CE approved) was used according to the manufacturer's protocol. The manufacturer's reported sensitivity was 98%. All assays were run following the manufacturer's instructions on the platforms DSX System (Palex Medical SA) and Triturus (Grifols Movaco SA) (Supplement B).
For molecular diagnosis of SARS-CoV-2 infection, nasopharyngeal swabs were processed by automatized extraction using the MagNa Pure Lc instrument (Roche Applied Science, Mannheim, Germany), and real time reverse transcription polymerase chain reaction used the SARS-Cov-2 nucleic acid detection Viasure kit (CerTest Biotec S.L.); this was performed following the manufacturer's instructions (Supplement B).
IgG results interpretation.All samples corresponding to all HCW were analyzed per hospital protocol. The results of the tested samples were determined by calculating the ratio of the optical density (OD) value of the sample to the OD value of the cut-off. (Cut off) ratios≥1.1 were considered positive, ratios≥0.9 to <1.1 were considered borderline, and ratios<0.9 were considered negative. HCW with a positive IgG and presence of symptoms older than 14 days were assumed to be infected but no longer contagious. Those with positive IgG and symptoms within the past 14 days were considered to be actively infected and potentially contagious; they underwent RT-PCR examination from nasopharyngeal swabs.12 If the RT-PCR results were positive, then the HCW were offered sick leave and sent home for quarantine. HCW with IgG negative were considered susceptible to SARS-CoV-2 infection.13 Regardless of the seroprevalence study, all symptomatic HCW attending to the OHU were tested by RT-PCR. Asymptomatic workers were not routinely tested by RT-PCR.
Statistical analysisData are reported as mean (± standard deviation), median (interquartile range), or percentage as appropriate. Categorical variables were compared using Pearson's chi2 test or Fisher's exact test. Continuous variables were compared using Student's t-test or the McNemar test as appropriate.
Factors associated with a positive SARS-Co2 IgG were evaluated by univariate logistic analysis. Independent factors associated with seropositivity were evaluated by multivariate logistic analysis in a model including clinically relevant variables as well as variables associated in the univariate model (p<0.01). Hypothesis testing was based on a two-tailed test of significance, and we considered statistical significance at p<0.05. Statistical analysis was performed with the Statistical Package for Social Sciences (SPSSPC v 20, IL, USA).
Study approval and ethicsAll participants enrolled into the study voluntarily, and written informed consent was required to use the data for analysis. Participation in the study or results were not reported to the employers. The study protocol was approved by the independent ethics research committee of the hospital (reference number 20/69). We stated that results would not be used to generate an immunological passport in the hospital.
ResultsAll 2919 HCW HUFA were invited to participate in the study between April 14–27, 2020. Of these, 278 (9.5%) workers did not come to be tested because of sick leave, working at home, or they declined the invitation. In addition, 51 HCW (1.8%) denied consent to use their data for investigational purposes and were removed from the analysis. Thus, data from 2590 (98%) HCW tested were analyzed. This group included 1915 females (73.9%); the mean age was 43.8 (SD 11.1) years. Previous relevant clinical condition was present in 998 HCW (38.5%) distributed as follows: tobacco use 21%, chronic lung disease or asthma 8%, obesity 6.0%, high blood pressure 6.9%, diabetes mellitus 2.1%, and other cardiovascular diseases 2.0% (Table 1). A total of 2369 (91.5%) participants reported some degree of direct exposure to SARS-CoV-2. Self-reported exposure was occupational in 1946 (75%), contact with affected colleagues in 1710 (66%), and non-occupational in 290 (12.2%) HCW. Among HCW with occupational exposure, 72% of them referred to adequate PPE use.
IgG resultsOverall, the SARS-CoV-2 IgG was positive in 818 HCW (31.6%), negative in 1743 (67.3%), and borderline in 29 (1.1%). There were no differences among the IgG-positive rate for sex (31.6% women vs 33% men, p=0.482) or age (mean age for positive cases 43.9 years [11.4 SD]) vs 43.6 years [11.2 SD] for negative cases), p=0.719) respectively.
