Proper management of the inflammatory process in Crohn's disease (CD) results in lower rates of complications. The objective of this study was to investigate the performance of isolated and combined use of fecal calprotectin (FC) and serum levels of C-reactive protein (CRP) as markers of inflammatory activity in CD and the possibility of their use as a therapeutic target.
Patients and methodsPatients with CD and indication for colonoscopy were prospectively enrolled in the study and allocated according to the presence or absence of endoscopic inflammatory activity. The correlation between FC and CRP levels and the Simplified Endoscopic Score of Crohn's Disease (SES-CD) was performed, and the accuracy of these markers was evaluated for the diagnosis of inflammatory activity, when used alone or in series.
ResultsEighty colonoscopies were performed in patients with CD. The FC cut-off value of 155μg/g showed high sensitivity (96%) and accuracy (78%) for the diagnosis of endoscopic activity. For CRP, the value of 6.7mg/L demonstrated sensitivity of 75% and specificity of 67%. The sequential usage of these markers (FC+CRP) showed greater specificity (82%) when compared to the use of these markers alone. Depending on the probability of inflammatory activity, different scenarios were used to evaluate the performance of these markers and an algorithm is proposed.
DiscussionCombined analysis of FC and CRP, when performed consecutively, allows decisions to be made with a high degree of certainty and even eliminates the need for colonoscopy in many situations.
Un adecuado control del proceso inflamatorio en la enfermedad de Crohn (EC) supone menores tasas de complicaciones. El objetivo de este estudio es evaluar la utilidad de la calprotectina fecal (CF) y los niveles séricos de la proteína C-reactiva (PCR), aisladamente o en combinación, como marcadores de actividad inflamatoria en la EC, así como la posibilidad de ser utilizados como objetivo terapéutico.
Pacientes y métodosSe incluyeron prospectivamente en el estudio pacientes con EC e indicación para colonoscopia siendo distribuidos de acuerdo a la presencia o no de actividad inflamatoria endoscópica. Se determinó la correlación entre CF y niveles de PCR con el índice SES-CD, y se evaluó la precisión de estos marcadores en el diagnóstico de la actividad inflamatoria, utilizados individualmente o en combinación.
ResultadosSe realizaron un total de 80 colonoscopias en pacientes con EC. Para la CF, el punto de corte de 155μg/g mostró una elevada sensibilidad del 96% y una especificidad del 78% en el diagnóstico de actividad endoscópica. En cuanto a la PCR, el valor de 6,7mg/l proporcionó una sensibilidad del 75% y una especificidad del 67%. El uso combinado de estos marcadores (CF+PCR) obtuvo mayor especificidad (82%) cuando se comparó con su utilización individual. De acuerdo al riesgo de actividad inflamatoria, se manejaron diferentes escenarios para evaluar la eficacia de estos marcadores y se propuso un algoritmo de uso.
DiscusiónLa monitorización conjunta de CF y PCR, cuando se realiza de forma consecutiva, permite tomar decisiones con un mayor grado de certeza, eliminando, incluso, la necesidad de colonoscopia en muchas situaciones.
Crohn's disease (CD) is a recurrent chronic disease which evolves with periods of activity and remission. It is often manifested by abdominal pain, fever, diarrhea, and weight loss.1,2 The clinical course of the disease is variable and the chronic inflammatory activity is responsible for the permanent structural damage of the intestine, even in the patients with prolonged clinical remission.3–7 Effective control of the inflammatory process has a direct effect on the healing of the intestinal mucosa. The healed mucosa, in turn, is related to lower rates of clinical recurrence, complications, hospitalizations or need for surgical treatment.8–13 Therefore, in order to achieve a good control of the disease, it is necessary to have methods that allow accurate assessment of the grade of intestinal inflammation, since in clinical practice, effective, objective and regular reassessments of the inflammatory process are necessary to guide the therapy.3,14,15
Ileocolonoscopy with biopsies of the intestinal segments remains as the standard procedure for evaluating the inflammatory activity in CD. However, despite the utility of the endoscopic exams, they present a number of drawbacks, as they are invasive, have a high cost and present risks related to the anesthetic procedure, possibility of bleeding and of intestinal perforation.16,17 Moreover, they are not always well accepted by patients.11
The ideal marker for evaluating inflammatory activity in CD should be less or non-invasive and have low cost, wide availability and good accuracy.17 In this context, serum measurement of C-reactive protein (CRP) and fecal measurement of calprotectin (FC) have been intensively studied.
