Effective vaccines against the SARS-CoV-2 are already available and offer a promising action to control the COVID-19 pandemic. IBD patients on biological agents accept the vaccine as well as an additional dose if recommended.
BackgroundVaccination against COVID-19 prevents its severe forms and associated mortality and offers a promising action to control this pandemic. In September 2021, an additional dose of vaccine was approved in patients with immunosuppression including IBD patients on biologic agents. We evaluated the vaccination rate and additional dose willingness in this group of at risk patients.
MethodsA single-center, cross-sectional study was performed among IBD patients on biologic agents and eligible for an additional dose of the COVID-19 vaccine. IBD clinical characteristics and type of vaccine and date of administration were checked in medical records. Acceptance was evaluated after telephone or face-to-face surveys in IBD patients.
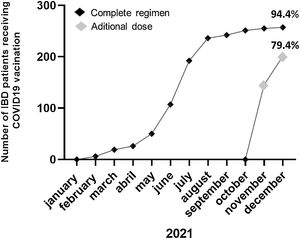
ResultsOut of a total of 344 patients, 269 patients (46.1% male; mean age 47±16 years; Crohn's disease 73.6%) were included. Only 15 (5.6%) patients refused the COVID-19 vaccine mainly (40%) for conviction (COVID-19 pandemic denial). 33.3% would re-consider after discussing with their doctor and/or receiving information on the adverse effects of the vaccine. Previous to the additional dose, the COVID-19 vaccination was present in 94.4% of patients (n=254). Adverse effects occurred in 53.9% of the cases, mainly pain in the arm (40%). Up to 94.1% of the patients agreed to an additional dose and 79.4% had already received the additional dose at the final time of the assessment.
ConclusionsIBD patients on biological agents accept the vaccine as well as an additional dose if recommended. Physicians in charge of IBD units should provide information and confidence in the use of the vaccine in these IBD patients.
La vacunación frente al COVID-19 constituye una acción prometedora para controlar esta pandemia. En septiembre de 2021, se aprobó una dosis adicional de vacuna en pacientes con inmunosupresión, incluidos los pacientes con enfermedad inflamatoria intestinal (EII) que reciben agentes biológicos. En este estudio se evaluó la tasa de vacunación y la disposición de recibir la dosis adicional de vacuna en este grupo de pacientes de riesgo.
MétodosSe realizó un estudio transversal unicéntrico con pacientes afectos de EII con tratamiento biológico y elegibles para una dosis adicional de la vacuna COVID-19. Se evaluó la aceptación y los efectos adversos de la vacuna mediante encuesta telefónica o presencial y se recopiló en las historias clínicas las características de la EII, el tipo de vacuna recibida y la fecha de administración.
ResultadosDe un total de 344 pacientes, 269 (46,1% varones; edad media 47±16 años; enfermedad de Crohn n=198) fueron incluidos. Solo 15 (5,6%) pacientes rechazaron la vacuna frente al COVID-19, el 40% por convicción (negación de la pandemia COVID-19). Antes de la dosis adicional, la vacuna COVID-19 se había administrado en el 94,4% de los pacientes (n=254). En el 53,9% de los casos presentaron efectos adversos, principalmente dolor en el brazo (40%). Hasta el 94,1% de los pacientes refería la aceptación de una dosis adicional de la vacuna y el 79,1% ya había recibido esta dosis adicional en el momento de la evaluación final.
ConclusionesLos pacientes con EII que reciben agentes biológicos aceptan la vacuna frente al COVID-19, así como una dosis adicional si se les recomienda. Los médicos responsables de las unidades de EII deben proporcionar información y confianza en el uso de la vacuna en estos pacientes.
