The updated Sydney system biopsy protocol (USSBP) standardizes the sampling of gastric biopsies for the detection of preneoplastic conditions (e.g., gastric intestinal metaplasia [GIM]), but the real-world diagnostic yield is not well-described.
AimTo determine whether regular application of USSBP is associated with higher detection of chronic atrophic gastritis (CAG), GIM and autoimmune gastritis (AIG).
MethodsWe performed a real-world retrospective study at an academic urban tertiary hospital in Chile. We manually reviewed medical records from consecutive patients undergoing esophagogastroduodenoscopy (EGD) from January to December 2017. Seven endoscopists who performed EGDs were categorized into two groups (USSBP ‘regular’ and USSBP ‘infrequent’) based on USSBP adherence, using minimum 20% adherence as the prespecified threshold. Multivariable logistic regression models were used to estimate the odds ratios (aOR) and 95% confidence intervals (CI) for the association between endoscopist groups and the likelihood of diagnosing CAG, GIM or AIG.
Results1206 patients were included in the study (mean age: 58.5; 65.3% female). The USSBP regular group demonstrated a higher likelihood of detecting CAG (20% vs. 5.3%; aOR 4.03, 95%CI: 2.69–6.03), GIM (12.2% vs. 3.4%; aOR 3.91, 95%CI: 2.39–6.42) and AIG (2.9% vs. 0.8%; aOR 6.52, 95%CI: 1.87–22.74) compared to infrequent group. Detection of advanced-stage CAG (Operative Link for Gastritis Assessment stage III/IV) was significantly higher in the USSBP regular vs. infrequent group (aOR 5.84, 95%CI: 2.23–15.31).
ConclusionsRoutine adherence to USSBP increases the detection rates of preneoplastic conditions, including CAG, GIM and AIG. Standardized implementation of USSBP should be considered in high gastric cancer risk populations.
El protocolo de biopsia del sistema de Sydney actualizado (Updated Sydney System biopsy protocol [USSBP]) estandariza la toma de muestras de biopsias gástricas para la detección de condiciones preneoplásicas (p. ej., la metaplasia intestinal gástrica (MIG)), pero el rendimiento diagnóstico en el mundo real no está bien descrito.
ObjetivoDeterminar si la aplicación regular del USSBP se asocia con una mayor detección de gastritis crónica atrófica (GCA), MIG y gastritis autoinmune (GAI).
MétodosEstudio retrospectivo del mundo real en un hospital terciario urbano académico en Chile. Revisamos manualmente los registros médicos de pacientes consecutivos sometidos a una endoscopía digestiva alta (EDA) desde enero hasta diciembre de 2017. Siete endoscopistas que realizaron EDA fueron categorizados en 2 grupos (USSBP «regular» y USSBP «infrecuente») según su adhesión al USSBP, utilizando un umbral predefinido de adhesión>20%. Se utilizaron modelos de regresión logística multivariable expresadas en odds ratio (OR) e intervalos de confianza del 95% (IC 95%) para la asociación entre los grupos de endoscopistas y la probabilidad de diagnosticar GCA, MIG o GAI.
ResultadosSe incluyeron 1.206 pacientes en el estudio (edad promedio: 58,5 años; 65,3% mujeres). El grupo USSBP «regular» demostró una mayor probabilidad de detectar GCA (20 vs. 5,3%; OR: 4,03; IC 95%: 2,69-6,03), MIG (12,2 vs. 3,4%; OR: 3,91; IC 95%: 2,39-6,42) y GAI (2,9 vs. 0,8%; OR: 6,52; IC 95%: 1,87-22,74) en comparación con el grupo USSBP «infrecuente». La detección de GCA en etapa avanzada (etapa III/IV de Operative Link for Gastritis Assessment [OLGA]) fue significativamente mayor en el grupo USSBP «regular» vs. USSBP «infrecuente» (OR: 5,84; IC 95%: 2,23-15,31).
ConclusionesLa adherencia rutinaria al USSBP aumenta las tasas de detección de condiciones preneoplásicas, incluyendo GCA, MIG y GAI. La implementación estandarizada del USSBP debería considerarse en poblaciones con alto riesgo de cáncer gástrico.
Gastric cancer (GC) is the fourth leading cause of cancer-related deaths worldwide.1 Commonly, in Western countries, GC is diagnosed in advanced stages, limiting treatment options and survival.2 According to the Correa histopathological cascade,3 noncardia gastric adenocarcinoma is preceded by chronic atrophic gastritis (CAG), with or without gastric intestinal metaplasia (GIM), and dysplasia. The most common trigger for the cascade is chronic Helicobacter pylori (Hp) infection. Autoimmune gastritis (AIG) is also associated with an increased risk of GC.4,5 Accordingly, screening strategies have focused on the detection and adequate follow-up or treatment of preneoplastic gastric conditions (CAG/GIM), dysplasia and early-stage GC.
Data describing the prevalence of preneoplastic GC conditions in Latin American populations are scarce. In Chile, GC-related mortality is high (18.7cases/inhabitants in 2020)6 and, accordingly, the Ministry of Health recommends performing an esophagogastroduodenoscopy (EGD) as a selective evaluation in patients aged 40 years or older who present with upper gastrointestinal symptoms, such as epigastric pain. Aligned with this recommendation, the National Association of Endoscopy of Chile recommends routinely assessing for preneoplastic gastric conditions in patients 40 years or older who are undergoing nonemergent EGD and, as part of this GC risk assessment, also advises consideration of the use of protocolized gastric biopsies following the updated Sydney system biopsy protocol (USSBP).7 Nevertheless, low adherence to these recommendations has been described8 which limits the quality and completeness of risk stratification, since gastric preneoplastic conditions require adequate mucosal sampling for diagnostic confirmation.