IgG results by area and professional categoryHigh and medium risk areas had higher rate of seropositivity (33.1%, [450/1359] and 33.8% [257/760]) than low risk areas (23.9%, [48/201]), p=0.007. The proportion of seropositive HCW among working areas included: ER (32.8%, 83/253), critical care (23.8%, 53/223), COVID-19 admitted patients (35.5%, 311/875), non-COVID-19 admitted patients (38.3%, 141/368), non-COVID-19 outpatient clinical care units (32.8%, 40/122), central units (29%, 85/293), administrative and management areas (24.9%, 56/225), and external workers 23.9%, 48/201; p<0.001 (Table 2).
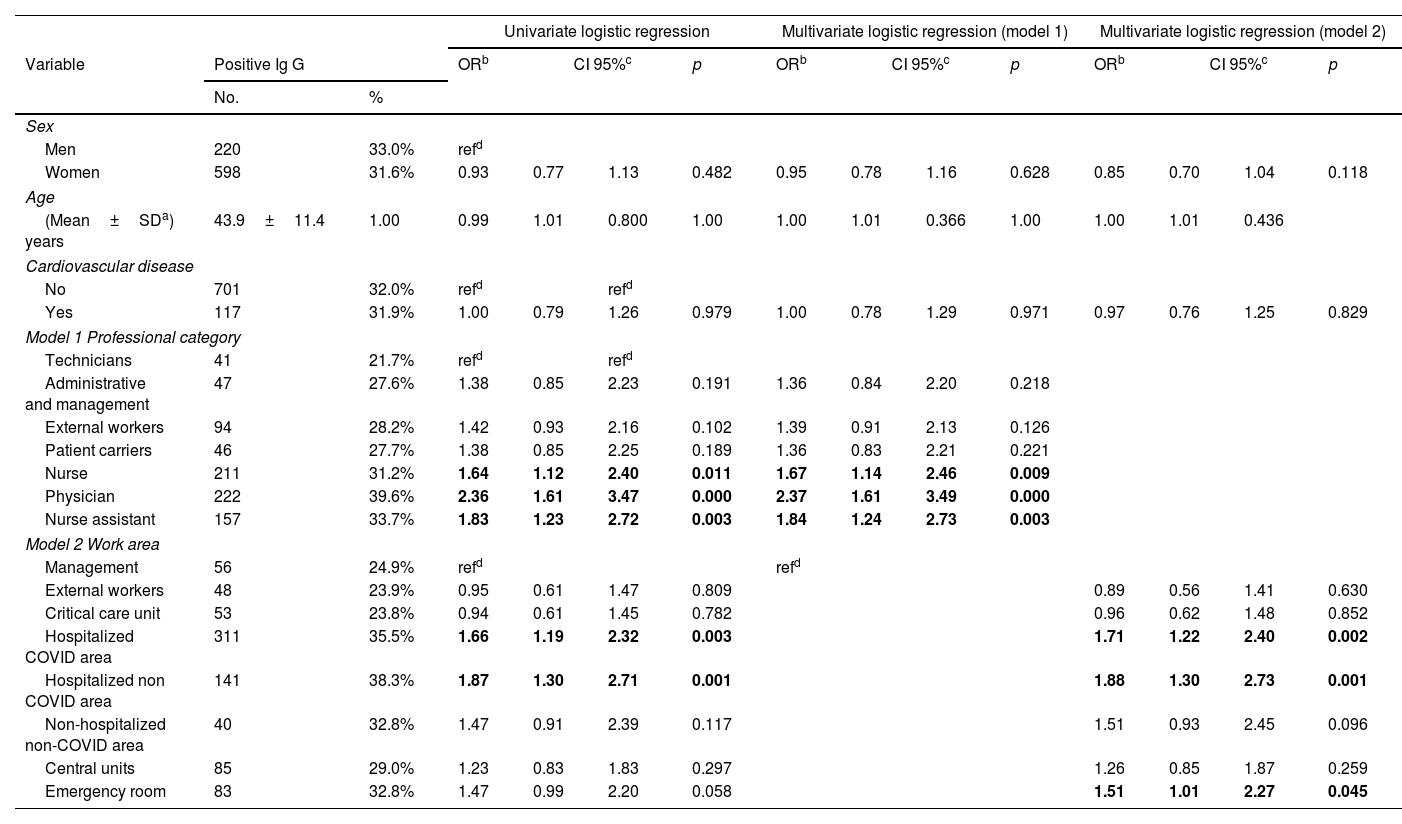
Univariate and multivariate analysis. Model 1 variables: sex, age, cardiovascular disease and professional category. Model 2 variables: sex, age, cardiovascular disease and work area. Central units included pharmacy, laboratory, radiology, pathology. External workers included cooks, food service, cleaners, ambulance drivers, store sellers, and watchmen.
