Familial adenomatous polyposis (FAP) is a hereditary colorectal cancer (CRC) syndrome caused by a germline pathogenic variant in the Adenomatous Polyposis Coli (APC) gene. However, a pathogenic mutation in this gene is not identified in 20% of patients. It is characterized by the early onset of multiple adenomas throughout the colon and rectum and is represented by two major phenotypes: the classic form, which is defined by the presence of over 100 colorectal adenomas, and if untreated, nearly all patients will develop CRC by the age of 40–50; and the attenuated variant, characterized by a reduced number of polyps (10–99), and presents a later and lower CRC risk. In terms of management, the current recommended treatment for the classic form, to prevent CRC, is surgical resection of the colon. In contrast, the attenuated form can be managed with endoscopic polypectomy. However, if this is not possible, a colectomy might be required, which significantly impacts quality of life and does not entirely eliminate the risk of neoplasia in the rectum or ileal pouch. Therefore, research into chemoprevention has become crucial. Currently, no primary chemopreventive interventions can replace surgery and endoscopic surveillance, though agents like sulindac, aspirin, erlotinib and celecoxib can be considered in selected cases, especially for secondary prevention.1 Other agents, such as imatinib, have also been suggested to play a potential role in chemoprevention.2
Here we describe the case of a 64-year-old man without family history of CCR nor polyposis, who was diagnosed with chronic lymphocytic leukemia (CLL), stage B according to Binet classification, in 2004. No treatment was started at that moment. In 2014, the CLL progressed to stage C, so fludarabine, cyclophosphamide, and rituximab were started, achieving complete response. Nevertheless, due to disease progression in 2022, venetoclax was started.
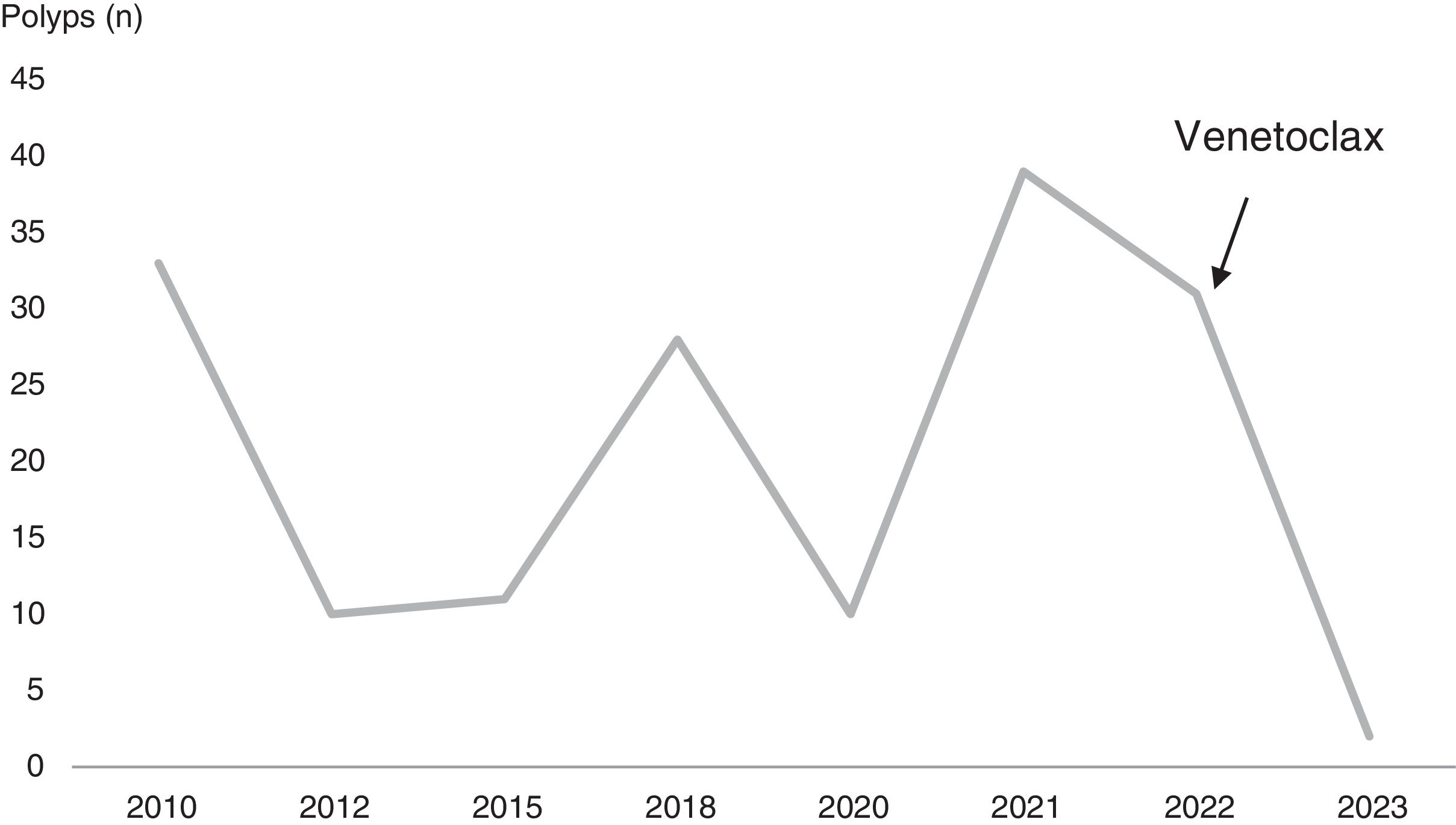
In parallel, the patient was diagnosed with FAP after a cumulative total number of 190 polyps were detected. All of the polyps were sessile, ≤5mm and were identified as tubular adenomas with low-grade dysplasia. Initially, in 2010 and at the age of 52, he presented 33 adenomas on colonoscopy, with a cancerous polyp in the ascending colon, so polypectomies and a right hemicolectomy were performed the same year. Over the following 13 years, the patient underwent scheduled colonoscopies, in which polyps (10–30 approximately in each colonoscopy) continued to be identified and resected (Fig. 1). Surprisingly, in June 2023, only two 5-mm sessile polyps were detected. Analyzing these events chronologically, venetoclax was initiated after the 2022 colonoscopy, in which 31 polyps were detected and resected. The medication has been maintained since then, suggesting its probable contribution to the evolution of the adenomatous polyposis. Regarding genetic testing, it was performed for APC and MUTYH genes in 2011, revealing a variant of uncertain clinical significance in the APC gene (c.7710A>T; p.(Ser2570=). In 2024, the test was repeated with no pathogenic variants detected.