Although EGD is the preferred method for the diagnosis of gastric preneoplastic conditions, conventional techniques with white light endoscopy are subject to low sensitivity compared to histologic diagnostic methods, especially in younger people.9,10 For this reason, histology is considered the gold standard. In experienced hands, image enhanced endoscopy, as well as magnification methods, may increase the diagnostic yield of CAG, especially if GIM is present.11,12 Nonetheless, their availability is limited and performance among endoscopists is highly variable.13
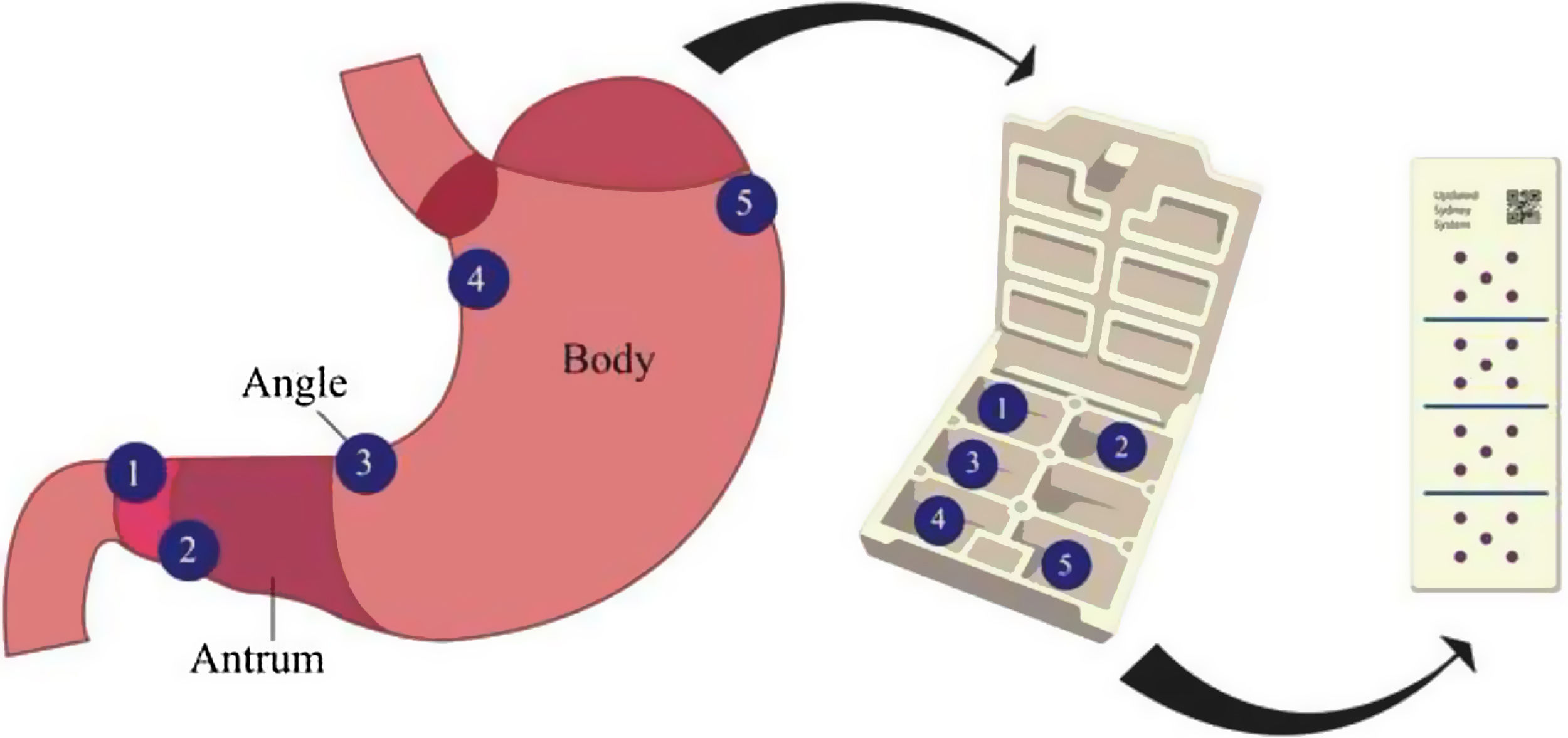
In this context, gastric mapping biopsies following the USSBP,14,15 which calls for separate sampling of five gastric locations from the antrum, incisura and corpus, could serve as an accurate stratification tool to assess the risk of progression of gastric preneoplasia to neoplasia, as well as further assess for Hp infection or AIG.16–18 Biopsies according to USSBP are needed to determine the Operative Link for Gastritis Assessment (OLGA) and Gastric Intestinal Metaplasia (OLGIM) staging for patients with CAG/GIM, which is one of the best predictors of progression to advanced neoplasia. While some clinical guidelines recommend the routine application of mapping gastric biopsies in patients at high risk of GC,19 it is still debatable whether routine implementation is associated with improved detection rates of preneoplastic conditions.
The aim of this study was to assess whether frequency of application of the USSBP is independently associated with higher diagnostic yield of preneoplastic conditions in a real-world clinical practice.
MethodsStudy design and settingsA single-center retrospective observational study was carried out between January and December 2017. The routine practice of seven volunteer experienced endoscopists (all performed>500 EGDs per year and had>7 years of experience of independent practice) from the Digestive Endoscopy Center at Hospital Clínico Universidad Católica de Chile was to assess their performance in the detection of CAG/GIM and AIG, as well as gastric neoplasia, defined as high-grade dysplasia or cancer. We included consecutive outpatients aged 40 years or older with a clinical indication for non-urgent EGD at our center during the predefined study period. Patients with a prior history of AIG, gastroesophageal varices, gastrectomy, bariatric surgery or prior gastric high-grade dysplasia or cancer were excluded. Patients were also excluded if they were already under endoscopic surveillance for CAG/GIM or if they had been previously evaluated with USSBP. Patients’ clinical data, family history of GC, endoscopic findings, pattern of gastric mucosal sampling, and histologic findings were manually abstracted from the medical records.
During the EGD, the decision to sample the gastric mucosa according to USSBP was made by each endoscopist. Endoscopists were divided into two groups according to percent adherence to the USSBP. In accordance with the National Association of Endoscopy of Chile recommendations, starting in April 2016 our endoscopy unit recommended routine consideration of the implementation of the USSBP. In the first year after recommended implementation, gastric biopsies were obtained according to USSBP in ∼20% of the non-urgent outpatient EGDs performed in patients aged 40 years or older (854 EGDs with USSBP out of total 4662 EGDs performed in eligible patients).20 Based on these observations, we set 20% as the a priori threshold for categorizing regular vs. infrequent application of the USSBP – that is, endoscopists who performed USSBP in at least 20% of EGDs performed among eligible patients were categorized in the “USSBP regular” group while those with <20% were categorized in the “USSBP infrequent” group. The outcome was a diagnosis of gastric preneoplastic conditions (CAG, GIM), AIG or neoplasia.
This study was performed in accordance with the ethical standards established in the Declaration of Helsinki and it was approved (ID 16-341) by the Ethics Committee of Hospital Clínico Universidad Católica de Chile.
Endoscopists and esophagogastroduodenoscopyAt our endoscopic center, since April 2016 all endoscopists have been receiving regular mandated instruction on the USSBP through information sessions, hands-on activities, supervised practice, and review of clinical cases. All endoscopists included in the study performed EGD routinely with the same equipment. The clinical units are equipped with either Olympus (GIF-H190/GIF-H170) or Fujinon (EC-600ZW) high-definition white-light EGDs. Narrow band imaging (NBI), Fuji Intelligent Chromo Endoscopy (FICE) or Blue Laser Imaging (BLI) were available for all patients, but these were applied regularly in most of the examens based on endoscopists’ clinical determination. Frequency of the application of virtual chromoendoscopy was not recorded.
