Povidone-iodine and hydrogen peroxide could be effective in against SARS-CoV-2.
MethodsA “non-interventional trial” in 88 patients (43±17 yrs., 55% men) with SARS-CoV-2 in nasopharyngeal swabs (RT-PCR). 31 received mouth rinses/gargling with povidone-iodine (every 8h, two consecutive days), 17 with mouth rinses/gargling of hydrogen peroxide, and 40 controls. Were repeated PCR in 3, 11 and 17 days.
ResultsAfter intervention the viral load (Log10 copies/ml) remained similar in povidone-iodine (4.3±2.7 copies/ml), hydrogen peroxide (4.6±2.9 copies/ml; p=0.40) and controls (4.4±3.0 copies/ml). The percentage of patients with a negative result in the second PCR was 27% in povidone-iodine group, 23% in hydrogen peroxide and 32% in controls; in the third PCR, 62%, 54% y 58% respectively; and in the fourth PCR, 81%, 75% y 81%.
ConclusionOur results do not support the clinical usefulness of mouth rinses/gargling with povidone-iodine or hydrogen peroxide in patients with COVID-19.
La povidona yodada y el peróxido de hidrógeno podrían ser eficaces contra el SARS-CoV-2.
MétodosEstudio observacional de seguimiento prospectivo (EPA-AS) en 88 pacientes (43±17 años, 55% varones) con SARS-CoV-2 en muestras nasofaríngeas (RT-PCR). 31 recibieron enjuagues/gargarismos con povidona yodada cada 8 horas dos días consecutivos, 17 con la misma pauta de peróxido de hidrógeno, y 40 controles sin enjuagues. Se repitió PCR a 3, 11 y 17 días.
ResultadosTras la intervención no hubo diferencias en la carga viral: povidona yodada (4,3±2,7 copias/ml), peróxido de hidrógeno (4,6±2,9 copias/ml; p=0,40), controles (4,4±3,0 copias/ml). El porcentaje de pacientes con 2ª PCR negativa fue 27% povidona yodada, 23% peróxido de hidrógeno y 32% controles; en la 3ª PCR 62%, 54% y 58% respectivamente y en la 4ª PCR, 81%, 75% y 81%.
ConclusiónNuestros resultados no apoyan la utilidad de los enjuagues de estos dos antisépticos en pacientes con COVID-19.
The oral cavity plays an important role in the transmission of SARS-CoV-21,2. The oral mucosal epithelial cells express ACE-2 receptors and the salivary glands are a reservoir for the virus3. For this reason, various scientific societies have proposed the use of antiseptic oral solutions to reduce transmission4,5. Povidone-iodine (PVI) has already shown significant in vitro antiviral activity against MERS and SARS-CoV6,7 and more recently against SARS-CoV-28. Hydrogen peroxide (H2O2) is also effective against SARS-CoV-2 on inanimate surfaces9 and in cell cultures10. However, the in vivo efficacy of either oral antiseptic has not been clarified in this pandemic11,12. Therefore, we aim to determine the utility of mouth rinses and gargles with PVI and H2O2 in reducing the oropharyngeal viral load of SARS-CoV-2 in patients with COVID-19.
Materials and methodsThis was an observational prospective follow-up study (EPA-AS), classified by the Spanish Agency of Medicines and Medical Devices (AEMPS), in 88 patients (inpatients or outpatients) with COVID-19 confirmed by RT-PCR in nasopharyngeal samples (Ct <35 N or R gene; VIASURE SARS-CoV-2 Real Time PCR Detection Kit, Certest, Zaragoza, Spain). The patients were diagnosed at our hospital between May and November 2020. Patients under 18 years of age, patients with a decreased level of consciousness, presence of mouth ulcers or wounds, thyroid diseases, hypersensitivity to PVI or hydrogen peroxide, who were pregnant or breast-feeding or on treatment with lithium, were excluded. Inclusion in the study occurred less than 24h after the first positive PCR determination. Of the 88 patients (mean age 43±17 years, range 19-86 years, 55% male), 31 carried out PVI (1%) oral solution rinses and gargles every 8h (100mg/ml; 30s) for 2 consecutive days; 17 carried out rinses and gargles with H2O2 (1.5%) with the same regimen and 40 were part of the control group without rinses or gargles. After the intervention, PCR was performed after 3 (2–4), 11 (9–13) and 17 (14–19) days in all patients.