The design of effective vaccines against the SARS-CoV-2, induces an immune response developing high-affinity antibodies or effector T cells, prevents its severe forms and associated mortality, and offers a promising action to control the COVID-19 pandemic. Several vaccines against COVID-19 are already available and recommended in global immunization programs for the entire population over 5 years of age.1 Vaccination is especially important in vulnerable cohorts, particularly those considered immunocompromised which include a subset of patients with IBD using biologic agents, small molecules, immunomodulators, and/or corticosteroids. Previous studies have shown that immunosuppressive drugs in IBD patients may reduce the effectiveness of some vaccines and therefore the vaccination protocol e.g. hepatitis B or influenza may be accelerated or the dose may be increased in these patients to adjust to this situation.2 Recently, a prospective observational study included in the ENEIDA registry (53,682 patients from 73 centers) reviewed infection rate and severity of infection due to COVID-19 in IBD patients and the results were compared with data of the general population in Spain. This study demonstrated that IBD did not worsen COVID-19 prognosis, even when immunosuppressants and biological drugs were used and age and comorbidity were the prognostic factors for more severe COVID-19 infection in IBD patients.3 At present, international experts recommend the vaccination of IBD patients at the earliest opportunity possible, without delay due to immunosuppressive drugs, and according to national guidelines.4
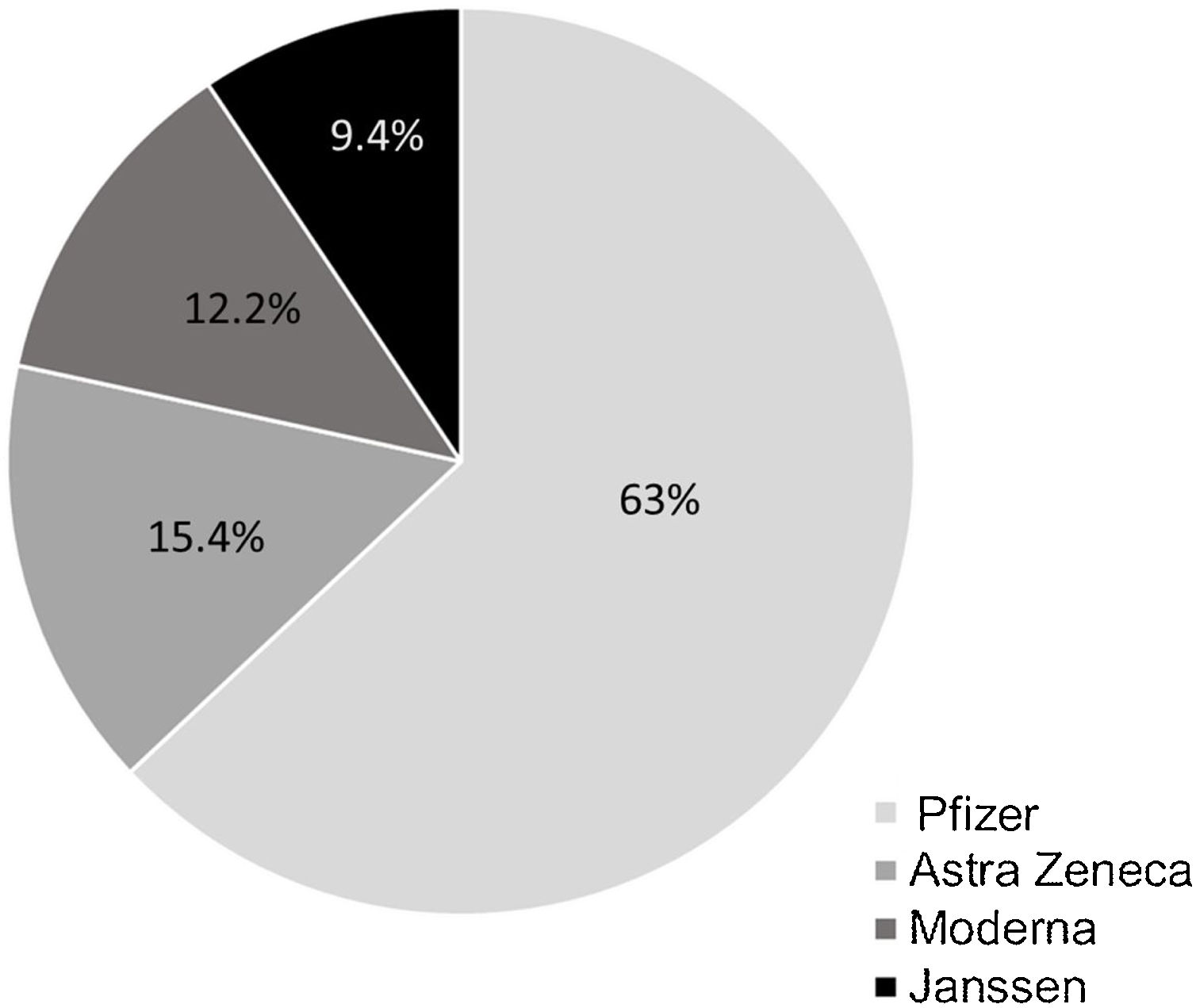
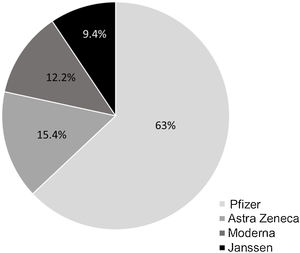
In Spain, the COVID-19 vaccination campaign started in December 2020 and in February 2021 was extended to the general population after targeting priority groups. The goal of the global vaccination strategy against COVID-19 remains to achieve immunization of up to 90 percent of the population. The vaccines used (4 vaccines approved by European Medicines Agency (EMA): BNT162b2 (Pfizer/BioNTech, US), mRNA-1273 (Moderna, Cambridge, US), AZD1222 (AstraZeneca, UK), and Ad26.COV2-S (Janssen Pharmaceutical Companies of Johnson & Johnson, US) and the time at which the population can be vaccinated depends on their age and associated diseases. In September 2021, an additional dose of vaccine was approved in patients with immunosuppression including IBD patients on biologic agents.1 This study aimed to evaluate the vaccination rate and additional dose willingness in this group of-at risk patients.
MethodsA single-center, cross-sectional study was performed among IBD patients on biologic agents and eligible for an additional dose of COVID-19 vaccine between 19th October and 21th December 2021. Patients were identified by the list provided by the Pharmacy service and only patients with a confirmed diagnosis of IBD and regular follow-up in our IBD unit were included.