Following patient sedation (using a combination of benzodiazepines and opiates in most patients), the endoscopists performed esophageal, gastric and duodenal assessment and reported main findings. Endoscopic features of CAG, GIM and AIG were registered when the endoscopist described in the endoscopic report based on the appearance of gastric mucosa with high-definition white light endoscopy. Also, endoscopic time in minutes was recorded for all procedures, from the time of endoscopic insertion until removal.
Gastric biopsies sampling methodIn all patients, gastric biopsies were sampled with a standard 3.0mm biopsy forceps (Endo-Flex®, GmbH, Germany). To qualify as adherence to USSBP the following gastric biopsy protocol needed to occur: two tissue samples obtained from the antrum (within 2–3cm from the pylorus from lesser and greater curvature), two from the corpus (one from lesser curvature about 4cm proximal from the angle and one from greater curvature about 8cm distal to cardia) and one from the angle (incisura angularis).14,15 Tissue samples were stored in a custom cassette designed for this purpose (Fig. 1) to ensure that the anatomical location of each biopsy was correctly identified, and to avoid additional cost for the patients related to the number of distinct pathology jars. While those who did not adhere to this protocol, it was considered as a single tissue sample obtained from antrum, corpus or angle. Additional gastric biopsies could be collected outside the USSBP if there was any finding during the EGD. In addition, one or two antral samples were collected for rapid urease testing (Pronto Dry®, Medical Instruments Corporation, France) to assess active Hp, as clinically appropriate; this was recorded but not considered part of USSBP adherence. As noted below, active Hp was also assessed on histology.
Histological classification of gastric biopsiesGastric samples were evaluated independently by two experienced pathologists (JT and JCR) at the same hospital. Each pathologist evaluated about 50% of samples from each study group and they were blinded to the endoscopist group, but not to the biopsy collection method. Sample sets with non-definitive findings for CAG or GIM were reviewed by both pathologists to achieve a final consensus diagnosis. Only a complete set of biopsies with at least one sample from each anatomical segment (antrum, incisura angularis and corpus) was considered as USSBP. Biopsies were assessed for CAG according to OLGA staging system,14 grouping them in OLGA 0, OLGA I–II and high-risk stages OLGA III–IV. GIM was further classified as complete or incomplete types based on hematoxylin and eosin (H&E) evaluation and according to anatomical extent (antral-restricted vs. corpus-extended). Histological findings were reported for each anatomical location (antrum, incisura angularis and corpus). The most advanced discrete histology observed in the set of biopsies was used as the global diagnosis.
Histologic characteristics of AIG were defined according to the following criteria: the presence of inflammatory infiltrate associated with mucosal atrophy, with or without metaplastic glands, involving the corpus, but with preserved glandular structure or only mild inflammatory infiltrates in the antrum, without any evidence of antral mucosal atrophy.21 Hyperplasia of enterochromaffin-like cells was an additional criterion to support histological AIG diagnosis, but was not mandatory. The presence of Hp infection did not rule out AIG diagnosis. Although, serum anti-parietal cells and anti-intrinsic factor antibodies were not routinely measured on all patients, they were considered for the diagnosis of AIG when available.
Active Hp infection was determined based on positive rapid urease test or presence of Hp organisms on Giemsa stain.
Finally, as an exploratory analysis, findings of low- and high-grade gastric dysplasia, gastric adenocarcinoma, gastric neuroendocrine tumor and gastric lymphoma were recorded in both groups as well, understanding that the incidence of these advanced lesions would be low.
Statistical analysisCategorical variables were expressed as proportions (%) and continuous variables were expressed as mean or median and interquartile range (IQR) and compared between the two endoscopist groups using Chi-square test and Mann–Whitney U test, respectively.
For the primary analysis, we used unconditional logistic regression to evaluate the association between endoscopist group (USSBP regular vs. infrequent) and histologically diagnosed CAG, GIM, or AIG, adjusting for patients’ age, sex, active Hp infection status (positive vs. negative) and EGD indication; estimates were expressed as adjusted odds ratios (aOR) and confidence interval (95%CI). We also separately evaluated this same model, but additionally adjusted for endoscopic features of CAG or GIM and endoscopic time. As a sensitivity analysis different threshold for the definition of USSBP regular vs. infrequent groups were evaluated and presented in supplementary material (Supplementary Table 1). We also used unconditional logistic regression to conduct a secondary analysis where the exposure was whether gastric biopsies were obtained according to USSBP vs. collected in a non-protocolized manner (e.g., ‘random gastric biopsies’), irrespective of the endoscopist group. The outcome was the same as for the primary analysis—that is, diagnostic yield of CAG, GIM and AIG. We assessed the demographic, clinical and endoscopic variables associated with the endoscopists’ decision to perform vs. not perform USSBP using logistic regression.
We used linear regression and Pearson correlation tests to evaluate the association between the USSBP performance rate among endoscopists as a continuous value and the detection of CAG/GIM. We also evaluated the sensitivity and specificity of EGD findings vs. histological diagnosis of CAG with or without GIM.
A p-value≤0.05 was considered statistically significant. All statistical analyses were conducted using STATA v14.2 (Statacorp, College Station, TX, USA).
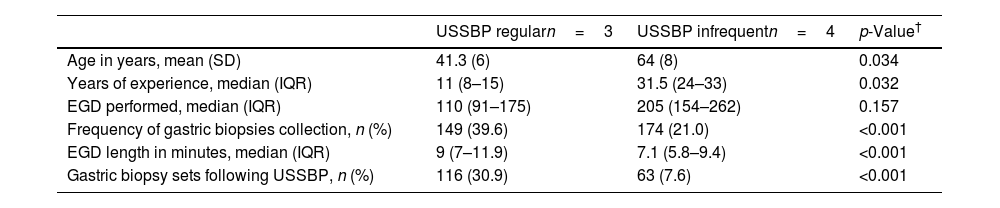
ResultsEndoscopists’ characteristics and gastric mucosal sampling methodsMean age across the seven endoscopists was 54 years (standard deviation, SD±14); additional characteristics are provided in Table 1. The 7 endoscopists included in this study performed a total of 1206 EGDs among eligible patients aged 40 years and older during the study time frame. Gastric biopsies were collected in 26.8% (n=323) of the patients. Significant differences were observed in the frequency of gastric biopsies sampling between the endoscopists (p<0.001), ranging from 9.6% to 45.5% of the performed EGDs. Also, significant differences in the application of USSBP were observed between the endoscopists (p<0.001), ranging from 4.7% to 38.2% of the EGDs. Therefore, 3 endoscopists were allocated to the USSBP regular group (≥20% application) and 4 endoscopists assigned to the USSBP infrequent group (<20% application).