Venetoclax is a B cell leukemia/lymphoma 2 (BCL-2) inhibitor that induces apoptosis in cancer cells. It binds to the BH3-binding groove of BCL-2, displacing pro-apoptotic proteins and leading to cell death. Originally, venetoclax demonstrated significant efficacy in CLL and small lymphocytic lymphoma (SLL), with high response rates and prolonged progression-free survival (PFS).3 BCL-2 expression is also observed in various solid tumors, including CRC, particularly in the early stages of tumorigenesis. Studies suggest that BCL-2 inhibition could prevent adenoma formation, serving as a potential preventive strategy against CRC in high-risk populations such as those with FAP.4
Other BCL-2 inhibitors have been explored in solid tumors with mixed results. For instance, oblimersen combined with irinotecan in metastatic CRC showed moderate efficacy but increased toxicity. Although venetoclax is considered a promising treatment for hematologic malignancies, its efficacy in solid malignancies is still under investigation. A phase II study tested venetoclax with fulvestrant versus fulvestrant alone in estrogen receptor-positive, HER2-negative, advanced or metastatic breast cancer patients. The combination did not significantly improve the clinical benefit rate or PFS.5
One requirement for a chemopreventive agent is low or no toxicity. The safety profile of venetoclax, based on data from patients with CLL, includes diarrhea (41%), neutropenia (40%), nausea (39%), anemia (31%), fatigue (28%) and upper respiratory tract infection (25%).6 Consequently, this may limit its use as a chemopreventive drug but its potential benefits in reducing polyp burden in FAP patients could be significant.
To this date, no trials have evaluated the efficacy and safety of venetoclax in CRC. Additionally, we have not found any clinical cases about this possible association. In this case, we believe the positive response in polyp burden might be due to venetoclax, given that BCL-2 is overexpressed in numerous solid tumors, including CRC. However, we cannot directly infer causality. Therefore, further studies that analyze the chemopreventive effect of venetoclax on colorectal adenomas and CRC should be considered in order to confirm its benefit.
FundingFrancesc Balaguer is responsible for managing the projects with reference numbers “PI19/01867”, “PI22/00470”, and “ICI22/00063” each of which receives funding from the Instituto de Salud Carlos III (ISCIII) and are additionally co-funded by the European Union.
Patient consentWe have obtained a signed patient consent to publish this case report.
Conflict of interestThe authors who have taken part in this article declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.