Demographic characteristics and data related to IBD diagnosis were collected from clinical records.
Adherence to medical visits (the patient attends the established appointments with the requested complementary tests performed), changes in treatment, emergency room visits, hospital admissions, and the need for surgery that occurred in the 12 months before their inclusion (during the pandemic) were reviewed.
Acceptance and safety of vaccination and willingness of the additional dose were evaluated by a face-to-face or telephone survey after consent to participate in the study. IBD patients accepted to be included and completed the survey during the medical consultation or were contacted by phone and they were asked to participate in the study (giving verbal consent) and completed a telephonic survey in a period between 19th October and 12th November 2021. We developed a short questionary (9-items) including 7 closed questions (yes/no or pre-defined response) to reduce non-response rates. If the patients were hesitant or refused to be vaccinated they were asked about their reasons and whether there was any action to change their decision. The questionnaire is included in Appendix A. The side effects of the vaccine after the initial regimen (2 doses for Pfizer, Moderna, and Astra Zeneca and 1 dose of Jannsen) were described. The survey was conducted by a non-IBD staff member to avoid interference in the physician-patient relationship with the assessment of the survey.
The type of COVID-19 vaccine and date of administration was confirmed in medical records. If the patient had received the additional dose of vaccine it was also checked; the revision was carried out two separate times during the study (at the end of November and December 2021). The infection for SARS-CoV-2 was also revised and when it occurs (before or after vaccination) and any complication related to the COVID-19 infection (hospital admission, need for ICU, or death).
For statistical analysis, SPSS 26 (IBM Corp., Armonk, NY) and Prism 6 (GraphPad, La Jolla, CA) were used. Significance testing was performed using the Pearson χ2 test for comparing frequencies and the Student test – test for comparing the mean values of the 2 groups. The results were considered to be significant at a p level below 0.05.
The study was approved by the Clinical Research Ethics Committee of the Hospital Universitario de Canarias (code: VAC-COVID-GI: October 2021).
ResultsOut of a total of 344 patients, 269 patients received at least one vaccine dose and were included. We were not able to contact 72 patients and 3 refused to participate in the study. The description of demographic and clinical characteristics is shown in Table 1; 198 (73.6%) were Crohn's disease patients, gender distribution was similar (51.8% female) with a mean age of 47±16 years.