Endoscopist characteristics stratified by updated Sydney system biopsy protocol regular vs. infrequent use.
| USSBP regularn=3 | USSBP infrequentn=4 | p-Value† | |
|---|---|---|---|
| Age in years, mean (SD) | 41.3 (6) | 64 (8) | 0.034 |
| Years of experience, median (IQR) | 11 (8–15) | 31.5 (24–33) | 0.032 |
| EGD performed, median (IQR) | 110 (91–175) | 205 (154–262) | 0.157 |
| Frequency of gastric biopsies collection, n (%) | 149 (39.6) | 174 (21.0) | <0.001 |
| EGD length in minutes, median (IQR) | 9 (7–11.9) | 7.1 (5.8–9.4) | <0.001 |
| Gastric biopsy sets following USSBP, n (%) | 116 (30.9) | 63 (7.6) | <0.001 |
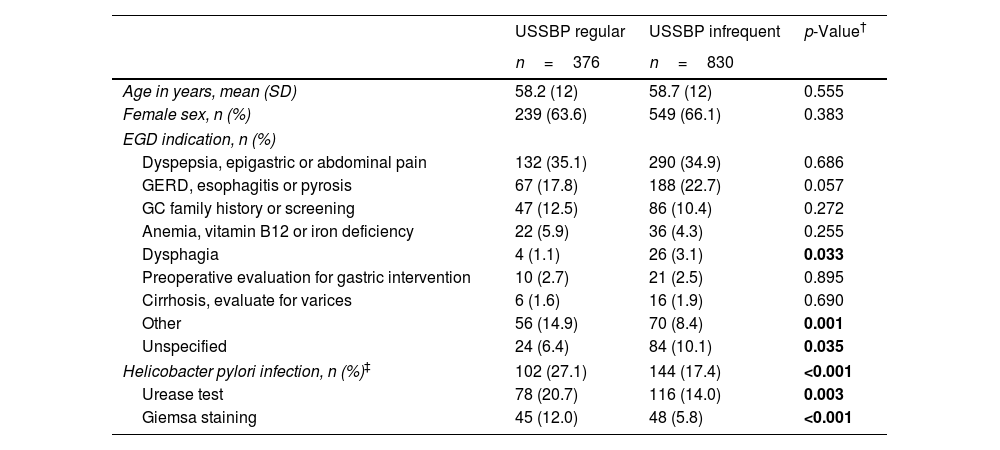
For the 1206 patients included in the study, the mean age was 59 (SD±12) years old and 65.3% were female. The study sample represents ∼30% of the overall EGD performed during 2017 at our center. Of the total number of patients, 31.2% (n=376) were categorized in the USSBP regular group and 68.8% (n=830) in the USSBP infrequent group. Detailed baseline characteristics of participants by endoscopist group are summarized in Table 2. There were no significant differences in demographic variables of patients included in each group. However, EGD indications were slightly different (but not statistically significant) between groups; dysphagia (1.1% vs. 3.1%; p=0.33) and unspecified symptoms (6.4% vs. 10.1%; p=0.35) were less frequently reported in the regular vs. infrequent group.
Patient characteristics by endoscopy group.
| USSBP regular | USSBP infrequent | p-Value† | |
|---|---|---|---|
| n=376 | n=830 | ||
| Age in years, mean (SD) | 58.2 (12) | 58.7 (12) | 0.555 |
| Female sex, n (%) | 239 (63.6) | 549 (66.1) | 0.383 |
| EGD indication, n (%) | |||
| Dyspepsia, epigastric or abdominal pain | 132 (35.1) | 290 (34.9) | 0.686 |
| GERD, esophagitis or pyrosis | 67 (17.8) | 188 (22.7) | 0.057 |
| GC family history or screening | 47 (12.5) | 86 (10.4) | 0.272 |
| Anemia, vitamin B12 or iron deficiency | 22 (5.9) | 36 (4.3) | 0.255 |
| Dysphagia | 4 (1.1) | 26 (3.1) | 0.033 |
| Preoperative evaluation for gastric intervention | 10 (2.7) | 21 (2.5) | 0.895 |
| Cirrhosis, evaluate for varices | 6 (1.6) | 16 (1.9) | 0.690 |
| Other | 56 (14.9) | 70 (8.4) | 0.001 |
| Unspecified | 24 (6.4) | 84 (10.1) | 0.035 |
| Helicobacter pylori infection, n (%)‡ | 102 (27.1) | 144 (17.4) | <0.001 |
| Urease test | 78 (20.7) | 116 (14.0) | 0.003 |
| Giemsa staining | 45 (12.0) | 48 (5.8) | <0.001 |
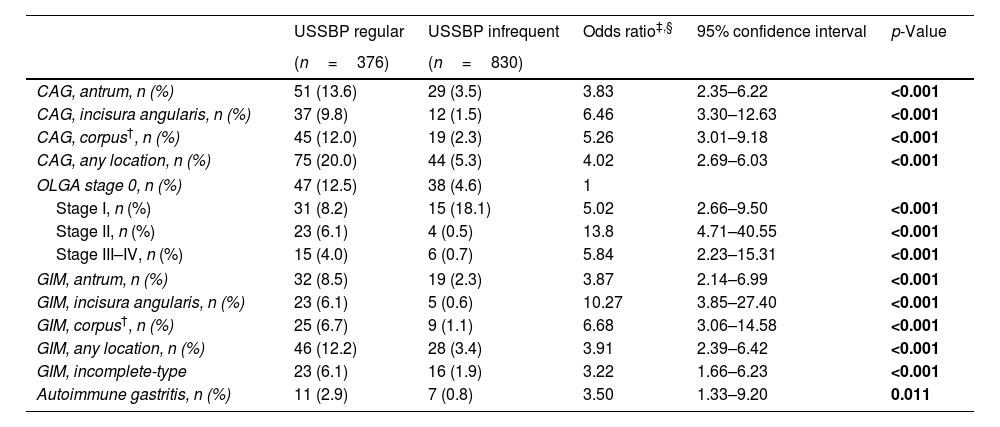
Detailed characteristics of CAG and GIM among endoscopists group are summarized in Table 3.
