COVID-19 pandemic has produced an increased burden for motility laboratories due to the need to implement measures to minimize infection risk during examinations. International Societies have proposed algorithms for evaluation of active infection risk using symptom questionnaires or performing COVID-19 specific detection tests. The aim of the present study is to evaluate prospectively the independent value of a symptom-based questionnaire and RT-PCR test to detect COVID-19 infection before a digestive motility examination.
Patients/MethodsAll patients referred for a motility study during a 4 month period with high incidence of COVID-19 in the community were prospectively evaluated with a symptom-questionnaire administered by phone one week before the examination, and a PCR test performed 48h before the examination, following international guidelines recommendations.
ResultsThe symptom questionnaire could be obtained from 435 patients, 7 patients referred COVID-19 symptoms, but only 1 of them had a positive PCR. From 481 PCR tests performed, 8 were positive. Only 1 patient had reported symptoms in the previous questionnaire, and 2 additional patients developed COVID-19 symptoms later. Hence, 435 telephonic questionnaires should be done for one COVID-19 case detection (detection tax 0.22%); and 60 PCR should be performed for one COVID-19 case detection (detection tax 1.66%).
ConclusionsThe use of screening strategies prior to a motility exploration results in a low rate of infection detection, especially the use of subjective symptom questionnaires, and the correct protection measures during motility explorations with aerosol generation remain the cornerstone to prevent COVID-19 infections.
La implementación de medidas para minimizar el riesgo de infección por COVID-19 durante las exploraciones de motilidad digestiva ha producido una carga asistencial relevante. El objetivo del presente estudio es evaluar prospectivamente el valor independiente de un cuestionario basado en síntomas y una prueba de RT-PCR para detectar la infección por COVID-19 antes de una prueba de motilidad digestiva.
Pacientes y métodosLos pacientes derivados para estudio de motilidad durante un período de 4 meses con alta incidencia de COVID-19 fueron evaluados prospectivamente con un cuestionario telefónico de síntomas una semana antes de la exploración y un test de PCR realizado 48 h antes de la prueba.
ResultadosEl cuestionario de síntomas se pudo obtener de 435 pacientes, 7 pacientes refirieron síntomas de COVID-19 (solo uno de ellos tuvo PCR positiva). De 481 pruebas PCR realizadas, 8 dieron positivo. Solo un paciente había informado síntomas en el cuestionario anterior y 2 pacientes adicionales desarrollaron síntomas de COVID 19 más tarde. Se debieron realizar 435 cuestionarios para la detección de un caso de COVID-19 (tasa de detección del 0,22%) y 60 PCR para la detección de un caso de COVID-19 (tasa de detección del 1,66%).
ConclusionesEl uso de estrategias de screening previo a una exploración de motilidad resulta en una baja tasa de detección de infecciones, especialmente el uso de cuestionarios de síntomas subjetivos. Las correctas medidas de protección durante las exploraciones de motilidad con generación de aerosoles siguen siendo la piedra angular para prevenir infecciones por COVID-19.
Corona VIrus Disease 2019 (COVID-19) pandemic has forced important restructuring in health-care attendance. The virus responsible for COVID-19, SARS-Cov2, has its highest load in the nasopharynx. Virus enters into the cells via the angiotensin-converting enzyme 2 (ACE2) receptor, which is expressed in the blood vessels of the lungs, brain, skin, and digestive system. In the digestive tract, ACE2 is widely expressed on esophageal epithelial cells,1 on gastric glandular cells and on enterocytes in small bowel and colon.1 Main transmission is via aerosols and droplets generation although virus can also be spread indirectly via contaminated surfaces.2 The virus is present in feces, but fecal-oral transmission has not been considered as clinically relevant due to denaturation of virus protein capsid in contact with intestinal secretions.3
All diagnostic examinations which imply near contact with potentially infectious biological material, especially those examinations able to generate aerosols like intubation of gastrointestinal tract during a digestive motility examination, had been considered to have a high infection risk. To minimize the risk of infection during motility examinations of the gastrointestinal tract, many scientific societies (ESNM, ASENEM, ANMS, ANMA) have developed guidelines with recommendations on how to perform those examinations during the pandemic.4–7 All proposed algorithms include recommendations to perform a previous patient selection (evaluating the indication and establishing the priority, and clinical implication of the examination), evaluation of active infection risk (by means of reporting symptoms, identifying clinical signs of infection or performing COVID-19 specific detection tests) and measures that should be taken to perform motility testing with safety for health-care professionals and patients (using PPE (personal protection equipment), sterilizing used material). However, due to the lack of studies evaluating the real efficacy of the different detection and protection measures in different phases of the COVID-19 pandemic, each society has published its own guidelines with its own particularities based mainly on current knowledge of the virus dissemination pathways and common rules to prevent general transmission of infections in general.
The aim of the present study was to evaluate prospectively the independent value of reverse transcription polymerase chain reaction (RT-PCR) test and a symptom-based questionnaire before performing a digestive motility examination during different phases of incidence of the COVID-19 pandemic in an area with high impact of COVID-19 infection in north of Spain.
Patients and methodsParticipants and study designAll consecutive patients referred to the Digestive Motility Lab for performing an outpatient esophageal manometry, a 24-h pH-measurement, anorectal manometry or anorectal biofeedback from 15th November 2020 to 28th February 2021 were prospectively included.
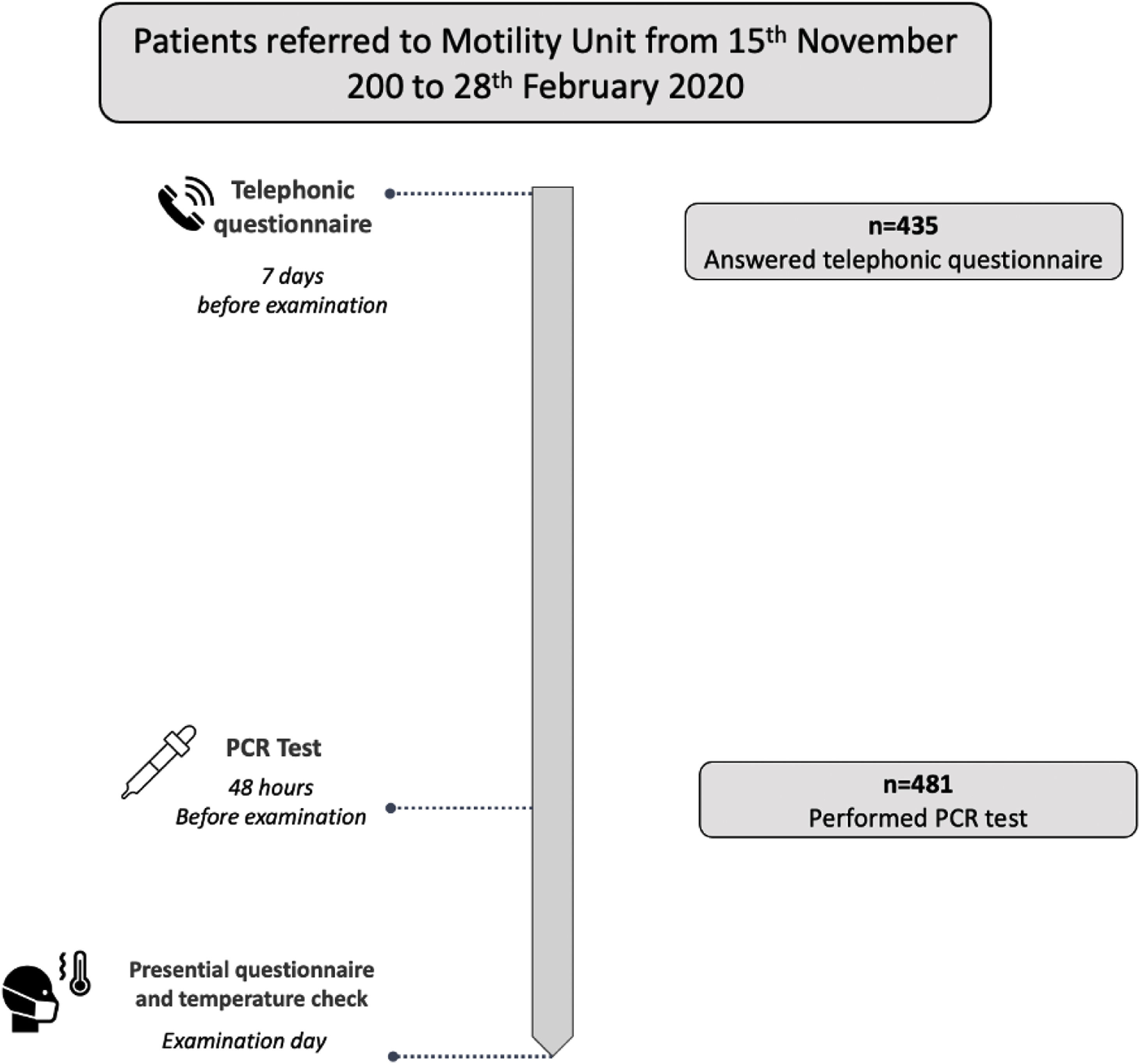
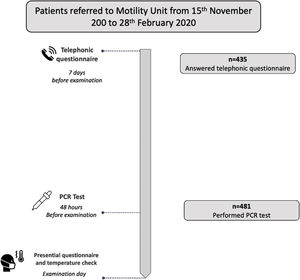
In each patient, a risk evaluation of COVID-19 infection was performed before the examination following international recommendations4,5 in three consecutive steps. (1) Seven days before the appointment date, clinical evaluation of possible infection was done using a symptom-questionnaire administered in a telephonic call performed by a trained nurse. The questionnaire evaluated the presence of symptoms compatible with acute COVID-19 infection and epidemiological background (contact with COVID-19 ten days before, or previous SARS-Cov 2 infection; Fig. 1). (2) Two days before the appointment date a RT-PCR test for COVID-19 detection was performed using a nasopharyngeal swab. To avoid unnecessary travel and lost of work, patients performed tests in their nearest qualified laboratory of the Public Catalan Health Care System. The specific nucleic acid amplification test technique used depended on each laboratory standardized protocol. According to the RT-PCR result, patients were classified as negative (no active infection) or positive (active infection). In the later case the appointment was canceled and the exploration postponed, regardless the absence of symptoms in the questionnaire. In these patients, a follow-up telephone call was done 2 weeks later to register the presence of late symptoms and infection evolution before a new appointment was scheduled. (3) The day of the examination, temperature was checked at arrival to the hospital, and a new short version symptoms questionnaire was done before entering to the examination room (Fig. 2). The presence of fever or any symptom in the questionnaire performed the appointment day entailed a postponement of the examination.
Short title: Patients flowchart. Description: All patients referred to the Motility Unit for a digestive motility examination were contacted by a trained nurse with a telephonic call on average 7 days before examination date. 435 patients answered our telephonic call. During this call a clinical evaluation was done, with symptoms and epidemiological background evaluation. 48h before de the examination day, a PCR test was done in 481 patients. The day of the examination, before entering to the examination room, a presential symptoms questionnaire was done and temperature was checked.
The number and percentage of patients who reported symptoms in the telephonic questionnaire and the number and percentage of patients with a positive result in PCR test was calculated as means±standard deviation or medians with interquartile ranges depending upon distribution of data. The incidence of COVID-19 infections in the community during the study period was recorded; data was obtained from official resources from the regional Health Department, Generalitat de Catalunya (https://dadescovid.cat). In the positive PCR group, the number of patients who reported symptoms in the telephonic questionnaire and the number of patients who developed symptoms after the telephonic call was calculated.
The accuracy of the telephonic questionnaire to diagnose SARS Cov-2 infection was evaluated in comparison to actual gold standard (PCR). Specifically, sensitivity, specificity, positive and negative likelihood ratio, positive predictive value and negative predictive value and their 95% CI were calculated. An analysis of concordance between telephonic questionnaires and PCR was carried out, obtaining the percentage of observed agreement and Cohen's kappa (k) index. To evaluate both tests efficiency, the number of PCR and telephonic questionnaires needed to detect one positive case was calculated. Values of p<0.05 were considered statistically significant. All statistical analyses were performed using Stata software (version 11.0; College Station, Texas, USA).
ResultsStudy populationFrom 15th November 2020 to 28th February 2021, 481 patients who underwent a PCR test before a scheduled motility examination were included in our study. Patients who canceled the appointment before the motility examination, or who did not attend to the laboratory the scheduled examination day were not included. There was a predominance of women (76% of the patients), with a mean age of 57±16 years. The scheduled examinations were isolated esophageal manometry (16%), combined esophageal manometry and pH-measurement (22%), and anorectal manometry or anorectal biofeedback training (62%).
Telephonic questionnairesThe previous telephone contact for acquisition of the symptom questionnaire could be performed in 435 (90.4%) of the patients who underwent a PCR test. The phone contact was performed 7±2 days before the motility examination. Only 7 patients (1.6%) referred some symptom compatible with COVID-19 acute infection: 3 patients referred dyspnea, 2 cough, 1 cough and fever and 1 patient anosmia and dyspnea. Only one of these patients with symptoms, who referred isolated cough, had a positive PCR test, whereas the remaining six patients had a negative PCR test.
During the evaluation of the epidemiological background with the telephonic call, no patient reported a COVID-19 contact 10 days before. Seventeen patients had a history of previous positive SARS Cov-2 PCR; and no patient had got vaccine immunization yet.
COVID-19 PCR testEight patients from the 481 who performed PCR had a positive test (1.6%). All 8 patients with positive PCR had done the previous symptom-questionnaire. Five of them had asymptomatic infections (the patients did not report symptoms either in the telephonic questionnaire prior to the test, or during the call performed after the positive result was known). Among the 3 patients with positive result and symptoms, only one patient reported symptoms (isolated cough) in the telephonic questionnaire performed 7 days before the scheduled study date; the other 2 patients reported symptoms during the telephonic call performed after knowing the positive PCR result.
Clinical detection of symptoms at arrival to the Motility UnitAmong the 473 patients with negative PCR for SARS Cov-2, no patient referred symptoms upon arrival to Motility Unit, and fever was detected in no patient. Likewise, no staff member developed COVID-19 related symptoms during the study period.
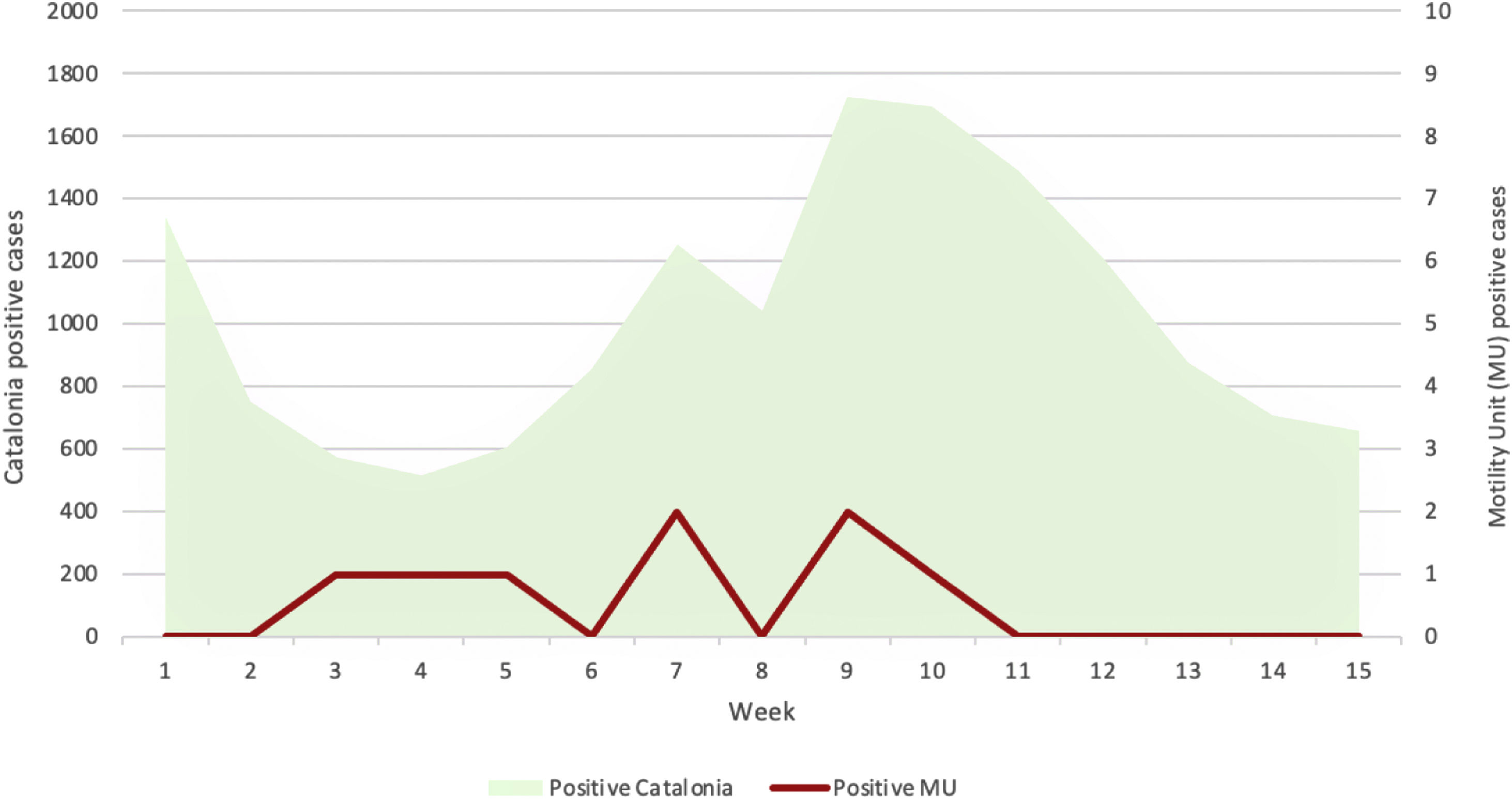
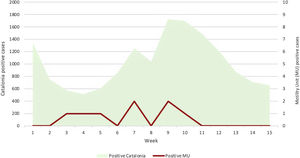
Effect of variations in the local epidemiological situation on screening resultsThe 15 weeks study period started when the 3rd wave of cases in Catalonia (our referral area) was declining and included the period between 3rd and 4th waves and almost the whole 4th wave of COVID-19 cases. Hence, the incidence of positive cases in Catalonia varied between 517 confirmed cases (with PCR or antigen test) for 100,000 habitants during the plateau between incidence waves, and 1724 cases/100,000 habitants in the peak of the 4th wave (Fig. 3). Despite these great variations in incidence, the number of positive cases detected by PCR was between 0 and 2 cases/week. Identifying correlation between the pandemic evolution in our referral area and the number of positive tests in our Unit is difficult due to the low prevalence of positive cases detected by us. However, the number of positive cases detected in patients who need a digestive motility examination seems to curse independently of the pandemic evolution in our area (Fig. 3).
Short title: Compared COVID19 incidence evolution. Description: Incidence of positive cases in Catalonia (our referral area) and incidence of positive cases detected by PCR prior to a digestive motility exploration during the 15 weeks study period (from 46th week of 2020 to 8th week of 2021). The community incidence varied between 517 and 1724 confirmed cases (with PCR or antigen test) for 100,000 habitants during the successive waves of the pandemic, but the detection of positive PCR confirmed infections prior to the examination remained low: 0–2 cases/week. No parallel evolution seems to occur between both curves.
The sensitivity and specificity of the telephonic questionnaire performed one week before the examination for detecting acute SARS-Cov2 infection was 12.5% (CI 95%: 2.2–47.1) and 98.6% (CI 95%: 97.0–99.4), respectively. The positive predictive value of the questionnaire was 14.3% (CI 95%: 2.6–51.3) and the negative predictive value was 98.4% (CI 95%: 95.0–98.3). The positive likelihood ratio is 8.93 and the negative likelihood ratio is 0.89. Kappa index between telephonic questionnaires and PCR test was 0.118 (no concordance was shown).
Regarding efficacy of the interventions, 435 telephonic questionnaires should be done for one COVID-19 case detection (detection tax 0.22%); and 60 PCR should be performed for one COVID-19 case detection (detection tax 1.66%).
In our sample, no suspected infection was detected by the questionnaire and temperature measurement performed at arrival to the Motility Unit. In case of COVID-19 infection, the uncertain percentage of asymptomatic cases in the population and the variability in incidence makes it difficult to calculate the pre-test probability, and secondarily the post-test probability.
DiscussionThe present prospective study comparing the efficacy of the different strategies recommended by international guidelines to minimize COVID-19 infection risk during the examination of gastrointestinal motility, have shown that the sensitivity of a previous questionnaire obtained by a phone call to predict active infection during the procedure is very low, and could probably be eliminated from the recommendations for performance of motility explorations during COVID-19 pandemic. Likewise, despite high prevalence of infections in the community during the whole study period, and the lack of vaccination at the time of the study, the number of positive PCR performed prior to the motility explorations was also low, and remained stable despite high fluctuations in the incidence of the infection in the community. Since the beginning of the pandemic, re-starting healthcare assistance in potentially infective diagnostic examinations has become a challenge for health-care professionals. During the first wave of the pandemic with a complete lockdown in most countries, multiple scientific societies elaborated protocols and recommendations to re-start the activity in procedures with a high infection risk. However, these recommendations were based on current knowledge of the virus dissemination pathways and common rules to prevent general transmission of infections in general.4,8,9 Hence, there is a need to evaluate the real efficacy of these measures in clinical practice.
Our study compares risk evaluation obtained by a questionnaire administered to all patients via a phone call before the examination with specific COVID-19 test performed to all patients referred for motility examinations, independently of their calculated risk of COVID-19 infections (both strategies are recommended by different guidelines4,6). The specific questionnaire used for clinical risk evaluation was the one recommended by the European Guidelines,4 and included both typical COVID-19 symptoms and epidemiological data of the patient (Fig. 1). The specific test for COVID-19 diagnosis chosen was RT-PCR from nasopharynx swab. The PCR obtained from nasopharyngeal swab is the gold standard test in the diagnosis of acute SARS-Cov2 infection, according to its sensitivity and specificity (the highest compared to other test like antigens test or antibodies test).10 PCR from nasopharyngeal swab sensitivity ranges from 85 to 90% in COVID-19 symptomatic patients. However, in asymptomatic patients, when the viral load is low, the rate of false negative cases has been reported to increase from 5 to 40% depending on the series.11 These false negative cases are the main reason for recommending clinical evaluation before examinations, combined with a specific COVID-19 test.
In our study, we found that risk evaluation using questionnaires prior to the motility examinations has a low positive-case-detecting capacity, as well as a low positive predicting value of infection when symptoms were present. This results are in the same way that the results obtained in other studies performed in endoscopy units.12 The reasons for the low performance of the questionnaire may be multiple: Responses to questionnaires depend on patient's own perception, the presence of less well-recognized symptoms (like diarrhea) and their willingness to be true. Interestingly, while during the study period there was a high prevalence of COVID-19 in the community, no patient referred a COVID-19 contact or suspicious contact during the survey, and only 7 patients of 435 surveyed (1.7%) referred any symptom. Hence, we must consider that different factors like the fear for having a severe disease, the need to be isolated in quarantine, the delay of a very expected examination for the patient, among others could influence the reliability of the self administered questionnaires.
By contrast, objective determination of infection by PCR had a much higher detection rate. Hence, 435 questionnaires are needed to detect one infection, whereas 60 PCR test are needed to detect one infected patient during periods with relatively high incidence of infection in the population, as those present during our study.
We have to consider that both prevention strategies result in increased costs for motility examinations. Hence, having a phone call with all patients before the examination carries a work overload for the personnel who make the telephonic contacts and perform the questionnaire. Likewise, scheduling a PCR test for all patients before the examination supposes increased costs, both direct costs related to the test itself, as well as indirect costs related to travel related expenses when an extra visit to health care facilities is needed. Hence, it is important to consider the impact of these strategies to minimize infection risk on the final performance of motility examinations. In our study, the results of the questionnaire obtained by the previous phone call did not change the final decision about performing or not a diagnostic examination or how health-care staff protect themselves during the examination. In fact, when a risk for infection is detected by the previous phone call the next step should be to confirm infection with a PCR test. However, a strategy based on performing PCR test only to patients with positive symptom questionnaires should result in a false feeling of safety because most of the SARS Cov-2 infection, even though infrequent, will not be detected by this strategy. On the other side, even though detection of SARS Cov-2 infections by PCR detect a low but relevant number of infections prior to the examinations, the rate of false negative results that has been reported in different series11,13 may also produce a false feeling of safety that need to be considered. Hence, probably the impact of performing a PCR test on the procedure will be finally minimal, because the protection measures (personal protection equipment, social distance, ventilation) should be maintained until the pandemic goes in real remission. In fact, it has been reported from endoscopic studies, with an aerosol generation and risk for infection similar or greater than motility examinations, that the rate of infections among personnel involved in these examinations is very low when the protection measures during the procedure are used properly,14 and for that reason, some of the last published recommendations propose to perform endoscopic examinations without a previous PCR test in case of patients with low COVID infection risk.9 Hence, the final decision on performance of a previous PCR seems not straight forward, and will depend on the local circumstances of each center, and the central factor for protection against infection remains the use of all the protection measures during the examination at the Motility Lab.
One of our study limitations is that the phone contact was made one week before the diagnostic examination (4 days before PCR test) while guidelines recommend performing the call the closest possible to the examination day, in order to avoid false negative cases (patients with no referred symptoms because they are in the infection asymptomatic phase). However, in our study, no patient with positive PCR developed symptoms in the interval between the phone call and the exploration date, and only 2 patients with positive PCR referred acute infection symptoms after the phone call was made. However, we cannot exclude that some patients could have been infected in the period between the questionnaire and the PCR, or even in the period between the PCR and the exploration. Likewise, we have included in our study only those patients who performed the PCR-test. So we don’t know if some patients canceled their PCR-test and their exploration because they developed symptoms that could not be registered. Hence, it seems plausible that the number of potential patients suffering infection was underestimated using the actual detection protocol. However, for the aim of the protocol, that is to minimize infection risk during motility explorations, this underestimation seems not to be clinically relevant.
Our data has to be considered within the fluctuating pandemic conditions in our referral area during the study period. Catalonia suffered the third and fourth COVID-19 waves, and the interval in between waves, during the study, so we were able to collect data from high and lower incidence periods. The rate of positive PCR test in the community varies with the different stages of the pandemic, being a rate lower than 5% considered as the cut-off for control of the pandemic in the community.15 By contrast with the high rates of positive PCR test reported in the community during the study period, we had a low rate of positive test in our study (1.6%). These differences may be related to the differences in the patients being tested: In our study we tested asymptomatic patients, whereas the community data relates to patients with suspected COVID-19 infection. However, this is interesting to note that we found no differences in the positivity rate during the different stages of the pandemic, probably due to the low rate of positive tests in our study.
Another important limitation of our study, shared with all clinical practice guidelines published up to now, is that our data was obtained before massive vaccination of the population was started. During our study period, no patient had been vaccinated, and all personnel in the Motility Lab received at least one vaccine dose, so the impact of vaccination on our screening data cannot be considered, and needs to be explored in future studies. Likewise, during the pandemic new variants of the virus are emerging, and the protective effects of the vaccines have been reported to wane progressively within a few months, especially in older people. New variants of the virus have also led to different incubation and clearance periods, and variations in predominant symptoms that could modify the predictive ability of the questionnaire used. Our study was performed when the beta variant was the prevalent one in our community, so the impact of the new variants cannot be considered. Taking together all the new variations in the evolution of the pandemic, it seems that strategies to perform motility explorations with maximal safety during COVID-19 pandemic should remain necessary for a longer period of time than desired, making our data relevant even in the next future.
In conclusion, we have shown that during periods with high prevalence of COVID-19 infection in not vaccinated populations, the efficacy to detect SARS Cov-2 infection using a subjective symptom questionnaire obtained via a phone call one week before a motility exploration is very low, leading to no changes in the way the motility studies are performed. Objective evaluation of infection using a PCR test to all patients referred for motility explorations have a higher efficacy, but with a rate of infection detection under 2% despite high prevalence of infection in the community. Hence, the use of screening strategies prior to a motility exploration results in a low rate of infection detection, especially the use of subjective symptom questionnaires, and the correct protection measures during motility explorations with aerosol generation remain the cornerstone to prevent COVID-19 infections and should remain as the main recommendation to perform with safety motility explorations.
Authors’ contributionAA: acquisition of data; analysis and interpretation of data; drafting of the manuscript, statistical analysis. JS: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content.
Sources of fundingThis research has not received specific aid from public sector agencies, the commercial sector or non-profit entities.
Conflict of interestAA none; JS none.
The authors thank Arantza Sanvicens for help with statistical calculations, and Maria José Pérez, Adoración Nieto and Purificación Rodriguez for technical support.