Data and clinical evolution of patients with IBD and use of biologic agents (n=269).
| Alln=269 | Vaccinatedn=254 | No-vaccinatedn=15 | p | |
|---|---|---|---|---|
| Age (years); mean (SD) | 47 (16) | 48 (16) | 41 (16) | 0.35 |
| Sex (female); n (%) | 145 (53.9) | 138 (54.8) | 8 (53.3) | 0.91 |
| Type IBD; n (%) | 0.47 | |||
| Crohn's disease | 198 (73.6) | 185 (72.8) | 13 (86.7) | |
| Extension Crohn's disease L1; L2; L3; L4 | 93 (47); 30 (15); 59 (30); 16 (8) | |||
| Ulcerative colitis | 66 (24.5) | 64 (25.2) | 2 (13.3) | |
| Extension ulcerative colitiss B1; B2; B3 | 40 (60.6); 20 (30.3); 6 (9.1) | |||
| Indeterminate colitis | 5 (1.9) | 5 (2) | 0 (0) | |
| Extension indeterminate colitis extensive | 5 (100) | |||
| Type of biological treatment; n (%) | 0.34 | |||
| Infliximab | 110 (40.9) | 103 (40.6) | 7 (46.7) | |
| Adalimumab | 54 (20) | 50 (19.7) | 5 (33.3) | |
| Ustekinumab | 80 (29.7) | 77 (30.3) | 3 (20) | |
| I | 24 (8.9) | 24 (9.4) | 0 (0) | |
| Biological+IMM treatment; n (%) | 75 (27.9) | 71 (28) | 4 (26.7) | 0.91 |
| Adherence to follow-up visits during a pandemic; n (%) | 260 (97) | 245 (96.5) | 15 (100) | 0.45 |
| Change of treatment during a pandemic; n (%) | 113 (42.2) | 104 (40.9) | 9 (60) | 0.14 |
| Hospital admissions during a pandemic; n (%) | 25 (9.3) | 25 (9.4) | 1 (6.7) | 0.71 |
| Surgery during a pandemic; n (%) | 8 (3) | 7 (2.8) | 1 (6.7) | 0.38 |
| COVID-19 infection during pandemic; n (%) | 7 (2.6) | 6 (2.4) | 1 (6.7) | 0.30 |
IMM: immunomodulators.
Among the 12 months before the vaccination assessment, up to 42.2% (n=113) of patients required a change in their usual medical treatment, 9.3% (n=25) patients were admitted to the hospital for IBD flares or complications, and 3% (n=8) required surgery for IBD during the pandemic.
Only 15 (5.6%) patients had not been vaccinated at initial evaluation and when considering the reason: 26.7% for fear of the vaccine's adverse effects, 33.3% for doubts about a “new-design” vaccine, and 40% for conviction (COVID-19 pandemic denial). 46.7% of the cases would not change their opinion, but 33.3% would re-consider after discussing with their doctor and/or receiving information on the adverse effects of the vaccine. 13.3% did not want to evaluate these options (no answer) and 6.7% (n=1) would consider changing the decision after the call was received.
A total of 7 (2.6%) COVID infections had been reported in the included patients at the final assessment (December 2021). Only one of the cases occurred after administration of 2 doses of Pfizer but without the additional dose while the other 6 infections were succeeded before receiving any dose of vaccine. In none of the cases was any complication requiring hospital admission (Appendix B).
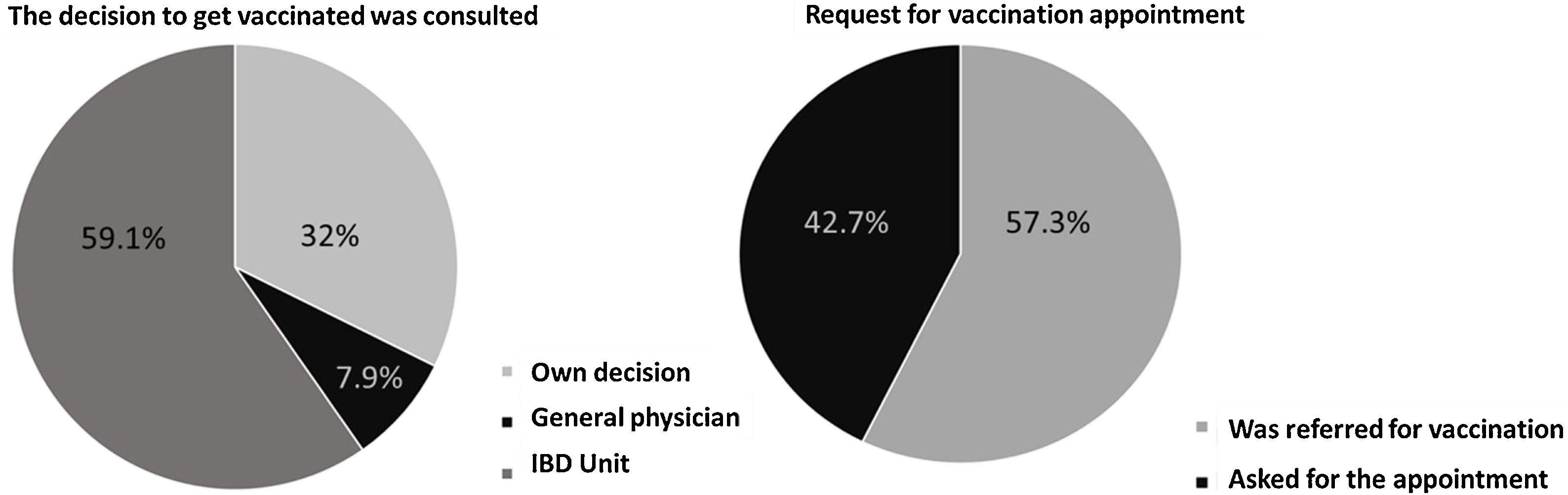
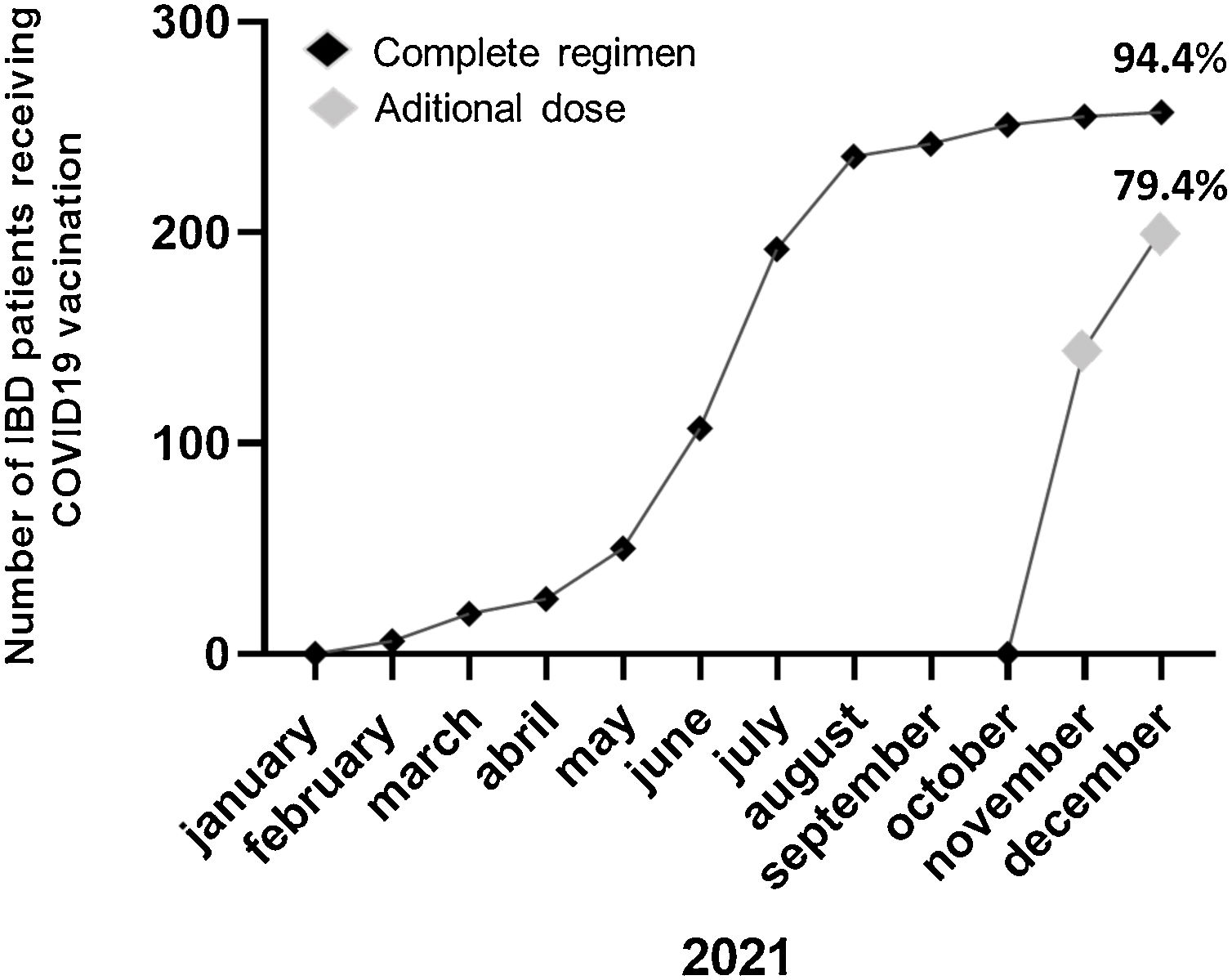
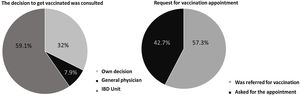
Prior to the additional dose, COVID-19 vaccination was present in 94.4% of patients (n=254) (complete initial regimen using 2 doses of Pfizer, 2 doses of Astra-Zeneca, 2 doses of Moderna or 1 dose of Janssen, as shown in Fig. 1). 42.7% of patients personally requested the appointment for vaccination and 59.1% contacted the IBD Unit to discuss the decision to be vaccinated (Fig. 2). 46% of the patients reported fear of vaccination due to the type of biological treatment they were receiving. Adverse effects occurred in 53.9% of the cases, mainly pain in the arm (at point of vaccine administration) (40%); influenza-like condition (29.3%), headache (14.6%), and fever (13.8%). Of the patients with the previous infection who received the vaccine (n=6), 2 presented sequelae, one presenting with pain in the arm and the other with headache, with no other complications. Only 5% of patients related a worsening of the disease to vaccination.
Up to 94.1% (239/254) of the previously vaccinated patients agreed to an additional dose and 57.2% had received this additional dose at the first time of the assessment reaching 79.4% of patients at the end of December 2021 (Fig. 3). The main reasons for refusing the additional dose of vaccine (5.6%; 15/254) were the side effects produced by the previous doses (46.6%), not having enough information about receiving new doses of COVID vaccination (40%) and that they did not consider this additional dose of vaccine as necessary (13.3%). During the final review of the vaccination, it was found that 2 (2/15; 13,3%) of the patients who had not been vaccinated due to doubts about the vaccine's side effects (n=1) or the use of a new-design vaccine (n=1) had finally received 1 dose by the end of December 2021.