Chronic atrophic gastritis with or without gastric intestinal metaplasia according to anatomical location and its association to endoscopist group.
| USSBP regular | USSBP infrequent | Odds ratio‡,§ | 95% confidence interval | p-Value | |
|---|---|---|---|---|---|
| (n=376) | (n=830) | ||||
| CAG, antrum, n (%) | 51 (13.6) | 29 (3.5) | 3.83 | 2.35–6.22 | <0.001 |
| CAG, incisura angularis, n (%) | 37 (9.8) | 12 (1.5) | 6.46 | 3.30–12.63 | <0.001 |
| CAG, corpus†, n (%) | 45 (12.0) | 19 (2.3) | 5.26 | 3.01–9.18 | <0.001 |
| CAG, any location, n (%) | 75 (20.0) | 44 (5.3) | 4.02 | 2.69–6.03 | <0.001 |
| OLGA stage 0, n (%) | 47 (12.5) | 38 (4.6) | 1 | ||
| Stage I, n (%) | 31 (8.2) | 15 (18.1) | 5.02 | 2.66–9.50 | <0.001 |
| Stage II, n (%) | 23 (6.1) | 4 (0.5) | 13.8 | 4.71–40.55 | <0.001 |
| Stage III–IV, n (%) | 15 (4.0) | 6 (0.7) | 5.84 | 2.23–15.31 | <0.001 |
| GIM, antrum, n (%) | 32 (8.5) | 19 (2.3) | 3.87 | 2.14–6.99 | <0.001 |
| GIM, incisura angularis, n (%) | 23 (6.1) | 5 (0.6) | 10.27 | 3.85–27.40 | <0.001 |
| GIM, corpus†, n (%) | 25 (6.7) | 9 (1.1) | 6.68 | 3.06–14.58 | <0.001 |
| GIM, any location, n (%) | 46 (12.2) | 28 (3.4) | 3.91 | 2.39–6.42 | <0.001 |
| GIM, incomplete-type | 23 (6.1) | 16 (1.9) | 3.22 | 1.66–6.23 | <0.001 |
| Autoimmune gastritis, n (%) | 11 (2.9) | 7 (0.8) | 3.50 | 1.33–9.20 | 0.011 |
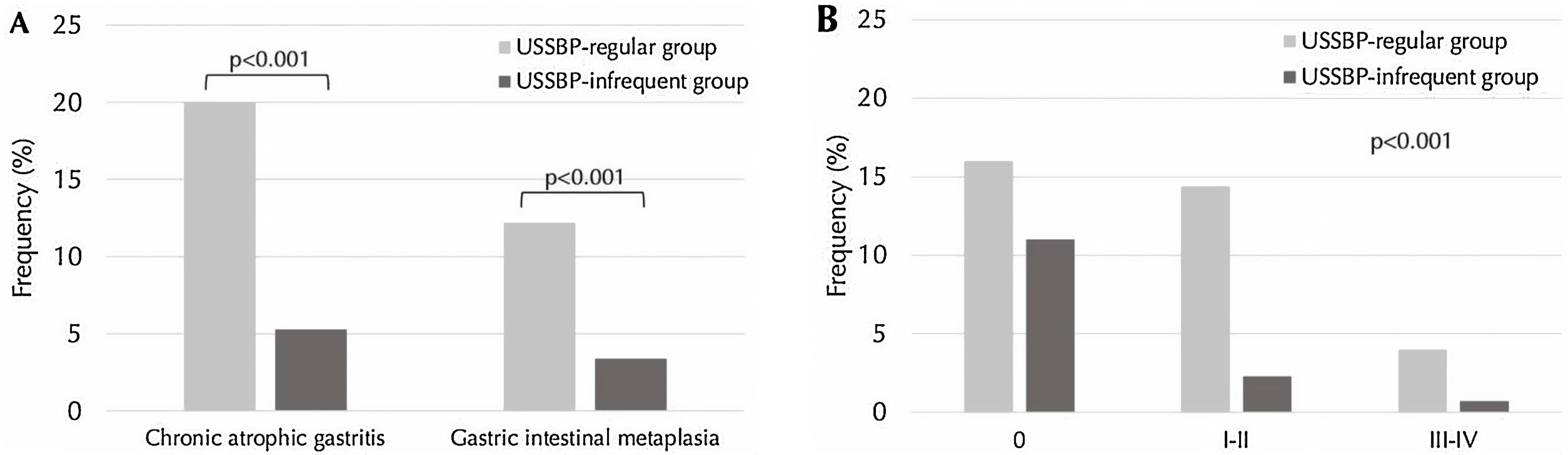
CAG was more often diagnosed in the USSBP regular group (20%; n=75/376) compared to the USSBP infrequent group (5.3%; n=44/830) (p<0.001) (Fig. 2A). Similarly, the distribution of OLGA stages was significantly different between the endoscopist groups (p=0.032; Fig. 2B): OLGA stages III–IV were more often detected in the USSBP regular group compared to the USSBP infrequent group (4.0% vs. 0.7%, p<0.001). On multivariable analysis, the USSBP regular group were 4-fold (aOR 4.03, 95%CI: 2.69–6.03) more likely to diagnose CAG compared to the USSBP infrequent group. This association was preserved even after adjusting for endoscopic features of CAG and endoscopic time (aOR 3.82, 95%CI: 2.45–5.94).
Differences in frequency of detection of chronic atrophic gastritis (CAG) and gastric intestinal metaplasia (GIM) in updated Sydney system biopsy protocol (USSBP) regular group compared to infrequent group. Panel A shows differences in frequency of overall CAG and GIM. Panel B shows differences in frequency by OLGA stages.
GIM was histologically diagnosed in 12.2% (n=46) of the USSBP regular group compared to 3.4% (n=28) in the USSBP infrequent group (p<0.001) (Fig. 2A). On multivariable analysis, the USSBP regular group were 3.9-fold (aOR 3.91, 95%CI: 2.39–6.42) more likely to diagnose GIM compared to the USSBP infrequent group, and 2.4-fold more likely after additionally adjusting for endoscopic features of GIM and endoscopic time (aOR 2.42, 95%CI: 1.40–4.19). Multivariable logistic regression models of histological diagnosis of CAG and GIM, according to gastric anatomical location and histological type, among endoscopist group are summarized in Table 3.
According to endoscopists group, characteristics of AIG were more often observed in the USSBP regular group (2.9%; n=11) compared to the USSBP infrequent group (0.8%; n=7), with an aOR of 6.52 (95%CI: 1.87–22.74).
Results of sensitivity analysis evaluating different thresholds for the definition of USSBP regular vs. infrequent groups are presents in Table S1.
