Fungal urinary tract infections predominantly affect the critically ill premature infant and those with urogenital tract abnormalities. Fungal balls are an uncommon complication which require prompt detection and treatment to prevent morbidity and mortality. The evidence on the management of fungus balls in young infants with Candida urinary tract infections is very scarce.
MethodsCase reports and review of the literature.
ResultsWe report two immunocompetent young infants with urogenital abnormalities that received local amphotericin B deoxycholate, and systemic therapy, for the treatment and prevention of Candida urinary tract infection-associated fungus balls. We identified 21 similar cases in the literature, with very limited data about drug compounding, optimal dosages, dwell times and length of treatment. Different management strategies are discussed.
ConclusionsAmphotericin B deoxycholate local irrigations were safe and effective for the therapeutic management and prophylaxis of Candida fungus balls in young infants, in combination with systemic antifungal therapy.
Las infecciones urinarias fúngicas afectan preferentemente al prematuro gravemente enfermo o al afecto de malformaciones genitourinarias. Las bolas fúngicas son una complicación infrecuente que requiere un diagnóstico y tratamiento precoces para evitar morbimortalidad asociada. La evidencia científica disponible sobre el manejo de las bolas fúngicas por Candida en lactantes pequeños es muy escasa.
MétodosCasos clínicos y revisión de la literatura.
ResultadosSe presentan 2 lactantes inmunocompetentes con malformaciones urogenitales que recibieron tratamiento local con anfotericina B desoxicolato junto a terapia sistémica para el tratamiento y la profilaxis del desarrollo de bolas fúngicas por Candida. Identificamos 21 casos similares en la literatura, con muy pocos datos disponibles sobre la preparación y posología, tiempos de permanencia y duración del tratamiento. Se discuten las distintas estrategias de manejo.
ConclusionesLa irrigación local con anfotericina B desoxicolato resultó segura y eficaz en el tratamiento y en la profilaxis de las bolas fúngicas por Candida en el lactante pequeño, junto con el tratamiento antifúngico sistémico.
Fungal complicated urinary tract infection (cUTI) is a rare condition in children, that is reported in patients with risk factors (prematurity, urinary tract abnormalities, and the use of indwelling urinary devices or broad-spectrum antibiotics).1 The most commonly involved microorganism is Candida spp. Fungal cUTI seldom leads to the development of renal fungus balls, which are necrotic debris mixed with proliferating mycelia in the renal collecting system that can cause obstruction of the urinary tract, challenge the success of conventional antifungal treatments and lead to higher morbidity and mortality.2 In adults, a combined medical and surgical therapeutic approach is recommended, including systemic antifungals and local irrigation with amphotericin B deoxycholate (AMBD).3 Data about the management of fungus balls in neonates and very young infants are scarce.
We report two cases of immunocompetent neonates with urogenital abnormalities presenting with cUTI caused by Candida albicans that were successfully treated with systemic antifungals and local AMBD.
MethodsAMBD (Amphotericin B for Injection USP; X-Gen Pharmaceuticals Inc.; Horseheads, NY) is presented in a vial of 50mg powder for sterile concentrate which needs to be reconstituted with 10mL of sterile water for injection (SWI), yielding a concentration of 5mg/mL.4 For local irrigation use, AMBD needs to be further diluted with SWI or dextrose 5%. Concentrations as high as 0.5–1mg/mL have been reported to be chemically stable for up to 120h when stored at 4°C.4
Report 1A 3-week-old male neonate with a prenatal diagnosis of severe right hydroureteronephrosis was admitted because of Klebsiella oxytoca sepsis-meningitis of urinary origin; cefotaxime was started. On day 8 of admission, the treatment was switched to vancomycin and meropenem due to clinical worsening. On day 10, Candida albicans grew both in blood and urine cultures. Intravenous fluconazole (12mg/kg/day) was initiated with good clinical response within 24h, and antibacterials were de-escalated to ceftriaxone. The patient remained afebrile and clinically stable, with negative control blood cultures but persistently positive urine cultures for Candida.