SARS-CoV-2 viral load was measured by quantitative PCR in all determinations (qRT-PCR; EDX SARS-CoV-2 Standard; Exact Diagnostics, Bio-Rad; E, N, ORF1a, RdRP and S genes). The viral load was expressed as Log10 of the mean of the viral loads of both genes (N and R) and Ct values. The processing of the samples and the interpretation of the results was a blinded study. Serological studies (IgG) were performed by chemiluminescence immunoassay (VirClia, Monotest, Vircell, S.L., Granada, Spain).
Informed consent was requested and the study was approved by the Cantabrian Medicines Research Ethics Committee.
The results were expressed as the mean and standard deviation (SD) for the quantitative variables and n (%) for the qualitative variables. The Kolmogorov–Smirnov test was used to determine the distribution of the variable. The Student’s T-test or Mann–Whitney U test was used for the comparison of quantitative variables and the Chi-squared or Fisher’s test was used for qualitative variables. The SPSS 23.0 software statistical package was used (Chicago, IL, USA). Statistical significance was considered to be p<0.05.
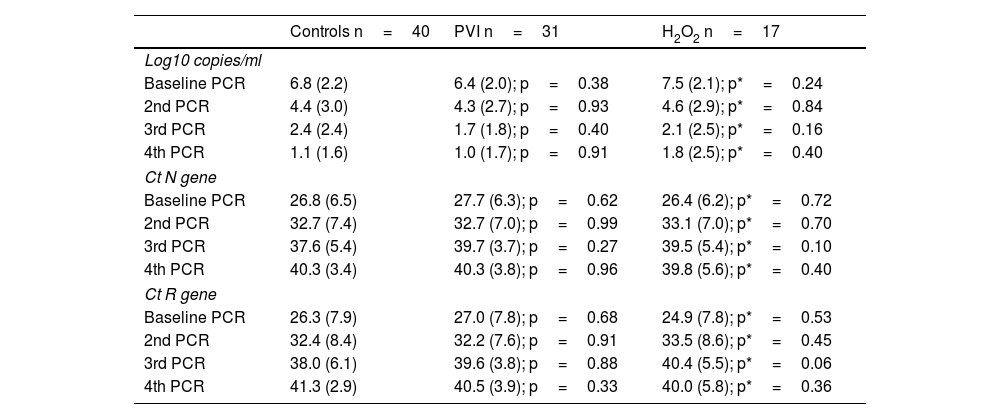
ResultsTwo thirds of the patients were seen on an outpatient basis (76%). The 3 groups began with the same viral load in the first positive PCR (PVI 6.4±2.0 copies/ml, H2O2 7.5±2.1 copies/ml and controls 6.8±2.2 copies/ml). There were no differences in viral load after rinses in the second determination (PVI 4.3±2.7 copies/ml, H2O2 4.6±2.9 copies/ml and controls 4.4±3.0 copies/ml). Neither were there differences in the third and fourth successive PCR determinations (Table 1). The Ct values (N and R gene) were similar in the 3 groups at all times (Table 1).
Viral load in the 3 groups.
| Controls n=40 | PVI n=31 | H2O2 n=17 | |
|---|---|---|---|
| Log10 copies/ml | |||
| Baseline PCR | 6.8 (2.2) | 6.4 (2.0); p=0.38 | 7.5 (2.1); p*=0.24 |
| 2nd PCR | 4.4 (3.0) | 4.3 (2.7); p=0.93 | 4.6 (2.9); p*=0.84 |
| 3rd PCR | 2.4 (2.4) | 1.7 (1.8); p=0.40 | 2.1 (2.5); p*=0.16 |
| 4th PCR | 1.1 (1.6) | 1.0 (1.7); p=0.91 | 1.8 (2.5); p*=0.40 |
| Ct N gene | |||
| Baseline PCR | 26.8 (6.5) | 27.7 (6.3); p=0.62 | 26.4 (6.2); p*=0.72 |
| 2nd PCR | 32.7 (7.4) | 32.7 (7.0); p=0.99 | 33.1 (7.0); p*=0.70 |
| 3rd PCR | 37.6 (5.4) | 39.7 (3.7); p=0.27 | 39.5 (5.4); p*=0.10 |
| 4th PCR | 40.3 (3.4) | 40.3 (3.8); p=0.96 | 39.8 (5.6); p*=0.40 |
| Ct R gene | |||
| Baseline PCR | 26.3 (7.9) | 27.0 (7.8); p=0.68 | 24.9 (7.8); p*=0.53 |
| 2nd PCR | 32.4 (8.4) | 32.2 (7.6); p=0.91 | 33.5 (8.6); p*=0.45 |
| 3rd PCR | 38.0 (6.1) | 39.6 (3.8); p=0.88 | 40.4 (5.5); p*=0.06 |
| 4th PCR | 41.3 (2.9) | 40.5 (3.9); p=0.33 | 40.0 (5.8); p*=0.36 |
Mean (SD) or n (%).
Ct: cycle threshold; RT-PCR: real-time polymerase chain reaction.
Mann–Whitney: p (PVI and controls); p* (H2O2 and controls).
The percentage of patients whose PCR was negative in the second determination was 27% in PVI, 23% in H2O2 and 26% in controls. These percentages were similar in the third PCR (62%, 54%, and 58%) and fourth PCR (81%, 75%, and 81%, respectively) (Fig. 1).
There were no differences in the immune response. 87% of the patients with PVI rinses developed immunoglobulin G, compared to 82% in H2O2 and 95% in the controls (mean time of 74±31, 78±33 and 81±48 days, respectively).
DiscussionPVI and H2O2 rinses and gargles did not change the oropharyngeal viral load of patients with COVID-19. These oral antiseptics did not accelerate the negativisation of the PCR for SARS-CoV-2. PVI is an iodophor that owes its antiviral action to free iodine, altering cell membranes and protein synthesis13. However, its recognised in vitro activity against SARS-CoV-28 does not seem to be evident in vivo, although we only found one clinical trial in this regard, conducted in 12 patients with COVID-19 who after receiving rinses and gargles with PVI (1%) did not have a modified oropharyngeal viral load of SARS-CoV-2 compared to the untreated group11. Furthermore, H2O2 has also shown efficacy in vitro in inactivating SARS-CoV-2, albeit less effectively than PVI10. Its antiviral potency seems to be due to oxidation14. To date, the use of this antiseptic in COVID-19 patients has been a matter of great debate15,16. Our work is the first observational study with a control group that evaluates the utility of H2O2 mouth rinses in COVID-19 patients and we do not see a clear reduction in viral load in the short or in the medium term.
We only found one other study, without a control group, in 12 hospitalised COVID-19 patients who received H2O2 (1%) mouth rinses, with no change in their oropharyngeal viral load at 30min13.
Our study has limitations, as it is not randomised. However, we included a large number of patients with a control group and evaluated the utility of two commonly used oral antiseptics.
We conclude that rinses and gargles of PVI and H2O2 do not seem to have a clear utility in reducing the oropharyngeal viral load of SARS-CoV-2. We believe that the recommendation for the use of these and other oral antiseptics should be based on scientific evidence, hence more studies are needed to assess their efficacy in this pandemic.
FundingThis work was financed with a Research Grant awarded by the Consejo Superior de Investigaciones Científicas [Spanish National Research Council] (CSIC. 202050E106). Spain.
Conflicts of interestThe authors declare that they have no conflicts of interest.
To the Spanish National Research Council and Javier Xercavins, from the Ministry of Science and Innovation. To professors Jesús Agüero and J.A. Riancho (University of Cantabria), Spain.