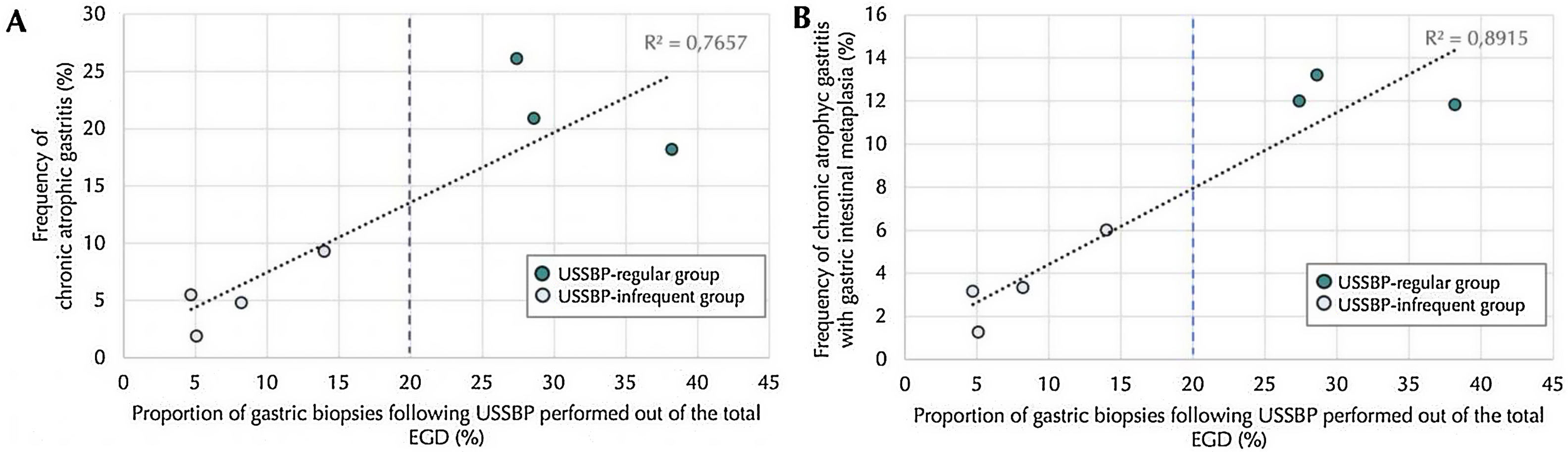
There was a positive linear correlation between proportion of EGDs where USSBP was performed, analyzed as a continuous variable, and the histologic diagnosis of CAG and GIM, with a Pearson R2 of 0.77 and 0.89, respectively (both p-values<0.001) (Fig. 3).
Correlation between histologically diagnosis of chronic atrophic gastritis (Panel A) and gastric intestinal metaplasia (Panel B) according to frequency of application of mapping gastric biopsies following updated Sydney system biopsy protocol (USSBP), out of the esophagogastroduodenoscopy performed by each endoscopist. The blue dotted line indicates the 20% cut-off point set to define the adhesion to USSBP.
One case of low-grade dysplasia was diagnosed in the USSBP regular group, and one case of high-grade dysplasia was diagnosed in infrequent group (0.27% vs. 0.12%; p=0.26). In addition, 1 case of gastric adenocarcinoma was diagnosed in the USSBP regular group and 5 in the infrequent group (0.27% vs. 0.6%; p=0.61), 1 case of gastric neuroendocrine tumor was found in the USSBP regular group and 2 in the infrequent group (0.27% vs. 0.24%; p=0.94), and 2 cases of gastric lymphoma were found in each group (0.53% vs. 0.24%; p=0.42). Each of these advanced lesions was visible endoscopically.
Association between gastric mucosal sampling method and histological diagnosis of gastric preneoplasiaCAG and GIM were more often diagnosed when USSBP was used compared to non-protocolized biopsies. According to sampling method (regardless of endoscopist group) CAG was found in 52% (n=93) of biopsies collected following USSBP compared to 18.1% (n=26) of non-protocolized biopsies (p<0.001); while GIM was diagnosed in 30.7% (n=55) of biopsies collected following USSBP compared to 13.2% (n=19) of non-protocolized biopsies (p<0.001).
On multivariable logistic regression, USSBP vs. non-protocolized biopsies were independently associated with higher likelihood of diagnosing CAG (aORs of 5.52, 95%CI: 3.17–9.62) and GIM (aOR 3.56, 95%CI: 1.94–6.54). Multivariable logistic regression models of CAG and GIM according to gastric anatomical location are summarized in Table S2.
Importantly, AIG was only diagnosed in patients with gastric biopsies sampled by USSBP, and no cases of AIG were diagnosed in patients who underwent non-protocolized biopsies.
Endoscopic findingsEndoscopic features of CAG were noted by endoscopists during the EGD in 16% (n=60) and 11.2% (n=93) of the patients in the USSBP regular and the USSBP infrequent group, respectively (p=0.022). Endoscopic suspicion for CAG demonstrated low sensitivity and high specificity for the histological diagnosis of CAG. The sensitivity of endoscopy for a histologically confirmed CAG diagnosis was significantly higher in the USSBP regular group compared to the infrequent group (52.0% (39/75) vs. 29.6% (13/44), p=0.022), while specificity was similar between the two groups (89.2% (66/74) vs. 86.2% (112/130), p=0.39).
Endoscopically suspected GIM was more frequently reported in the USSBP regular group (11.2%; n=42) compared to the infrequent group (1.5%; n=12) (p<0.001). As for CAG, endoscopic suspicion of GIM had low sensitivity and high specificity for the histological diagnosis of GIM. Interestingly, while sensitivity was higher in the USSBP regular vs. infrequent group (41.3% (19/46) vs. 17.9% (5/28), p=0.037), the specificity was significantly higher in the USSBP infrequent vs. regular group (98.0% (143/146) vs. 85.4% (88/103), p<0.001).
Endoscopic findings according to clinical indications for the EGD, irrespective of endoscopist group, are summarized in Table S3.
Endoscopic timeRegarding EGD procedure time, endoscopists in the USSBP regular group had slightly longer procedure times compared to the infrequent group (9 vs. 7.1min; p<0.001). Importantly, procedure time irrespective of endoscopist group was longer during EGDs where USSBP was applied compared to those without USSBP (7.3 vs. 10.1min; p<0.001), suggesting an increase in endoscopic time attributable to the application of USSBP as opposed to the individual endoscopist. Of note, endoscopic time was positive and independently associated to the histological diagnosis of CAG (aOR 1.05, 95%CI: 1.02–1.08) and GIM (aOR 1.05, 95%CI: 1.02–1.08).
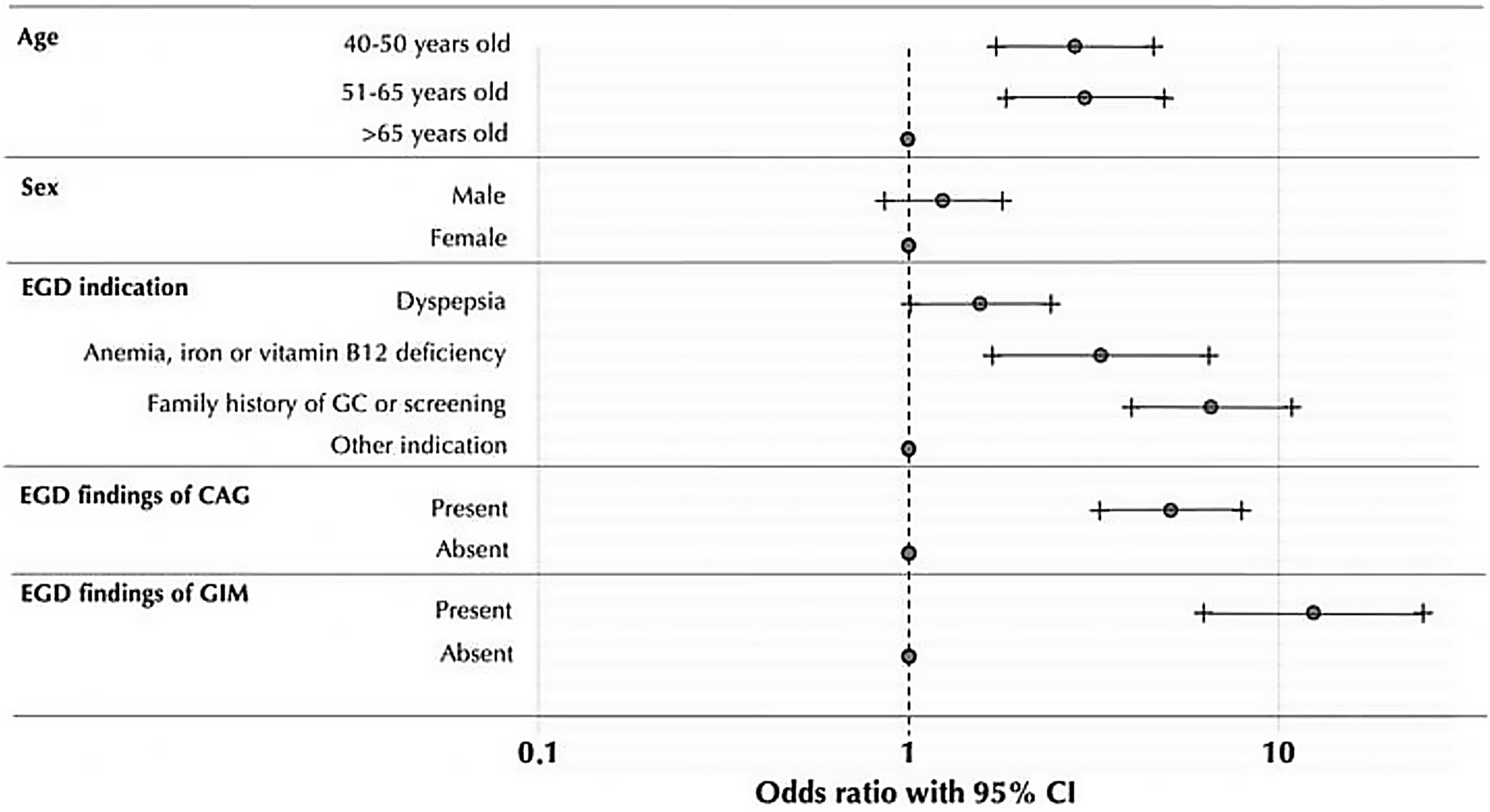
Demographic, clinical and endoscopic variables associated with the application of USSBPPatients in whom gastric biopsies were sampled following USSBP (regardless of endoscopist group) had a median age of 54 years (SD±11) and 60.9% were female. Patients who were>65 years old were less likely to have USSBP performed during EGD compared to patients in younger age groups. Considering the group of patients over 65 years as reference, the ORs for sampling gastric biopsies following USSBP in patients aged 40–50 and 51–65 years were 2.85 (95%CI: 1.74–4.66) and 3.03 (95%CI: 1.85–4.97), respectively. Other variables positively associated with the performance of the USSBP are provided in Fig. 4.
DiscussionCAG with or without GIM and AIG are preneoplastic gastric conditions associated with an increased risk of developing GC. Therefore, a standardized and optimal approach for their detection during EGD is needed. One of the proposed methods is obtaining mapping gastric biopsies according to USSBP; however, whether this approach adds to diagnostic yield in real-world practice is understudied. Here we report that endoscopists who regularly performed gastric biopsies following USSBP had 4-fold higher odds of detecting histologically confirmed preneoplastic conditions compared to their counterparts who performed USSBP less frequently. In support of the diagnostic yield of USSBP itself, this higher likelihood of a diagnosis of preneoplastic conditions was observed when USSBP were obtained as opposed to non-protocolized gastric biopsies irrespective of the endoscopist. Taken together, our results suggest that a higher diagnostic yield and more accurate risk stratification can be attained with this USSBP.
The best strategy for the detection of gastric preneoplastic conditions has not been established.18,22,23 Several reports have demonstrated that white light endoscopy is insufficient, while image enhanced endoscopy represents newer technology with promising results.9,24 However, widespread use of image-enhanced endoscopy is limited due to the need of more specialized level of training, time for implementation in regular endoscopy outpatient clinics and equipment cost. Gastric mapping biopsies following USSBP may complement endoscopic diagnostic methods, also when image-enhanced endoscopy methods are available.25 As expected, in our study high-definition white light endoscopy with non-routine application of image enhanced endoscopy methods demonstrated low sensitivities for histologically confirmed CAG/GIM diagnoses.
To date, based on the available evidence, histological assessment of gastric preneoplastic conditions and determination of anatomic involvement allows for the most accurate GC risk stratification. Contrasted with multiple random or non-protocolized biopsies, USSBP assures the collection of antrum, angle and corpus samples which allows assessment of OLGA or OLGIM stages, providing an objective grade and anatomic extent of CAG and GIM. An elevated risk of GC has been described among OLGA or OLGIM III–IV stages26,27 and corpus-extended GIM compared to antral restricted GIM.28,29 In our real-world study we observed that regular use of USSBP was associated with an independent 5-fold higher likelihood of diagnosing of OLGA III/IV vs. infrequent use. Moreover, a higher frequency of Hp infection was observed in the USSBP regular vs. infrequent group, which is at least in part attributed to higher number of gastric samples collected for USSBP since biopsies from these locations combined approaches 100% sensitivity for Hp diagnosis.30
The adoption of USSBP is highly variable across endoscopists worldwide. This situation may be attributed to the lack of uniform global recommendations for its application.19,22,23 In our study, we attempted to understand the main reasons why endoscopists decided to sample gastric biopsies following USSBP. As expected, endoscopic findings of CAG and GIM showed the strongest associations. We identified that EGD indication is another significant factor, particularly EGDs in patients for the evaluation of anemia, family history of GC, or if the indication is specifically GC screening. In terms of age, there was a lower implementation of USSBP in patients over 65 years, possibly due to the perceptions of a lower benefit of stratification risk of GC in the older group of patients. However, there is still controversy regarding the upper age limit for the assessment and endoscopic surveillance of preneoplastic gastric conditions, particularly since older age is associated with higher likelihood of harboring (pre)neoplasia.19,22,23
In addition to the lack of uniform clinical recommendation, there are other considerations for the application of USSBP within usual endoscopy practice. Important barriers to their implementation may be unawareness of patient's GC risk, endoscopy time, concerns about bleeding risk, increased cost, availability of pathologists with experience in OLGA/OLGIM staging and increase in pathologist workload. Related to adverse effects, it has been reported that taking multiple gastric biopsies does not increase the risk of bleeding.31 In terms of endoscopy time, we observed a minimal but statistically significant increase in procedural time by about 2–3min when USSBP was employed, although this did not translate to longer overall procedural room utilization time. This marginal increase in EGD procedure time must be considered in the context of the downstream benefit of several-fold the higher detection rates of gastric preneoplastic conditions and better GC risk assessment associated with USSBP vs. non-protocolized biopsies observed in this study. Notably, increased procedural time alone only minimally increased the diagnostic yield of gastric preneoplasia (aOR 1.05, 95%CI: 1.02–1.08). Furthermore, thanks to close collaboration with the pathologists, we implemented the use of a custom-designed plastic cassette to store and process the gastric biopsies within one single paraffined-embedded block. In our experience, this device facilitates both endoscopists and pathologists with respect to the correct identification, processing, and interpretation of gastric biopsies without accruing additional cost for patients. That said, cost might be one factor associated with lower likelihood of USSBP use in settings where pathology costs are additive based on the number of jars.