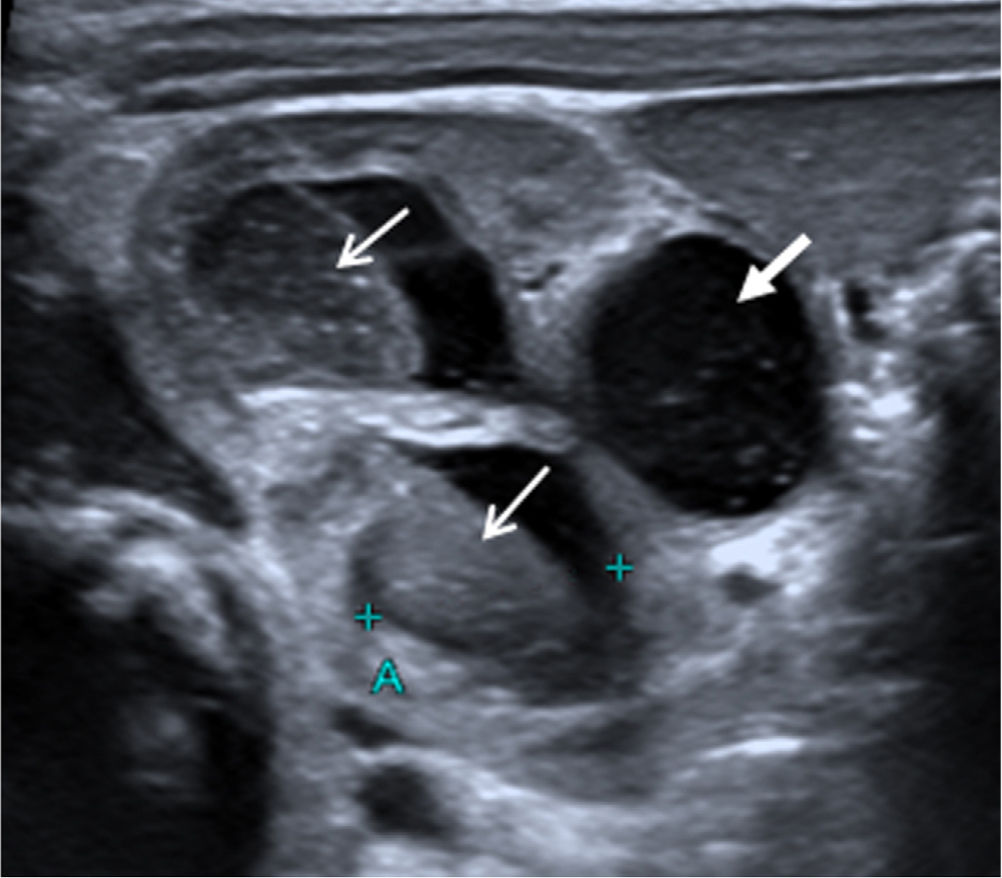
On day 12, renal ultrasound revealed multiple fungus balls in the right kidney with nephromegaly and an increase in the calyceal, pyelic and ureteral dilatation (Fig. 1). Creatinine plasmatic levels remained within normal limits. On day 14, local AMBD irrigations through a bladder catheter were added, aiming to reach the right kidney thanks to the vesicoureteral reflux. Based on the estimated bladder capacity of the patient, 3.5mg AMBD (1mg per kg of body weight) diluted in 25mL SWI syringes (AMBD concentration, 0.14mg/mL) was administered every 8h for 60min. Due to discomfort during dwell time, the instilled volume was reduced to 20mL (0.175mg/mL) and finally to 10mL (0.35mg/mL), with good tolerance. Three days later, renal ultrasound showed complete resolution of fungus balls, with persistence of right hydroureteronephrosis and grade V vesicoureteral reflux in the voiding cystourethrogram; negative urine cultures were obtained. Local antifungal treatment and oral fluconazole were maintained for 14 days and 5 weeks, respectively. A 3-week regimen of ceftriaxone was completed and the patient was discharged with oral fluconazole and prophylactic cotrimoxazole. At the 14th month of life, no recurrent UTIs had occurred.
Report 2A full-term male newborn with postnatal diagnosis of bladder exstrophy and epispadias underwent primary bladder exstrophy closure with cystostomy and bilateral ureteral permanent catheterization on the 2nd day of life. As per local protocol, he received prophylactic intravenous amoxicillin-clavulanate after the surgery.
On day 9 of life, vancomycin and meropenem were started due to suspicion of sepsis, and 24h later Candida albicans was isolated both in urine and in surgical wound cultures, but not in blood cultures. The renal ultrasound showed a slight pyelic-calyceal dilatation. Intravenous fluconazole (12mg/kg loading dose followed by 6mg/kg/day) was initiated, together with local AMBD irrigations to prevent the development of fungus balls. Based on the volume of the ureterostomies (2.5mL), syringes of 2.5mg AMBD (1mg per kg of body weight) in 5mL SWI (0.5mg/mL) were prepared, and 2.5mL were instilled through each ureteral catheter every 8h, during 20min.
Local AMBD treatment was maintained for 7 days, with optimal tolerance, and ureterostomies were then removed. Renal function remained always within normal limits. Fluconazole treatment was continued for 14 days and renal ultrasound showed normal sized renal calyces 18 days after surgery. The patient was discharged on day 33 of life with prophylactic oral amoxicillin-clavulanate. At the 8th month of life, no recurrent infections had been observed.