| Univariate logistic regression | Multivariate logistic regression (model 1) | Multivariate logistic regression (model 2) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Positive Ig G | ORb | CI 95%c | p | ORb | CI 95%c | p | ORb | CI 95%c | p | ||||
| No. | % | |||||||||||||
| Sex | ||||||||||||||
| Men | 220 | 33.0% | refd | |||||||||||
| Women | 598 | 31.6% | 0.93 | 0.77 | 1.13 | 0.482 | 0.95 | 0.78 | 1.16 | 0.628 | 0.85 | 0.70 | 1.04 | 0.118 |
| Age | ||||||||||||||
| (Mean±SDa) years | 43.9±11.4 | 1.00 | 0.99 | 1.01 | 0.800 | 1.00 | 1.00 | 1.01 | 0.366 | 1.00 | 1.00 | 1.01 | 0.436 | |
| Cardiovascular disease | ||||||||||||||
| No | 701 | 32.0% | refd | refd | ||||||||||
| Yes | 117 | 31.9% | 1.00 | 0.79 | 1.26 | 0.979 | 1.00 | 0.78 | 1.29 | 0.971 | 0.97 | 0.76 | 1.25 | 0.829 |
| Model 1 Professional category | ||||||||||||||
| Technicians | 41 | 21.7% | refd | refd | ||||||||||
| Administrative and management | 47 | 27.6% | 1.38 | 0.85 | 2.23 | 0.191 | 1.36 | 0.84 | 2.20 | 0.218 | ||||
| External workers | 94 | 28.2% | 1.42 | 0.93 | 2.16 | 0.102 | 1.39 | 0.91 | 2.13 | 0.126 | ||||
| Patient carriers | 46 | 27.7% | 1.38 | 0.85 | 2.25 | 0.189 | 1.36 | 0.83 | 2.21 | 0.221 | ||||
| Nurse | 211 | 31.2% | 1.64 | 1.12 | 2.40 | 0.011 | 1.67 | 1.14 | 2.46 | 0.009 | ||||
| Physician | 222 | 39.6% | 2.36 | 1.61 | 3.47 | 0.000 | 2.37 | 1.61 | 3.49 | 0.000 | ||||
| Nurse assistant | 157 | 33.7% | 1.83 | 1.23 | 2.72 | 0.003 | 1.84 | 1.24 | 2.73 | 0.003 | ||||
| Model 2 Work area | ||||||||||||||
| Management | 56 | 24.9% | refd | refd | ||||||||||
| External workers | 48 | 23.9% | 0.95 | 0.61 | 1.47 | 0.809 | 0.89 | 0.56 | 1.41 | 0.630 | ||||
| Critical care unit | 53 | 23.8% | 0.94 | 0.61 | 1.45 | 0.782 | 0.96 | 0.62 | 1.48 | 0.852 | ||||
| Hospitalized COVID area | 311 | 35.5% | 1.66 | 1.19 | 2.32 | 0.003 | 1.71 | 1.22 | 2.40 | 0.002 | ||||
| Hospitalized non COVID area | 141 | 38.3% | 1.87 | 1.30 | 2.71 | 0.001 | 1.88 | 1.30 | 2.73 | 0.001 | ||||
| Non-hospitalized non-COVID area | 40 | 32.8% | 1.47 | 0.91 | 2.39 | 0.117 | 1.51 | 0.93 | 2.45 | 0.096 | ||||
| Central units | 85 | 29.0% | 1.23 | 0.83 | 1.83 | 0.297 | 1.26 | 0.85 | 1.87 | 0.259 | ||||
| Emergency room | 83 | 32.8% | 1.47 | 0.99 | 2.20 | 0.058 | 1.51 | 1.01 | 2.27 | 0.045 | ||||
Physicians were the most likely infected professional category (39.6%, 222/561) followed by nurse assistant (33.7%, 157/466), nurses (31.2%, 211/676), external workers (28.2%, 94/333), patient carrier (27.7%, 46/166), administrative and management staff (27.6%, 47/170), and finally technicians (24.1%, 41/170); p<0.001 (Table 2).
Participants who referred to the use of inappropriate PPE were 27.0%. The rate of seropositivity was 42.0% (219/522) versus 27.6% (374/1354) in cases with referred appropriate PPE use; p<0.001. However, this difference disappeared when the sample was stratified by previous attention to the OHU.
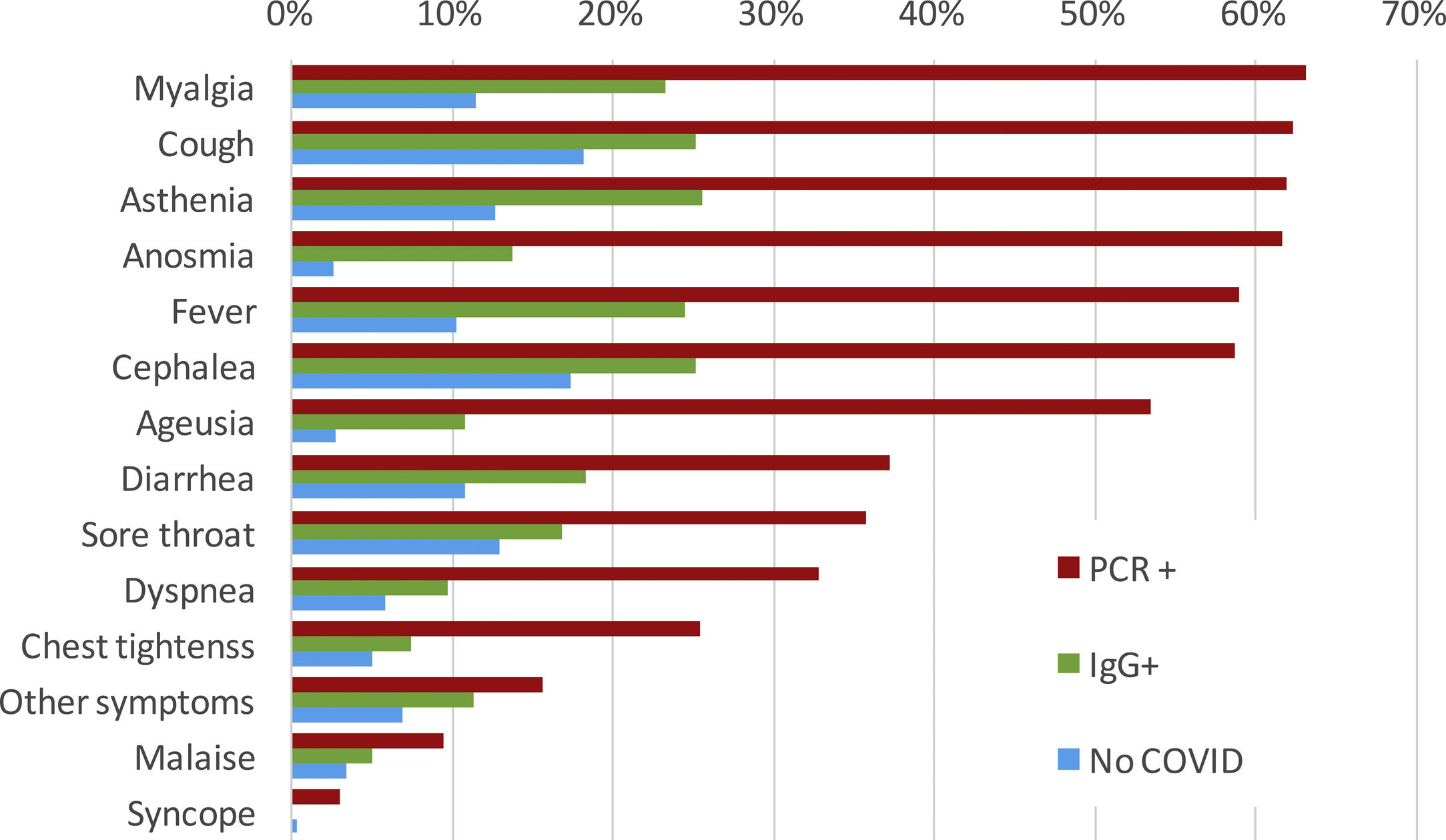
SymptomsThere were 397 out of 818 (48.5%) IgG-positive HCW who had not requested attention at the OHU prior to the seroprevalence study. Among them, 193 (48.6%) recalled minor symptoms in the study interview that they had not attributed to potential SARS-CoV-2 infection. Conversely, during the period of COVID-19 clinical care, 421 out of 818 HCW (51.5%) attended to the OHU because of symptoms suggestive of COVID-19 and were tested by RT-PCR for SARS-CoV-2; of these, 306/421 (72.7%) tested positive. A small proportion (48 cases/421 [11.4%]) of HCW were further evaluated at the ER, and 25 (5.9% [25/421]) required hospital admission. The most common symptoms among RT-PCR-confirmed COVID-19 cases were myalgia (63.1%), cough (62.2%), asthenia (61.9%), anosmia (61.7%), fever (59.0%), headache (58.7), and ageusia (53.4%) (Fig. 2). Those HCW with COVID-19 diagnosed solely by serology presented symptoms less frequently. Of note, some symptoms were also reported among HCW without COVID-19.
Forty-two out of 818 IgG positive HCW referred to mild symptoms in the 14 days previous to the serology evaluation and were thus tested by RT-PCR for SARS-CoV2. Of these, eight HCW were positive, and they were sent home for quarantine.
RT-PCR resultsWe found that 90.2% (306/339) of HCW with a previously positive RT-PCR were SARS-CoV-2 IgG positive, 9.1% (31/339) were negative, and two were indeterminate. Median time between positive RT-PCR and sample collection with negative IgG test was 21 days (IQR 14–26). These patients were followed longitudinally. Conversely, 29.6% (115/388) of HCW with negative SARS-CoV-2 RT-PCR had a positive IgG test.
Multivariate analysisWe evaluated factors associated with SARS-CoV-2 seropositivity by logistic regression analysis. Variables included in the logistic regression analysis were age, sex, cardiovascular disease, professional category (model 1), and work area (model 2). Two models were conducted because of significant interactions between professional categories and work areas. Regarding professional categories, HCW with a significantly increased probability of SARS-CoV-2 IgG positive were physicians (OR 2.37, CI95% 1.61–3.49), nurses (OR 1.67, CI95% 1.14–2.46), and nurse assistants (OR 1.84, CI95% 1.24–2.73). Regarding working areas, COVID-19 hospitalization areas (OR 1.71, CI95% 1.22–2.40), non-COVID-19 hospitalization areas (OR 1.88, CI95% 1.30–2.73), and ER (OR 1.51, CI95% 1.01–2.27) were significantly associated with increased risk of seropositivity (Table 2).
DiscussionThis is one of the few studies of SARS-CoV-2 seroprevalence of all HCW regardless of whether they were direct or indirect hospital employees. We found a relatively high proportion (30%) of HCW with a positive IgG for SARS-CoV-2 versus other centres in Europe.8,14,15 A recent study of the Spanish population showed a national prevalence of 5% but 11.3% in Madrid.16 A partial explanation for the higher prevalence at our hospital is the higher exposure to the virus in the city of Alcorcón. Data from the Madrid Regional Government shows that Alcorcón had a slightly higher incidence of COVID-19 (1055,05/100,000 inhabitants) than the region of Madrid.17 Furthermore, a study from a large hospital in Barcelona showed a prevalence in a sample of HCW of 11.6% doubling the seroprevalence of the general population14 and strengthening the notion of hospitals as a place of risk for SARS-CoV-2 infection among workers. Other European HCW seroprevalence studies ranged from 6.4%15 to 19.1%8 according to population seroprevalence rates. The temporal profile of SARS-CoV-2 RT-PCR positivity suggests that both community and hospital exposure have similarly contributed to HCW infection. Since the HCW seropositivity rate is essentially double that of the population, 50% of the infection may be related to in-hospital exposure. Besides a high community seroprevalence, another reason that may help to explain our results are (1) a relatively late use of universal masking9 due to PPE shortages and (2) excessive workload—a well-known risk factor for infection18 (Fig. 1).
Unsurprisingly, HCWs with no direct contact with patients such as external workers (23.9%) and non-clinical workers (25.8%) had lower seroprevalence than average as has been reported,8,10,19 however, there is still much higher than the general population in Madrid.16 These data suggest a role for nosocomial transmission in non-clinical workers.20 In these HCW cases, other HCWs are the likely source of infection rather than the patients.
Regarding clinical workers (all of them direct employees of the hospital), the rate of positive IgG was virtually identical among workers with direct contact with COVID-19-patients and those taking care of non-COVID-19 patients similar to other settings.11 These data suggest that the non-COVID-19 clinical areas are indeed an unrecognized potential source for SARS-CoV-2 infection among workers both from asymptomatic colleagues or patients not suspected of having SARS-CoV-2 infection.21 A recent meta-analysis estimates that nosocomial transmission is the source of SARS-CoV-2 infection in about 44% of cases.22
Universal COVID-19 screening was not an early standard practice, and it is conceivable that a substantial proportion of the so-called non-COVID-19 patients may be actually subclinical or unnoticed COVID-19 cases.23 This could be a reason for early infection in a relatively high proportion of HCW (Fig. 1). Our results are in agreement with a high rate of nosocomial transmission reported among workers in a dialysis unit in New York.24 These data emphasize the need for universal screening of all in-hospital patients as recommend by the World Health Organization.25 In contrast, a similar proportion of seropositivity among clinicians taking direct care of COVID-19 patients may reflect that the isolation protocols and PPE appear sufficient to prevent high levels of nosocomial COVID-19 transmission in our setting.7 Of note, critical care workers had one of the lowest seropositivity rates in our study. Indeed, our hospital prioritized the use of the best available PPE for critical care units where virtually all patients were positive for COVID-19 at the peak of the outbreak.26
In addition, the infection timeline (Fig. 1) suggests a potential source of infection outside the hospital in the very beginning of the pandemic, when HCW were not aware of the risk. The clinical spectrum of COVID-19 in our workers resembles that described for the general population: about half of them are asymptomatic or paucisymptomatic27 and less than 60% had fever (Fig. 2). This means that most infected workers may remain undetected unless there is a universal screening.28 In retrospect, about 50% of seropositive workers attending to the serology study recalled minor symptoms that did not prompt a request for OHU evaluation. Thus, only about one fourth of IgG-positive workers were fully asymptomatic as reported in other studies.24 This fact shows the limitations of relying on symptom-based surveillance alone. Workers with overt symptoms suggestive of COVID-19 disease usually (83%) had a mild disease that could be managed in the outpatient setting. This is hardly surprising since there are few elderly active workers.