Since potential complications and the increased risk of gastric cancer related to AIG, its diagnosis demands histologic confirmation.32,33 USSBP ensures separate, and adequate sampling from the antrum and corpus for proper diagnosis of AIG. Limited representation of these anatomical subsites may lead to the underdiagnosis of AIG.30 In our study, AIG was more frequently diagnosed in the USSBP adherent group; in fact, no cases of AIG were diagnosed when non-protocolized biopsies were obtained. Nevertheless, is important to consider that usually CAG and GIM is restricted to the corpus in AIG, and it has been described a lower risk of AIG compared to CAG induced by Hp,34 therefore OLGA or OLGIM scale may not accurately reflect the risk of progression to GC in these patients.
In our real-world study, endoscopists applied the USSBP following their own clinical and endoscopic criteria because there is a lack of formal recommendations in this setting. Although more extensive evaluation is needed, our data suggest that if USSBP is applied in even as low as 20% of patients among a population where GC-related mortality is high, a better diagnostic yield of gastric preneoplastic conditions and AIG can be attained compared to EGD without USSBP, even after adjusting for endoscopic features of CAG/GIM. This threshold could vary among different regions, based on the regular practices of different endoscopic units. Future studies with larger number of endoscopist may assess whether a higher adherence to USSBP may lead to a better diagnostic yield. A recent study from Europe analyzed the relation between endoscopic biopsy rate (EBR) and the detection of gastric preneoplastic conditions, demonstrating an OR of 2.0 (95%CI: 1.7–2.4) and 2.5 (95%CI: 2.1–2.9) for the high and very high EBR, respectively.35
There are several strengths of our study. While previous studies have been limited by their inconsistent number of biopsies included in the protocol and low adherence to USSBP,17 our real-world endoscopy-based study included a well-characterized population according to manual chart review and where all histologic diagnoses were assessed independently by two experienced pathologists. Also, we additionally adjusted for endoscopic findings of CAG or GIM and endoscopic time in our analysis, since this could confound the association between USSBP and diagnostic yield; indeed, the same magnitude and strength of association was maintained. Finally, our study indirectly reflects other benefits of the implementation of USSBP. It was possible to identify greater number of high-risk patient (OLGA III–IV) to focus EGDs and avoid unnecessary follow-up in low-risk patients (OLGA 0), increase the detection of Hp and increase local awareness of the importance of recognition of gastric preneoplastic condition and the need of regular gastric biopsies collection.36
This study has the limitation of a retrospective study and not being a randomized controlled intervention; given the observational design, we were not able to control the frequency of possible confounders variables between study groups, such as the difference observed in the frequency of Hp infection. Nevertheless, regression models were adjusted for these possible confounding factors. Also, the single center design and a relatively small number of endoscopists limit generalization of our results, including to other countries/regions outside of Chile. Our study comprised only Chilean patients and therefore may not be generalizable to other populations, particularly those at lower risk for gastric cancer. On the other hand, we observed a relatively low frequency of gastric biopsies among the endoscopists included in our study. This may reflect the limitation of resources in this real-life setting. However, even with a low frequency of obtention of gastric biopsy we still observe a frequency of gastric preneoplastic conditions close to what we were expecting8 and significant differences in detection of CAG and GIM among the study groups. The endoscopist in the USSBP regular group reported a higher frequency of endoscopic features of CAG and GIM. This observation could indicate a greater awareness of gastric premalignant conditions or better training in recognizing these conditions, which could potentially influence our results. Accordingly, we adjusted the regression models for endoscopic findings, which may be a surrogate for these potential confounders.
In conclusion, application of the USSBP is associated with a higher diagnostic yield for gastric preneoplastic conditions, and the ability to assess severity using validated scoring systems with prognostic implications (e.g., OLGA), without significant added resource utilization. Our results suggest that adherence to the USSBP should be promoted in high-risk gastric cancer populations, and it could be measured and documented as a quality metric for gastric cancer screening exams to increase the detection of preneoplastic conditions and guide subsequent surveillance recommendations.
Authorship statementGuarantor of the article: Riquelme Arnoldo, MD, MMedEd.
Ethical considerationsThis study was approved by the local institute review board and all patients provided informed written consent.
All authors had access to the study data and reviewed and approved the final manuscript.
FundingGrant support: CONYCYT, FONISSA19I/0188 (AR); Conicyt-Fondap15130011 (AHC); FONDECYT1191928 (AHC); FONDECYT N° 11201338 (PA); PREVECAN project (AR); Residents’ project grant PUC PBN°25/16 (GL); ANID FONDAP152220002 (AR, JCR). FONDECYT1230504 (AR, GL, SCS, JCR, AC). European Union's Horizon 2020 research and innovation program grant agreement No 825832 (AR); ICX002027A (SCS), AGA Research Scholar Award (SCS); P30 DK120515 (SCS).
Conflict of interestsThe authors declare that they have no conflict of interest.
The authors very much appreciate the support by the Chilean Gastroenterology Society (Sociedad Chilena de Gastroenterología, SChGE) and the National Association of Digestive Endoscopy (Asociación Chilena de Endoscopía Digestiva, AChED). The authors also thank Valentina Riquelme affiliated with the Centro UC Síndrome de Down, Faculty of Medicine, Pontificia Universidad Católica de Chile for her contribution with illustrations.