DiscussionIn our study, the COVID-19 vaccination rate in group of patients with IBD using biological agents is very high (94.4%) and they accept to receive an additional dose of vaccine if it is recommended (94.1% of those previously vaccinated). In addition, the vaccine is safe with non-serious side effects in these IBD patients.
The evolution and the time elapsed in a pandemic is increasing the knowledge about immunization and adapting the recommendations on vaccination, including particular groups such as the immunosuppressed. A previous study has shown that seroconversion rates are lower after vaccination with a single dose of BNT162b2 or ChAdOx1 nCoV-19 vaccines in IBD patients treated with infliximab than with vedolizumab. However, a second exposure to the antigen, either after infection or by a second dose of vaccine led to effective seroconversion in most patients.5 We do not currently have available data on the effect of immunization of the additional dose of vaccine in patients with IBD and the use of biologics. Even though there were certain concerns that IBD patients may be at risk of sub-optimal vaccine response, a prior study demonstrated that the incidence of COVID-19 in IBD patients after vaccination is very low, including patients on immunosuppressive agents, and is similar to non-IBD population.6
In the present study we have evaluated the vaccination rate and the acceptance of the additional dose in the schedule established at the time of the evaluation (December 2021); with an initial complete regimen (1 or 2 doses depending on the type of vaccine) and an additional dose indicated by immunosuppression at least 28 days after the last dose (if the patient has not been infected by COVID-19).