DiscussionFungal UTIs are rare in healthy neonates but common in prematures, critically ill newborns and those with urological malformations. Candida-associated UTI in the neonate usually develops from candidemia or renal candidiasis, but ascending infection is also possible in patients with urological malformations.2Candida fungus balls in neonates and young infants are a rare complication. Therapeutic management is challenging, and the available literature in infants aged<3 months is scarce. Only 21 cases of young infants that received local AMBD to treat fungus balls have been reported (Suppl. Table 1). Systemic antifungal treatment was given to most patients; surgery and local streptokinase were required in 5 and 2 patients, respectively; outcomes were good in all cases, and residual minor anatomic defects were described only in 4 patients.
In adults and older children, combined treatment of fungus balls with surgery and systemic antifungals (fluconazole or AMBD, with or without flucytosine) is strongly recommended.3 Continuous or intermittent irrigation with AMBD (25–50mg in 200–500mL SWI) through a nephrostomy tube can be considered, and intermittent irrigation with saline or streptokinase and endoscopic removal have also been reported.3,5 Local irrigation of AMBD leads to high non-toxic AMBD concentrations in urine that clear candiduria within 24h.3,6 If feasible, removal or replacement of nephrostomy tubes or bladder catheters is recommended to prevent biofilm formation.
In a review on obstructive renal candidiasis in children, initial local irrigation with AMBD or fluconazole added to systemic treatment is recommended followed by local streptokinase/urokinase and surgical removal if response to treatment does not occur.2 AMBD concentrations of 0.05mg/mL and dwell times of 60–120min are recommended in instillations 2–4 times per day or in continuous irrigation.2,7,8 Systemic antifungals are always indicated. Except for Candida krusei and Candida glabrata infections, fluconazole remains the preferred agent because of its safety, achievement of high urinary concentrations, and availability in both oral and intravenous formulations.2
There are very limited data about local AMBD preparation, optimal dosages, dwell times and duration of treatments in neonates and infants with fungus balls (Suppl. Table 1). The use of strikingly wide ranges of AMBD concentrations (range: 0.0002–60mg/mL) has been reported, and the dwell times are not given in any of the case reports we identified. Continuous and intermittent irrigations (every 4, 5, 6 or 8h), most commonly through nephrostomy tubes, but also transureterally (by means of double-J stents or umbilical artery catheters), have been described. In 10 of the case reports, no details about local AMBD preparation were given and in 4 of these 10 cases the method of irrigation was not specified.
In agreement with recommendations in older children,2 we prepared AMBD irrigation volumes according to estimated bladder and ureteral catheter capacities in patients 1 and 2, respectively, with AMBD concentrations ranging from 0.14 to 0.5mg/mL. In patient 1, smaller irrigation volumes (with increasing AMBD concentrations) were required due to discomfort during dwell time. Other than this, local AMBD irrigation was safe and well tolerated. We administered local AMBD every 8h, together with systemic antifungal treatment in both patients. AMBD dwell times and treatment duration were 60minutes and 2 weeks in patient 1, and 20min and one week in patient 2. These differences were due to the original indication of local AMBD in our patients: the former developed fungus balls despite adequate systemic antifungal treatment and AMBD was administered through the urethral catheter aiming to reach the upper urinary tract through the patient's severe vesicoureteral reflux. In patient 2, local AMBD was given to treat urinary tract Candida infection, but also to prevent the development of fungus balls and to avoid manipulation and having to remove the ureteral catheters after recent surgery for bladder exstrophy. Currently, antifungal prophylaxis is only recommended in premature newborns weighing<1000g in nurseries with high rates of invasive candidiasis, but not in neonates or infants with other risk factors for fungal cUTI.3 The use of local AMBD in the prophylaxis of fungus balls in infants with congenital abnormalities of the urinary tract and indwelling catheters has not been previously described, but it may prove to be safe and effective in selected cases. In patient 2, local AMBD was stopped when ureteral catheters were removed.
In summary, local irrigation with AMBD was safe and proved useful in the treatment and prevention of Candida fungus balls in two neonates, in combination with systemic antifungal therapy. Prospective controlled studies are needed to better define the optimal drug concentration, posology and duration of treatment in very young patients at high risk.
Authors’ contributionsAll authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by ACE, MVF, EVA and ANJ. The first draft of the manuscript was written by ACE and ANJ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics approvalEthics approval was obtained from Hospital Sant Joan de Déu (Barcelona, Spain) Ethics Committee (reference ART 08-21).
Consent to participate and for publicationInformed consent was obtained from the parents.
Data sharingNot applicable.
FundingAntoni NOGUERA-JULIAN was supported by “Subvencions per a la Intensificació de Facultatius Especialistes” (Departament de Salut de la Generalitat de Catalunya, Programa PERIS 2016-2020) [SLT008/18/00193]. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone.