The occurrence of mucormycosis has been observed in individuals with COVID-19. However, there is limited information on the epidemiological factors, presentation, diagnostic certainty, and outcome of this infection in children. PubMed, MEDLINE, Scopus, Embase, Web of Science, LitCovid, and back-references of the identified manuscripts were systematically searched from December 2019 to March 2023. We have identified 14 cases of pediatric mucormycosis in patients with COVID-19. The median age of patients was 10.7 years. Among these cases, 10 were associated with active COVID-19. In 7 cases, the patients had pre-existing diabetes mellitus and concomitant diabetic ketoacidosis. Corticosteroids were administered to treat COVID-19 in 7 of the patients. The most common clinical presentation of the disease was rhino-orbital cerebral mucormycosis. Seven patients died (50%). Given the high mortality rate, clinicians should maintain a high level of clinical suspicion of mucormycosis in pediatric patients with COVID-19.
La emergencia de mucormicosis se ha observado en personas con COVID-19. Sin embargo, la información sobre los factores epidemiológicos, la presentación, la certeza diagnóstica y el resultado de esta infección en niños es limitada. Se realizaron búsquedas sistemáticas en Pubmed, MEDLINE, Scopus, Embase, Web of Science, LitCovid y referencias bibliográficas de los manuscritos identificados desde diciembre de 2019 hasta marzo de 2023. Se identificaron 14 casos de mucormicosis pediátrica en pacientes con COVID-19. La edad mediana de los pacientes fue de 10,7 años. De estos casos, 10 estaban asociados con COVID-19 activo. En 7 casos, los pacientes tenían diabetes mellitus preexistente y cetoacidosis diabética concomitante. Se administraron corticosteroides para tratar el COVID-19 en 7 de los casos. La presentación clínica más común de la enfermedad fue la mucormicosis rino-órbito-cerebral. Siete pacientes fallecieron (50%). Dada la alta tasa de mortalidad, es necesario mantener un alto nivel de sospecha clínica de mucormicosis en los pacientes pediátricos con COVID-19.
Mucormycosis is a set of invasive fungal infections caused by molds called mucormycetes, which belong to the Mucorales order. It is the third most common fungal infection in children after Candida and Aspergillus species. The primary risk factors for mucormycosis are immunosuppressive conditions like uncontrolled diabetes mellitus (DM), especially ketoacidosis, neutropenia, long-term corticosteroid use, prolonged hospitalization, and hematologic malignancies.1,2 The incidence of mucormycosis has increased due to the COVID-19 pandemic,3 and there have also been reports of increasing cases of mucormycosis in pediatric patients with diabetes associated with SARS-CoV-2 infection, with fatal outcomes.4 Although rare, mucormycosis infections have a high mortality rate of 40–80%, depending on the immune status and site of infection.5 COVID-19 seems to affect children differently from adults with respect to clinical manifestations and outcomes. Unlike adults, children seldom have significant respiratory symptoms and often remain asymptomatic.6 Severe illnesses, such as acute respiratory distress syndrome, myocarditis, acute renal failure, multisystem organ failure, and multisystem inflammatory syndrome (MIS-C), with comorbidities such as cardiopathy or cancer, and death have occurred in children, especially in those younger than 1 year.7 Among children with COVID-19 who were hospitalized, approximately 28–40% were admitted to the intensive care unit (ICU), 6–18% required invasive mechanical ventilation, and as many as 3% died.7 Many hypotheses have been formulated for the mechanisms underlying children's lower susceptibility to severe SARS-CoV-2 infection compared with adults, including an immature receptor system, specific regulatory mechanisms in the immune respiratory system, and cross-protection by antibodies for common viral infections in infant.8
The use of systemic corticosteroid for the treatment of COVID-19 can reduce mortality in people with the most severe courses of the disease9; however, together with immunological factors and other clinical factors, this treatment can also predispose patients to secondary fungal disease.10 Although COVID-19-associated pulmonary aspergillosis has been the primary focus of COVID-19, secondary infections in the literature, other fungal superinfections, including Candida spp. infections,10 rare mold infections (fusariosis), and COVID-19-associated mucormycosis, are likely to be underreported.11
With the resurgence of mucormycosis infections in the COVID-19 era, it is essential to develop new strategies for better treatment and prevention interventions. COVID-19-associated mucormycosis has become an epidemic during this global pandemic, especially in low- to middle-income countries where inadequate management, delayed diagnosis, and incorrect treatment plans are still prevalent.12 In this review, our objective was to gather and assess all available reports of pediatric COVID-19-associated mucormycosis and describe its clinical characteristics, presentation, diagnosis, management, and outcomes.
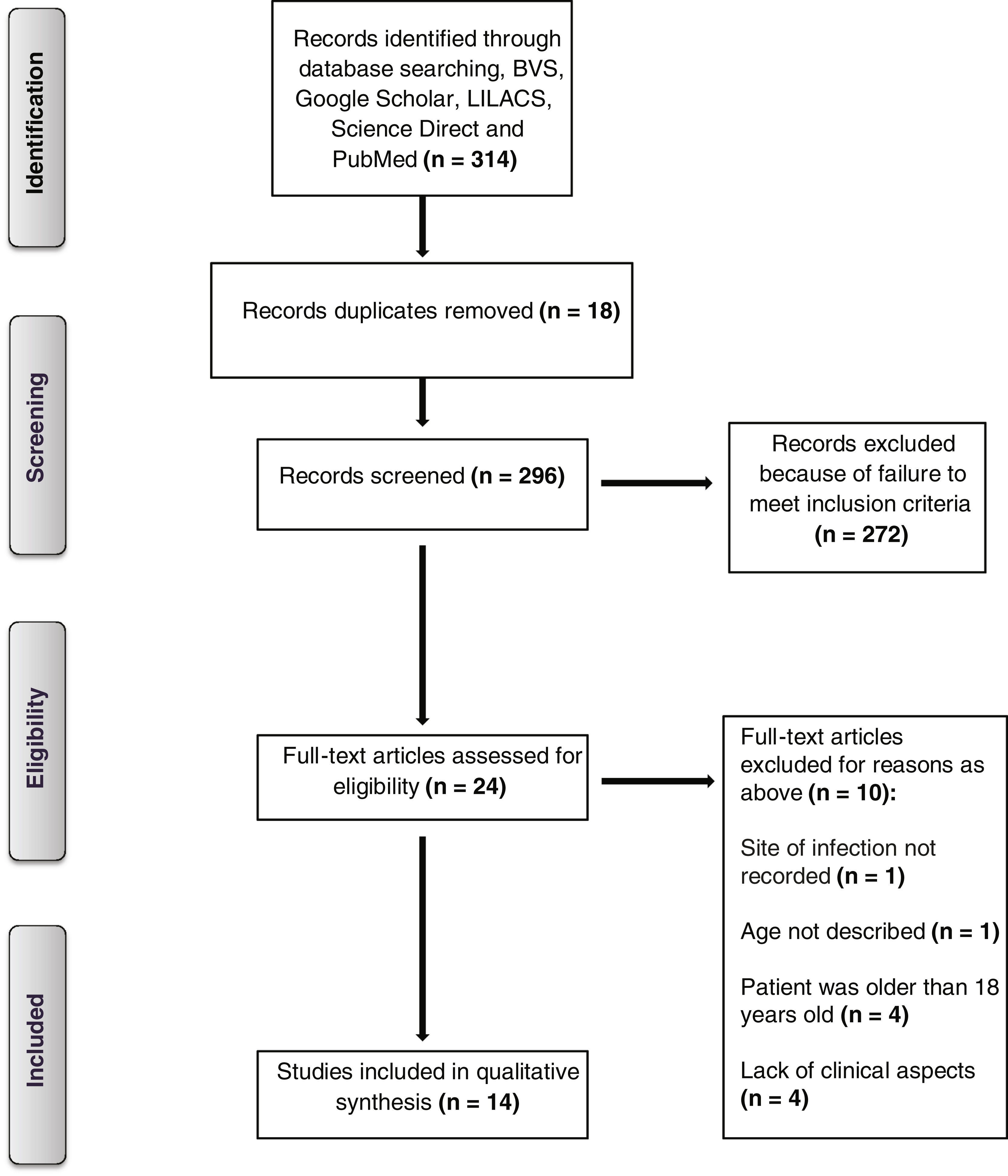
MethodsLiterature search and eligibility criteriaThe systematic review conducted for this study was registered with PROSPERO (registration number CRD42022336076) and adhered to the PRISMA guidelines to identify articles published in English, Portuguese, and Spanish languages between October 1, 2019, and March 31, 2023. We searched several databases, including Scopus, MEDLINE, LILACS, PubMed, Google Scholar, Science Direct, Web of Science, and LitCovid. In addition, we reviewed the reference lists of the identified manuscripts to find any additional articles. Our search terms included “(zygomyco* OR mucormycosis* OR Mucorales OR Rhizopus OR Absidia)” AND (COVID-19 OR SARS-CoV-2 OR coronavirus OR pandemic)” AND “(pediatric OR pediatric OR children OR child OR neonates)”. We also searched for any articles listed in the references of relevant publications.
Study selection and data extractionThe inclusion criteria for the case reports selected were13:
- (A)
The article was published between December 2019 and March 2023.
- (B)
The patients in the study ranged from newborns to 18 years old.
- (C)
The diagnosis of mucormycosis was confirmed through either histologic analysis or culture of the affected tissue. Clinical aspects were also taken into consideration to verify the diagnosis.
- (D)
The site of the infection at the time of diagnosis was recorded.
- (E)
Therapeutic intervention including antifungal therapy, surgery, or both, or a lack thereof, was documented.
- (F)
The article provided information about whether patients had an active or recovered COVID-19 infection at the time of their mucormycosis diagnosis.
Articles describing interventional studies and observational studies (prospective or retrospective) were not included due to the lack of available studies on this topic. In addition, all articles in non-English, non-Portuguese, or non-Spanish languages were excluded. The details of the individual reported cases were retrieved, reviewed, and analyzed.
Initially, all duplicates from the searches were removed. All titles and, in a second step, all remaining abstracts were screened by one author (L.B.A.) for possible eligibility. The full texts of all potentially eligible publications were rescreened by two authors (L.B.A. and L.R.); conflicting opinions about eligibility could be resolved in all cases by discussion. Data collection and analysis were performed by three authors (L.B.A.; L.R. and F.C.) The database search was updated regularly until March 31, 2023.
Demographic and epidemiological information (age at diagnosis, sex, and country), underlying conditions, COVID-19 on admission or previous COVID-19, ICU procedures, corticosteroid use, diagnosis of mucormycosis (radiological and mycological findings), sites of infection (rhino-orbital, pulmonary, and central nervous system [CNS] involvement), coinfections, clinical management (systemic antifungal therapy and surgical procedures), and outcomes were extracted from the literature.
DefinitionsMucormycosis associated with active COVID-19 was defined as when both COVID-19 and mucormycosis were present simultaneously, whereas recovered COVID-19 was defined as two weeks after the diagnosis of COVID-19.14
The patients were grouped into the rhino-orbital cerebral mucormycosis with CNS involvement, rhino-orbital cerebral mucormycosis without proof of CNS involvement, and others (e.g., pulmonary, intestinal, and disseminated mucormycosis) groups according to the site of infection.10 Disseminated infection was defined as involving two or more non-contiguous sites, as previously described.15
ResultsAs a result, a total of 14 cases of pediatric mucormycosis with associated COVID-19 infection were identified and featured in articles published from December 2019 to March 2023 (Fig. 1). The demographic and clinical data of the patients included in this study are shown in Table 1. The study included participants with a mean age of 10.7 years (range: 1.3–17 years), with eight girls and six boys. Among the cases reported, six were from India. Type 1 DM was the most frequent underlying disease, described in 7 cases (50%) followed by hematological malignancies in 3 (21%).