To prevent nosocomial transmission, both patients and HCW should be screened for SARS-CoV-2 infection regardless of the absence of typical symptoms for COVID-19 disease28,29 because asymptomatic transmission is increasing recognized as very relevant in SARS-CoV-2 spread21 In addition, other measures may be considered to reduce the risk of infection in HCW such as close contact limitation, universal mask use, hand hygiene reinforcing, training through web, limitation time in cafeteria, entry check point, use of telemedicine, etc.
Our study has some limitations that deserve consideration. First, we do not have data about Ig M or concurrent RT-PCR for asymptomatic HCW. However, our study was designed to create a picture of past exposure to the virus among our workers. Second, the samples were collected over two weeks so the interpretation of the prevalence must be related to the average prevalence at that time. Third, a recall bias could underrepresented paucisymptomatic HCW. Nonetheless, our work has several strengths. First, the quality of the technology we had used seems to be one of the highest sensitivities available (ELISA).30 Second, we had a virtually universal representation of all workers of the hospital (90%) including external employees. This was an evaluation that is rarely performed. We also identified the particular function of all employees in a time of changing roles for clinicians in the middle of the crisis. In addition, its close temporal vicinity with the serologic study in the Spanish population allows for a direct comparison.
Our seroprevalence study unmasked a high rate of infection previously unnoticed among HCW. Clinical care of COVID-19 unscreened patients is associated with a similar prevalence of SARS-CoV-2 antibodies as that described in COVID-19 facilities uncovering a relevant source for nosocomial SARS-CoV-2 transmission. In addition, apparently healthy HCW may also be another relevant source for SARS-CoV-2 transmission. HCW testing could reduce in-hospital transmission. Sero-surveys in hospitals may be helpful to design strategies that control the SARS-CoV-2 epidemic.
Contributors and authorshipVC was the author of the original proposal, designed the study, and obtained the support of Hospital Management. All authors contributed to the planning of the study. IG, MLC, MJG, and CN conducted the study and acquired the data. MV analyzed the data, interpreted the data, and drafted the manuscript. All authors reviewed and approved the final draft of the manuscript. Working Group Alcorcon COVID-19 investigators contributed to the design and performance of the study and gave final approval of the manuscript. EP contributed to the data analysis, and CG drafted the manuscript.
DisclosureAll authors have approved the final article.
FundingThere was no financial support.
Conflict of interestThe authors declare that they do not have competing interests.
We thank all workers of the Hospital Universitario Fundación Alcorcón for their everyday work and cooperation in this study. We celebrate their brave and generous fight during the COVID-19 epidemic of March–April/2020.
Working Group Alcorcón COVID-19 investigators: Alejandro Algora Weber; Juan Carlos Alonso Punter; Mar-a Teresa Alonso Salazar; Gregorio Bonilla Zafra; M. Mercedes Bueno Campaña; Camilo Carrión Pulido; Ana Isabel Díaz Cuasante; Aurora Fabero Jiménez; Rosa Mar-a Fariña García; Mar-a Isabel González Anglada; Carlos Guijarro Herraiz; M. Mercedes Izquierdo Patron; Susana Lorenzo Martínez; Margarita Mosquera González; Montserrat Pérez Encinas; Elia Pérez Fernández; Francisco Jos, Pérez Vega; Maria Esther Renilla Sánchez