Data on the intention to vaccinate against COVID-19 in patients with IBD are available. Dalal et al.,7 found that 80.9% of patients in their local center (Massachusetts, United States) had the intention to be vaccinated. In Italy, up to 80.4% of patients, belonging to the Italian association of IBD patients who responded to an anonymous survey were ready to be vaccinated for COVID-19.8 Remarkably, a collaborative study between the Spanish IBD Working Group (GETECCU) and the national patient association (ACCU Spain) showed that only 43% were willing to be vaccinated against COVID-19 but without an increasing concern about vaccination since the pandemic or fear due to IBD treatments.9 However, a multicenter survey performed in Germany showed that 58.5% of IBD patients planned to get COVID-19 vaccination or had already been vaccinated and those without IBD treatment were less willing to get vaccinated.10 These previous data were published in mid-2021 and it has been shown that increasing the time of the pandemic, with greater experience of use of the vaccines reduces their rejection. In our study, 46% of patients referred to the fear of the COVID-19 vaccine due to biological agents, but not affected the final decision about vaccination. The information offered by the IBD unit about the vaccine helped and was requested by almost 60% of these patients.
Spain is a country with a high acceptance of the use of vaccines despite its voluntary demand. The coverage of primo-vaccination between 2010 and 2020 has been about 95% of the population. When we consider a vaccine of annual use, such as the influenza vaccine, the rate of vaccination reaches 67.7% in 2021.11
In IBD patients, we dispose of consensus guidelines for providing vaccines and obtaining immunization to ensure health care maintenance for patients with IBD.12 A recent study using data from the US showed improving and higher immunization rates in IBD patients than the general population but still suboptimal with yearly influenza and pneumococcal vaccine uptake (48% and 75%, respectively).13 In Spain, adherence to the vaccination program was acceptable, raising a rate f 84.3% among IBD patients. However, the vaccination compliance was shown to be dependent on the type of treatment (decreased if not use of immunosuppressants) and adherence to IBD maintenance therapy. Concluded that optimization of patient information on the disease and emphasis on the need for adequate vaccination was necessary to improve adherence.14
Related to the vaccine against COVID-19, by January 2022, 90.8% of the population older than 12 years of age in Spain had received the complete vaccination regimen.1 As we found in our study, not only the vaccination rate but also the acceptance rate of the additional dose was very high in these patients, rising to 74.6% in just 2 months of the vaccination campaign.
The main limitation of the study is the reduced number of patients as a single-center study and represented a country with high vaccine acceptance and an acceptable rate of vaccination program adherence in IBD patients. In addition, we do not have data on patients who declined or could be enrolled in the study and who might have a differential adherence profile to COVID-19 vaccination, although it was not an objective of the study, the rate of COVID-19 infection in our sample is very low because it occurred before the arrival and dissemination of the omicron variant in our area. However, the strengths of the study are that the survey was conducted by physicians outside the IBD unit to avoid interfering with its development and that it reflects the real vaccination rate by reviewing the vaccination schedule of each patient.
Future studies will allow us to test the efficacy of the vaccination strategy for our IBD patients, including immunosuppressed patients, to ensure its indication and to provide the necessary information and confidence to patients.
In summary, IBD patients on biological agents accept the vaccine as well as an additional dose if recommended. Physicians in charge of IBD units should provide information and confidence in the use of the adequate scheme of COVID-19 vaccine in these IBD patients to reduce the fear and increased (or maintained) the desire to get vaccinated.
FundingNone declared.
IRB approval statusCómite Etico Hospital Universitario de Canarias (code: VAC-COVID-GI: October 2021).
Conflicts of interestLR has received educational and travel grants, and speaker fees: MSD, Pfizer, Abbvie, Takeda, Janssen, Shire Pharmaceuticals, Ferring, and Dr. Falk Pharma.
MHG has received research grants from Abbvie and Gilead and has participated in consultant advisories for Bayer and Intercept.
The rest of the authors do have not conflicts of interest to disclosure.