Pediatric mucormycosis in COVID-19 patients.
| Case | Age/gender | Country | Comorbidities | COVID-19 (active/recovered) | Treatment received for COVID-19 | Corticotherapy | ICU admission | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.3/F | Poland | B-cell acute lymphoblastic leukemia | Recovered | ND | Yes | No | 16 |
| 2 | 5/M | India | B-cell acute lymphoblastic leukemia | Active | ND | Yes | Yes | 17 |
| 3 | 7/F | Peru | Immunocompetent | Active | ND | Yes | Yes | 18 |
| 4 | 7/M | Rumania | Major beta-thalassemia, iron overload | Recovered | ND | ND | No | 19 |
| 5 | 9/F | India | Immunocompetent | Active | ND | Yes | Yes | 20 |
| 6 | 11/M | Bulgaria | Type 1 diabetes mellitus, diabetic ketoacidosis | Active | ND | Yes | No | 3 |
| 7 | 11/M | India | Type 1 diabetes mellitus, diabetic ketoacidosis | Recovered | ND | ND | No | 21 |
| 8 | 12/F | India | Type 1 diabetes mellitus, diabetic ketoacidosis | Recovered | ND | ND | No | 22 |
| 9 | 13/F | India | Type 1 diabetes mellitus, diabetic ketoacidosis | Active | ND | ND | No | 21 |
| 10 | 13/F | USA | Type 1 diabetes mellitus, diabetic ketoacidosis | Active | ND | ND | No | 23 |
| 11 | 13/M | USA | Morbid obesity, diabetes mellitus, diabetic ketoacidosis | Active | ND | Yes | Yes | 24 |
| 12 | 14/M | Iran | Acute myeloid leukemia | Active | ND | ND | Yes | 25 |
| 13 | 17/F | USA | Type 1 diabetes mellitus, diabetic ketoacidosis | Active | Remdesivir | Yes | Yes | 26 |
| 14 | 17/F | India | Immunocompetent | Active | ND | ND | Yes | 27 |
ND: not described.
Notably, only three patients (21.4%) had no identifiable underlying condition and were characterized as immunocompetent hosts. Multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 was observed in two immunocompetent hosts. All presented cases had COVID-19, most cases of mucormycosis were diagnosed during the active phase of COVID-19, with four patients (28.5%) diagnosed as having recovered COVID-19 and ten patients (71.4%) having active COVID-19. For COVID-19 treatment, only one patient received remdesivir, and seven out of seven cases with reported information received corticosteroids.
All patients were admitted to the hospital, and seven of them (50%) were admitted to the intensive care unit (ICU). All patients had mucormycosis, as documented by histopathology and/or culture (Table 2). Among the 14 patients, Rhizopus spp. were identified in four cases (28.5%). The distribution of the infection sites is shown in Table 2. The most common patterns of mucormycosis were rhino-orbito-cerebral mucormycosis (ROCM) (seven cases – 50%), without proof of CNS involvement occurred in three cases, disseminated (three cases – 21.4%) and pulmonary mucormycosis (three cases – 21.4%).
Summary of microbiological and clinical aspects of pediatric mucormycosis associated with COVID-19.
| Case | Laboratory diagnosis of mucormycosis | Species | Organ/site affected | Type of infection | Antifungal therapy | Other pharmacological treatments | Surgery | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Biopsy+culture | Rhizopus microsporus | Liver, lung, intestine | Disseminated | Amphotericin B liposomal+isavuconazole | ND | Yes | Survived |
| 2 | Biopsy | * | Brain, sinuses | ROCM | ND | ND | No | Died |
| 3 | Biopsy+culture | Mucor spp. | Eye | ROCM | Amphotericin B deoxycholate | Meropenem+vancomycin | ND | Died |
| 4 | Culture+PCR | Cunninghamella spp. | Heart, brain, lung, kidney, spleen | Disseminated | ND | No | Yes | Died |
| 5 | Biopsy | * | Nose | ROCM # | ND | ND | No | Died |
| 6 | Biopsy | * | Heart, trachea, lung | Pulmonary | ND | No | ND | Died |
| 7 | Biopsy+culture+MALDI-TOF MS | Rhizopus arrhizus | Brain, eye, sinuses | ROCM | Amphotericin B liposomal | Metronidazole+ceftriaxone | Yes | Survived |
| 8 | Biopsy | * | Intestine | Intestinal | Amphotericin B | ND | Yes | Survived |
| 9 | Biopsy+culture+MALDI-TOF MS | Rhizopus arrhizus | Brain, eye, sinuses | ROCM | Amphotericin B deoxycholate+amphotericin B liposomal | Metronidazole+ceftriaxone | Yes | Survived |
| 10 | Biopsy+culture | Rhizopus delemar | Brain, sinuses | ROCM | Amphotericin B liposomal+posaconazole+isavuconazole | Metronidazole+ceftriaxone+vancomycin | Yes | Survived |
| 11 | Biopsy | * | Trachea | Pulmonary | ND | ND | Yes | Died |
| 12 | Biopsy | * | Nose | ROCM # | Amphotericin B+caspofungin | ND | ND | Survived |
| 13 | Biopsy | * | Lung | Pulmonary | Amphotericin B+posaconazole | ND | Yes | Survived |
| 14 | Biopsy | * | Lung, liver, intestine | Disseminated | ND | No | Yes | Died |
ROCM: rhino-orbito-cerebral mucormycosis; ROCM #: without proof of CNS involvement; ND: information not described.
Of the 14 reported cases, eight patients (57.1%) underwent antifungal therapy. Among them, four patients received only amphotericin B preparation (with two receiving liposomal formulation (AMBl), one receiving the deoxycholate form, and one receiving both deoxycholate amphotericin B and a lipid formulation). Four patients received a combination of antifungals (one received AMBl+isavuconazole, one received AMB+posaconazole, one received AMB+caspofungin and one received AMB+posaconazole+isavuconazole). Four patients (28.5%) received other pharmacological treatments (two received metronidazole+ceftriaxone, one received meropenem+vancomycin and one received metronidazole+ceftriaxone+vancomycin). Surgery was performed in nine (64.2%) cases. Seven (50%) of the 14 children with mucormycosis associated with COVID-19 died (Table 2).
DiscussionTo our knowledge, this is the first published review on the clinical epidemiology and treatment of pediatric mucormycosis associated with COVID-19. Notably, mucormycosis associated with COVID-19 has been identified in various countries, particularly in individuals with uncontrolled DM. According to our review, India was the country with the highest number of cases, a finding in accordance with these in a previous report on the adult population.14 This situation can be explained by the fact that India already has the second largest population of DM in the world.14 Notably, in pediatric mucormycosis associated with COVID-19, DM is the most common risk factor, and most patients have uncontrolled or poorly controlled DM. The mechanism behind this observation is the hyperglycemia-induced inflammatory state that might be potentiated once antiviral immunity to SARS-CoV-2 is activated.10 In a scenario without COVID-19, hematological malignancies are the most common risk factor in Europe and the USA.1,13
Moreover, the majority of the children described here received systemic corticosteroid treatment, indicating that children with COVID-19 who receive corticosteroids may have a higher risk of developing COVID-19-associated mucormycosis. Corticosteroid therapy may hinder neutrophil migration, ingestion, and phagolysosome fusion, thereby increasing the risk of mucormycosis.10 Before the COVID-19 pandemic, rhinocerebral disease was the most common form of mucormycosis in both adults and children. However, in the pediatric population, rhino-orbito-cerebral is the most frequently observed pattern of mucormycosis.13
The diagnostic approach for COVID-19-associated mucormycosis follows the same principles as in other populations. Nearly all cases of mucormycosis associated with COVID-19 are classified as proven infections, in contrast to COVID-19-associated pulmonary aspergillosis (CAPA), which is usually considered a probable infection.28 Detecting the infection at an early stage is crucial to prevent tissue invasion and spreading. However, it is challenging even in high-risk patients since most cases of mucormycosis were diagnosed during the active phase of COVID-19 and they may display non-specific symptoms that are often misattributed to other infections.
The treatment of mucormycosis is a multi-faceted approach that includes antifungal medication, surgical intervention, immune system enhancement, and other supplementary therapies.29 The highest levels of therapeutic success have been achieved when aggressive surgical debridement and reversal of the underlying host factors are combined with medical management.30,31 The data presented in this review supports the use of AMB as a fundamental medical treatment for mucormycosis, which is consistent with findings from previous studies.13 A delay in AMB initiation is an independent predictor of poor outcomes in patients with mucormycosis, and the early initiation of AMB is associated with improved survival rates.31,32
Combination antifungal therapy may be used in pediatric patients with mucormycosis, as seen in four cases outlined in this review, although the efficacy of such therapy in children remains insufficiently investigated. The concomitant use of multiple antifungal agents can enhance their efficacy and potentially reduce resistance, leading to shorter treatment durations.33 Patients who received amphotericin B plus posaconazole or isavuconazole had favorable outcomes. However, an analysis comparing initial combination therapy and monotherapy for treating mucormycosis in adults with hematologic malignancy demonstrated no significant differences in the 6-week mortality rates of the two types of therapy.34 It is important to mention that isavuconazole has not yet been authorized for use in children. Out of the cases reported in this review, surgery was performed on nine patients, of whom six survived. Studies have shown that combining surgery with medical management has resulted in the highest levels of therapeutic success.35
Despite therapy, the mortality rate of patients with this condition is high. Of the 14 children analyzed in this review, seven (50%) died because of the association between mucormycosis and COVID-19. This rate is higher than the overall mortality rate reported in a context without COVID-19, which is 32%.13
This study has certain limitations. First, the sample size was small with only 14 cases reported in the literature. Therefore, the absence of a control group prevented us from making definitive conclusions regarding the risk factors associated with COVID-19-associated mucormycosis in the pediatric population. Second, there was heterogeneity in the reported cases with some providing detailed information while others not reporting important parameters. Additionally, the reported cases of mucormycosis may have been underrepresented due to the difficulty in making a microbiological or histopathological diagnosis, particularly in a pandemic setting. Finally, it could be challenging to define active and recovered COVID-19 and their relationship with the onset of mucormycosis due to the lower sensitivity of confirmatory RT-PCR tests and the occasional use of alternative diagnostic tests.
Conclusions and future perspectivesBased on our review of 14 recently published cases, it appears that pediatric mucormycosis associated with COVID-19 mainly affects immunocompromised children, particularly those with uncontrolled DM and ketoacidosis. Rhino-orbital cerebral disease remains the most common manifestation of the infection in children. Hence, it is imperative to screen high-risk patients, closely monitor their risk factors, and facilitate early diagnosis to expedite the commencement of antifungal therapy. Mucormycosis is a severe infection in children that can progress rapidly and cause angioinvasion. Further research is necessary to validate diagnostic testing and determine the best diagnostic strategy for at-risk children. Surgical debridement plays a crucial role, serving both for tissue diagnosis and as a treatment measure, and should be promptly pursued whenever feasible. Aggressive management is essential to treat these infections, including prompt initiation of appropriate antifungal therapy, aggressive surgical debridement, and immune reconstitution.
Authors’ contributionsAll authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Laura Batista Amaral, Fabianne Carlesse and Luana Rossato. The first draft of the manuscript was written by Laura Batista Amaral and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
FundingNone declared.
Competing interestsLBA and LR declare no conflict of interest. FC is speaker of Pfizer and United Medical.