Serum CRP, on the one hand, not only increases in cases of inflammation, infection and tissue damage, but also has a short half-life and has been proved to be useful in the successive evaluation of inflammatory processes.18 In addition, it is a widely available marker since it is identified by means of an easily performed blood test. However, it is not specific and can be elevated in response to any inflammatory and/or infectious process, which means that it is not an exclusive marker of inflammatory bowel disease. Furthermore, approximately 15% of the population does not present elevation of this protein, not even during current inflammatory conditions.18–20
On the other hand, fecal markers, such as FC and lactoferrin, are proteins found in the stools. Their measurements are considered useful because of the following characteristics: are non-invasive, present no risk to patients, have relatively low cost, and present high specificity for inflammatory bowel processes; in addition, their levels have a good correlation with endoscopic findings, as demonstrated in several studies.17,21 These markers are recommended by scientific societies such as Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) and European Crohn's and Colitis Organization (ECCO) for diagnosis and monitoring of IBD.22,23 However, the ideal cutoff value is still discussed24 and there are false positive results, as in patients with intestinal infections, colorectal neoplasia and use of nonsteroidal anti-inflammatory drugs.17
The use of CRP and FC in the diagnosis of inflammatory activity in CD is well established.16,18,24–26 However, the use of these two tests in series allows the creation of a third method of evaluation of the inflammatory activity, with sensitivity and specificity different than the ones found with the isolated use of each of them. In this case, there is loss of sensitivity and increased specificity when compared to the two separate exams. Nevertheless, there are still doubts regarding the best cutoff values and real benefit of the sequential use of these tests.
The present study aimed to define the best cutoff values of FC and serum CRP for the diagnosis of inflammatory activity of CD, as well as their respective sensitivity and specificity, using as reference, the Simplified Endoscopic Score of Crohn's Disease (SES-CD). We also calculated the sensitivity and specificity of the combined serial use of FC+CRP. Moreover, we suggest some hypothetical clinical scenarios to evaluate the performance of FC and CRP tests, used alone and consecutively, according to different prevalence of inflammation, proposing an algorithm to evaluate the inflammatory activity in CD.
Patients and methodsParticipantsFrom November 2011 to June 2016, a total of 65 patients >18 years old, who were previously diagnosed with CD (active or in remission) and had indication to undergo colonoscopy were prospectively included in this study. These patients were selected from the outpatients attended at the Intestine Outpatient Clinic and from those who were hospitalized in the Gastroenterology Ward of the Instituto Alfa de Gastroenterologia of the Hospital das Clínicas da Universidade Federal de Minas Gerais (IAG-HC/UFMG). Of the 65 cases included, 13 underwent colonoscopy on two occasions and the procedure was repeated in one patient at three different moments, totaling 80 endoscopic procedures. Those who presented any of the following criteria were excluded from the study: refusal to undergo colonoscopy; inadequate intestinal preparation that hindered the evaluation of the mucosa; incomplete examination due to technical difficulties; failure to submit stool samples for the FC test; use of non-steroidal anti-inflammatory drugs; diagnosis of colorectal malignancy; presence of ostomy; or history of previous ileocolectomy.
The present study was approved by the Research Ethics Committee of the Universidade Federal de Minas Gerais (protocol number ETIC 0070.0.203.000-11) and all the patients signed the inform consent form.
Clinical and laboratory evaluationBefore being referred to the colonoscopy, the patients selected for the study were submitted to an interview and a complete clinical examination, both carried out by one of the researchers. The Crohn's Disease Activity Index (CDAI) was used for quantification of inflammatory activity.27 The measurement of CRP serum concentrations and the determination of FC levels were performed at the same timepoint, during clinical evaluation, seven days before colonoscopy. CRP was measured by conventional assay at the laboratory of the institution and FC by enzyme-linked immunosorbent assay (ELISA) using a commercial kit (BÜHLMANN Laboratories AG®, Switzerland) according to the manufacturer's instructions. This test detection range varies between 30μg/g and 1800μg/g. Therefore, for purpose of the statistical analysis, these values were assigned to the samples whose concentrations were ≤30μg/g and ≥1800μg/g, respectively.
Endoscopic evaluationA single endoscopist (RMR), experienced in applying endoscopic scores with Fujifilm's high-definition device (Fujifilm Co., Japan), performed the colonoscopic examinations. He was blinded to the clinical evaluation of the CD inflammatory activity, as well as to the results of the CRP and FC tests. In order to quantify the endoscopic inflammatory activity, we used the Simplified Endoscopic CD Score (SES-CD)28 defining as “in remission” the patients with scores between 0 and 2 and “with active CD” those with SES-CD ≥3, as recommended by the International Organization of Inflammatory Bowel Diseases29 and by Koutroumpakis.30
Statistical analysisThe sample size was estimated in at least 77 patients, 51 of them with inflammatory activity. This would achieve a power of 81% to detect a sensitivity ranging from 74% to 89% in the diagnosis of CD inflammatory activity based on the FC test. The bilateral binomial test was used for this calculation, with significance level of 0.05 and prevalence of CD inflammatory activity of 66%. The sensitivity range was obtained from a meta-analysis that assessed the specificity and sensitivity of FC test in the diagnosis of CD activity.
Categorical data are presented as numbers and percentages and continuous data are expressed as median and interquartile range (IQR, P25−P75) as they do not have normal distribution. The Spearman's rank correlation test was used in the correlation analysis between the noninvasive markers of inflammatory activity and the endoscopic score. The Mann–Whitney test, in turn, was used for comparison of the medians between the two groups (patients in remission versus patients in activity). Finally, the Fisher's exact test, chi-square test and Z-test were used to analyze association of categorical variables between groups.
The cutoff values of CRP and FC were obtained by means of receiver operating characteristic (ROC) curves. For CRP, we selected the cutoff value that presented the highest sum of sensitivity and specificity, corresponding to the Youden Index. For FC, in addition to the highest Youden Index, the selected value was the one that favored sensitivity, given that it allowed the highest number of diagnosis of disease activity. Sensitivity and specificity of the combined serial use of FC and CRP, FC+CRP, were easily calculated, as follows: sensitivity=sensitivity of FC×sensitivity of CRP; and specificity=(specificity of FC+specificity of CRP)−(specificity of FC×specificity of CRP). According to the combined evaluation, the final result is considered positive when both tests show positive results. In contrast, if any of them shows a negative result, the final result is considered negative.
In order to evaluate the performance of each of the three tests (FC, CRP, and FC+CRP), probabilities of clinical activity were defined as mild (25%), moderate (50%) and severe (75%). The definition of these clinical activity probability values was based on the clinical presentation (diarrhea, abdominal pain, weight loss, anemia), impact of the disease on the patient's life, and course of CD, in each individual, as proposed by Peyrin-Biroulet.31 This approach allows discussing the usefulness of these tests in daily clinical practice.
After evaluating association between the outcome variable (SES-CD) and the possible explanatory variables (age, gender, disease location, age at diagnosis and behavior of CD) by means of univariate analysis, only the variable age was associated with the p value (p<0.20) selected for including a variable in the multivariate model. Therefore, there was no adjustment in the multivariate analysis.
P values <0.05 were regarded as statistically significant. Statistical analysis was performed using SPSS statistical package version 18 (SPSS Inc., Chicago, Illinois).
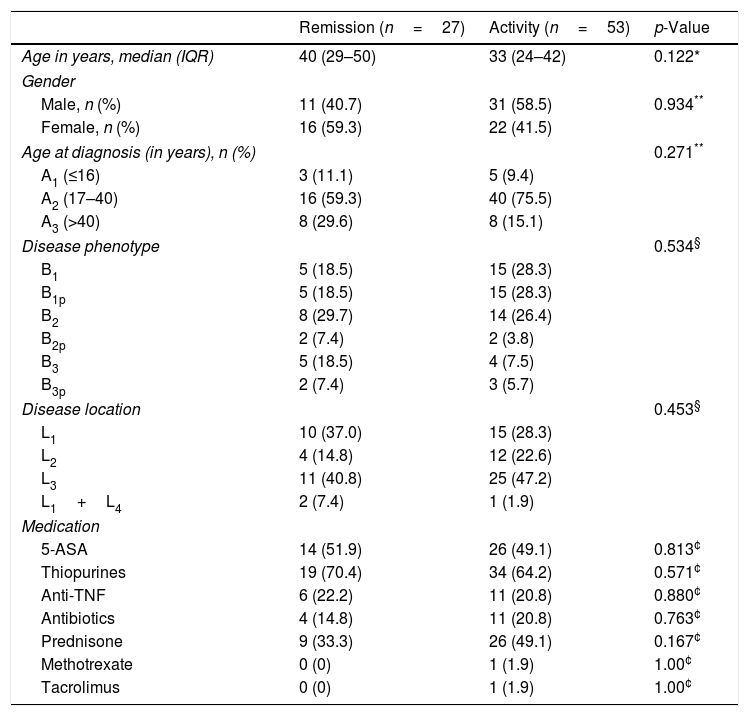
ResultsClinical characteristics of the patientsAmong the 65 patients with CD included in this study, 52.5% were males and the median age was 34 years (IQR, 25–44). Using the Montreal Classification32 and considering the baseline treatment of the patients, the epidemiologic and phenotypic profiles of CD are summarized in Table 1.
Clinical characteristics of the patients with Crohn's disease.
| Remission (n=27) | Activity (n=53) | p-Value | |
|---|---|---|---|
| Age in years, median (IQR) | 40 (29–50) | 33 (24–42) | 0.122* |
| Gender | |||
| Male, n (%) | 11 (40.7) | 31 (58.5) | 0.934** |
| Female, n (%) | 16 (59.3) | 22 (41.5) | |
| Age at diagnosis (in years), n (%) | 0.271** | ||
| A1 (≤16) | 3 (11.1) | 5 (9.4) | |
| A2 (17–40) | 16 (59.3) | 40 (75.5) | |
| A3 (>40) | 8 (29.6) | 8 (15.1) | |
| Disease phenotype | 0.534§ | ||
| B1 | 5 (18.5) | 15 (28.3) | |
| B1p | 5 (18.5) | 15 (28.3) | |
| B2 | 8 (29.7) | 14 (26.4) | |
| B2p | 2 (7.4) | 2 (3.8) | |
| B3 | 5 (18.5) | 4 (7.5) | |
| B3p | 2 (7.4) | 3 (5.7) | |
| Disease location | 0.453§ | ||
| L1 | 10 (37.0) | 15 (28.3) | |
| L2 | 4 (14.8) | 12 (22.6) | |
| L3 | 11 (40.8) | 25 (47.2) | |
| L1+L4 | 2 (7.4) | 1 (1.9) | |
| Medication | |||
| 5-ASA | 14 (51.9) | 26 (49.1) | 0.813¢ |
| Thiopurines | 19 (70.4) | 34 (64.2) | 0.571¢ |
| Anti-TNF | 6 (22.2) | 11 (20.8) | 0.880¢ |
| Antibiotics | 4 (14.8) | 11 (20.8) | 0.763¢ |
| Prednisone | 9 (33.3) | 26 (49.1) | 0.167¢ |
| Methotrexate | 0 (0) | 1 (1.9) | 1.00¢ |
| Tacrolimus | 0 (0) | 1 (1.9) | 1.00¢ |
Z test.
Age at diagnosis: A1: <16 years; A2: between 17 and 40 years; A3: >40 years.
Phenotype: B1: inflammatory; B2: stenosing; B3: penetrating; p: perianal.
Location: L1: terminal ileus; L2: colon; L3: ileocolonic; L4: upper gastrointestinal tract.
Anti-TNF, anti-tumor necrosis factor (infliximab, adalimumab); antibiotics (metronidazole, ciprofloxacin).
Comparing the SES-CD of the 80 colonoscopies with the CRP (r=0.525; p<0.001) and FC (r=0.450; p<0.001) levels, we found a moderate correlation.
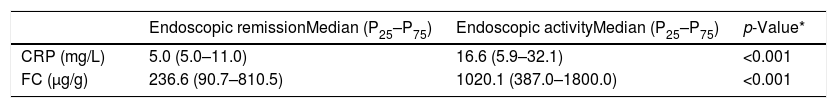
CRP and FC were evaluated in relation to their ability to diagnose inflammatory activity. The median values of these markers were compared between the patients with endoscopic remission (SES-CD=0–2) and those with activity (SES-CD>3) (Table 2). Both tests demonstrated significantly higher levels in the group with endoscopic activity.
Median values and interquartile range of C-reactive protein (CRP) and fecal calprotectin (FC) in patients with endoscopic remission and in patients with endoscopic activity (SES-CD>3).
| Endoscopic remissionMedian (P25–P75) | Endoscopic activityMedian (P25–P75) | p-Value* | |
|---|---|---|---|
| CRP (mg/L) | 5.0 (5.0–11.0) | 16.6 (5.9–32.1) | <0.001 |
| FC (μg/g) | 236.6 (90.7–810.5) | 1020.1 (387.0–1800.0) | <0.001 |
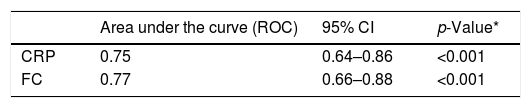
The values of the accuracy of CRP and FC tests for the diagnosis of inflammatory activity were obtained using the area under the ROC curve (Table 3).
Accuracy of C-reactive protein (CRP) and fecal calprotectin (FC) for the diagnosis of inflammatory activity in Crohn's disease.
| Area under the curve (ROC) | 95% CI | p-Value* | |
|---|---|---|---|
| CRP | 0.75 | 0.64–0.86 | <0.001 |
| FC | 0.77 | 0.66–0.88 | <0.001 |
CRP: C-reactive protein; FC: fecal calprotectin. CI 95%: 95% confidence interval.
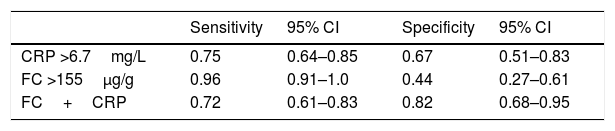
For CRP, the cut off value 6.7mg/L presented the best Youden Index. For FC, the cut off 155μg/g was associated with a very high sensitivity that allows the diagnosis of almost all patients with active CD that formed our sample. Based on these values, the sensitivity and specificity of the combined serial test of FC+CRP were calculated (Table 4). We observed that the combined evaluation of these two markers showed lower sensitivity and higher specificity when compared with each test individually.
Sensitivity and specificity of CRP and FC tests alone and combined in series (FC+CRP) for diagnosing inflammatory activity in Crohn's disease.
| Sensitivity | 95% CI | Specificity | 95% CI | |
|---|---|---|---|---|
| CRP >6.7mg/L | 0.75 | 0.64–0.85 | 0.67 | 0.51–0.83 |
| FC >155μg/g | 0.96 | 0.91–1.0 | 0.44 | 0.27–0.61 |
| FC+CRP | 0.72 | 0.61–0.83 | 0.82 | 0.68–0.95 |
CRP: C-reactive protein; FC: fecal calprotectin; FC+CRP: combined tests in series FC and CRP; 95% CI: 95% confidence interval.
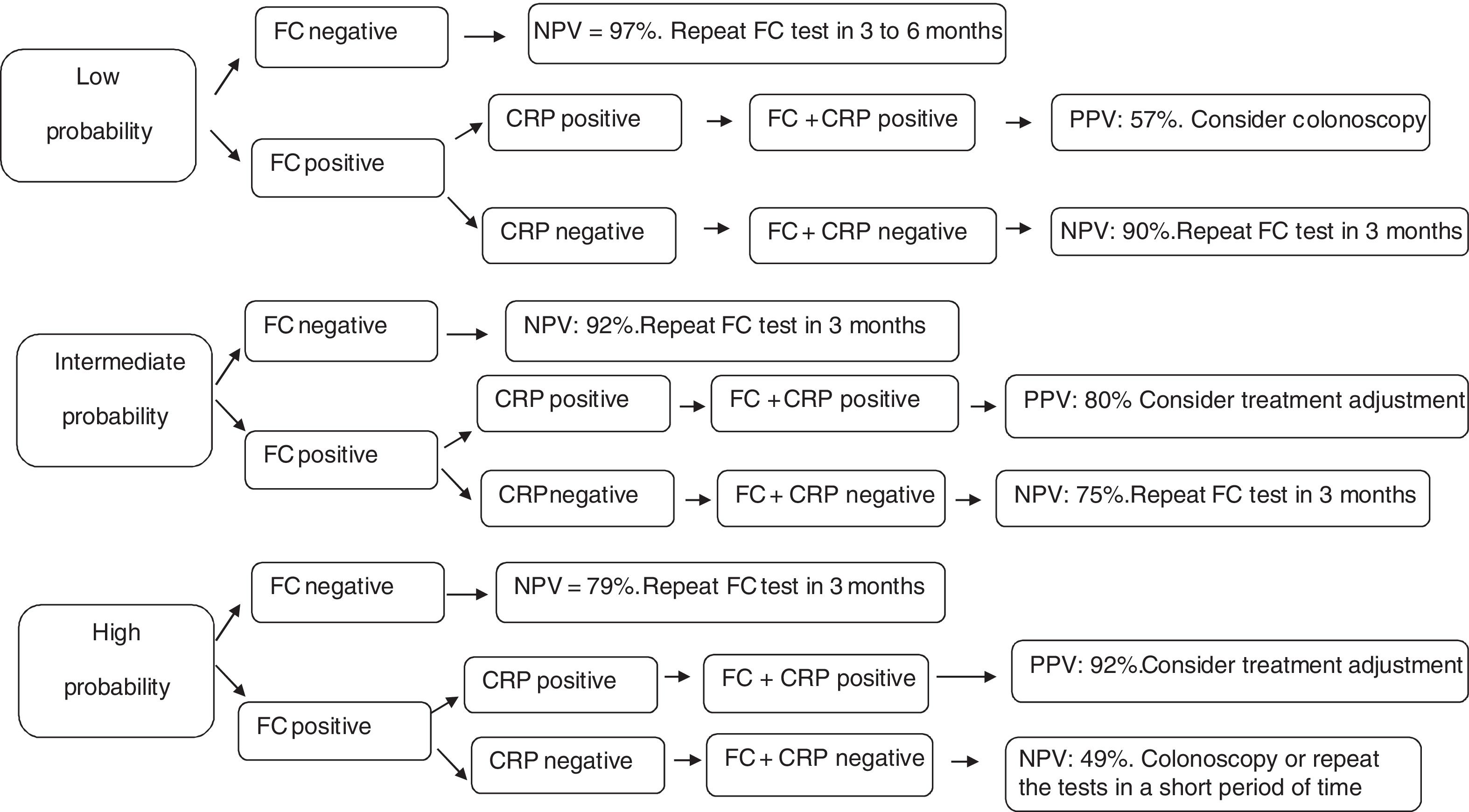
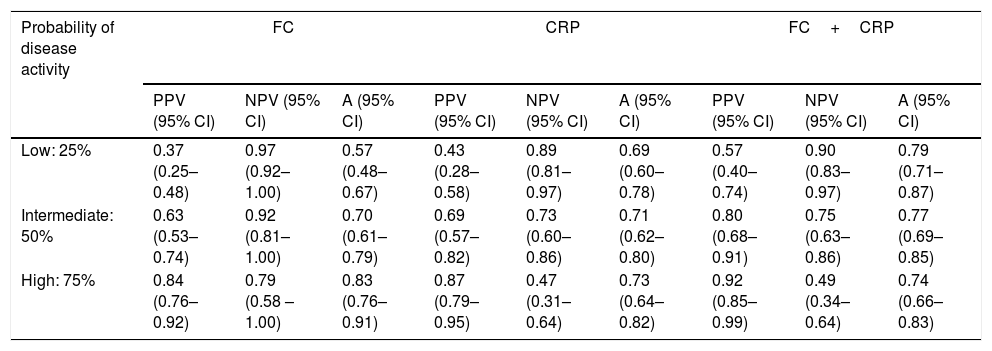
Using the hypothetical evaluation of three different scenarios, which were grouped into low probability of active disease (indicated by pretest probability of 25%), intermediate probability of active disease (indicated by pretest probability of 50%), and high probability of active disease (indicated by pretest probability of 75%), we calculated the predictive values as well as the accuracy of each of these markers in these scenarios (Table 5).
Hypothetical scenarios according to different probabilities of Crohn's disease activity and the respective values of positive predictive, negative predictive and accuracy.
| Probability of disease activity | FC | CRP | FC+CRP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PPV (95% CI) | NPV (95% CI) | A (95% CI) | PPV (95% CI) | NPV (95% CI) | A (95% CI) | PPV (95% CI) | NPV (95% CI) | A (95% CI) | |
| Low: 25% | 0.37 (0.25–0.48) | 0.97 (0.92–1.00) | 0.57 (0.48–0.67) | 0.43 (0.28–0.58) | 0.89 (0.81–0.97) | 0.69 (0.60–0.78) | 0.57 (0.40–0.74) | 0.90 (0.83–0.97) | 0.79 (0.71–0.87) |
| Intermediate: 50% | 0.63 (0.53–0.74) | 0.92 (0.81–1.00) | 0.70 (0.61–0.79) | 0.69 (0.57–0.82) | 0.73 (0.60–0.86) | 0.71 (0.62–0.80) | 0.80 (0.68–0.91) | 0.75 (0.63–0.86) | 0.77 (0.69–0.85) |
| High: 75% | 0.84 (0.76–0.92) | 0.79 (0.58 – 1.00) | 0.83 (0.76–0.91) | 0.87 (0.79–0.95) | 0.47 (0.31–0.64) | 0.73 (0.64–0.82) | 0.92 (0.85–0.99) | 0.49 (0.34–0.64) | 0.74 (0.66–0.83) |
PPV: positive predictive value; NPV: negative predictive value; A: accuracy; 95% CI: 95% confidence interval; CRP: C-reactive protein; FC: fecal calprotectin; FC+CRP: consecutive FC and CRP tests.
The diagnosis and follow-up of patients with inflammatory bowel disease is often complex and require a combination of clinical, laboratory, endoscopic, histopathological and radiological parameters.2,15,33,34 The recognition of inflammatory activity, especially in CD, is often delayed or underestimated because the methods currently available are non-specific or invasive.14,19,20,35 The existence of non-invasive tests that allow diagnosing inflammatory activity in patients with CD is highly valuable for clinical practice.18
The evaluation of the intestinal mucosa by colonoscopy remains the standard test for the diagnosis of inflammatory activity in CD. Mucosal healing is the therapeutic goal since it is associated with a better prognosis and a lower rate of complications.3,13,15,36 However, colonoscopy is invasive, has limited availability and high cost. Thus, it is necessary to find markers that improve diagnostic accuracy, are non-invasive and widely available.17 This study demonstrated a moderate correlation between CRP and FC levels and SES-CD values (r=0.525 and r=0.450, respectively). Similar results were reported by Sipponen et al. who evaluated 87 patients with CD and found a good correlation between SES-CD and FC (r=0.662) and CRP (r=0.522) values.25 Subsequently, other studies, such as those by Schoepfer et al.,16 Lobatón et al.,37 and Lin et al.,38 confirmed these findings.
In many studies, FC and CPR levels have been used individually to diagnose inflammatory activity in CD. Sensitivity and specificity values are diverse and vary according to the cut-off values selected.16,18,24,25,37–40 In this study, the median values of FC were significantly different between patients in remission and those in activity (236.6μg/g and 1020.1μg/g, respectively, p<0.001) and the same result was observed for CRP (median levels in remission: 5.0mg/L and in activity: 16.7mg/L; p<0.001). Similar results were also found by other authors.16,25,38,41
The analysis of the area under the ROC curve showed good accuracy of the CRP and FC levels to diagnose inflammatory activity in CD. The cut-off values of these markers were obtained from the ROC curves. The CRP value of 6.7mg/L presented the highest Youden Index and the FC value of of 155μg/g presented high sensitivity to detect inflammatory activity. In a recent meta-analysis, which included 19 studies in which FC and CRP tests were evaluated for detecting inflammatory activity in CD, the cut-off values of FC ranged from 6 to 280μg/g and those of CRP ranged from 5 to 10mg/L.42
In order to increase the diagnostic performance of the non-invasive tests in monitoring CD activity, some authors studied the usefulness of these markers in a combined manner. Langhorst et al. 43 proposed a combination of fecal markers (calprotectin and lactoferrin), serum CRP, and clinical parameters (CDAI).27 They considered as positive (presence of activity), when at least two of these three markers were present. They observed no improvement in the diagnostic accuracy of the combined tests when compared with the individual use of FC in patients with CD.43 Later, Bjorkesten et al.44 analyzed the efficacy of the combination of CRP, FC, CDAI, and the Harvey-Bradshaw Index (HBI)45 to detect inflammatory activity in CD. The use of HBI and FC simultaneously presented a diagnostic accuracy of 88%,44 which was superior to any other index used alone or in combination. Recently, Bodelier et al.46 used CRP and/or HBI to diagnose inflammatory activity in CD, when FC was not positive. They observed improvement of the sensitivity with the use of the combined score (CPR+HBI), which was especially useful in the patients with moderate values of FC (range 100–250μg/g).46 The combined use of CRP and FC was evaluated by Garcia-Planella et al.47 in a study including CD patients in posteperative setting. The cut-off values of FC and CRP of, respectively, 100mcg/g and 5mg/L, when used together, showed sensitivity of 82% and specificity of 53%. There was also an improvement in sensitivity and negative predictive value when these markers were used together, and the authors observed that colonoscopy could have been avoided in 39% of cases.
A recent study demonstrated that the treatment of patients with CD based on clinical evaluation and biomarkers (CRP and FC) presented better results when compared with treatments based on symptoms alone.9 However, in that study, there was no comparison between the use of the biomarkers alone and in combination. In addition, there was no stratification of the CD patients according to the clinical activity of the disease.
In the present study, the sequential use of FC and CRP revealed a good specificity (82%). This is especially useful because when used separately, they provided better sensitivity than specificity. Thus, the use of these markers in combination allows for the best definition of CD activity.
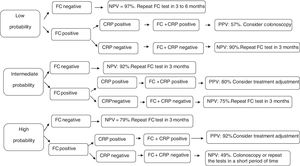
To investigate the efficacy of these tests, three clinical scenarios were established in the present study as proposed by Mosli et al.18 and an algorithm was developed (Fig. 1). A patient with low probability of active disease (pretest probability 25%) is the one that presents no or mild clinical symptoms (abdominal discomfort and/or increased evacuation frequency) without impairment of quality of life. On the other hand, high probability of active disease (pretest probability 75%), refers to the patients who present diarrhea, weight loss, abdominal pain, impaired quality of life, and frequent relapses. Intermediate probability of active disease (pretest probability 50%) is recognized as the intermediate scenario in comparison to those described above.
Follow-up decision algorithm of patients with Crohn's disease considering the pretest probability of clinical disease activity. PPV: positive predictive value; NPV: negative predictive value; CRP: C-reactive protein; FC: fecal calprotectin; FC+CRP: combined tests in series FC and CRP. FC negative: FC<155mcg/g; FC positive: FC>155mcg/g; CRP negative: CRP<6.7mg/L; CRP positive: CRP>6.7mg/L.
In the first cenario, only patients with both biomarkers positive should undergo colonoscopy. The remaining may be followed up by repeating CF test in three to six months. Considering the patients with high probability of active disease, only those with negative CF test could be followed up by repeating the biomarkers measurements. Colonoscopy or treatment ajustment shoud be strongely considered for the others. Finally, patients with intermediate probability of active disease should undergo treatment ajustment only if both biomarkers are positive. In the other situations, they may be followed up by repeating the markers in three months.
This study has potential limitations. Firstly, we did not perform serial measurements of FC, which could increase the accuracy of this test. Secondly, we did not use enterography to examine the small intestine. This could provide more reliable conclusions about CD inflammatory activity. However, all patients included in this study had ileal or ileocolonic involvement, allowing us to infer that ileocolonoscopy was sufficient to diagnose inflammatory activity. Lastly, the number of patients in endoscopic remission should be higher as evidenced by the large confidence intervals identified for the values of specificity. However, this number of patients was estimated before the beginning of the study.
In conclusion, this study showed that FC and CRP tests are useful to differentiate patients in remission from those in activity, because these tests presented good accuracy in the diagnosis of inflammatory activity in CD. In addition, the combined analysis of these exams, when they are performed consecutively (FC+CRP), allows decision-making with a great degree of certainty, eliminating the necessity of colonoscopy in many situations. Further studies are desirable to evaluate the efficacy of the proposed algorithm in a “treat-to-target” strategy implementation.
FundingNone.
Conflicts of interestNone declared.






 155mcg/g; CRP negative: CRP<6.7mg/L; CRP positive: CRP>6.7mg/L.' title='Follow-up decision algorithm of patients with Crohn's disease considering the pretest probability of clinical disease activity. PPV: positive predictive value; NPV: negative predictive value; CRP: C-reactive protein; FC: fecal calprotectin; FC+CRP: combined tests in series FC and CRP. FC negative: FC<155mcg/g; FC positive: FC>155mcg/g; CRP negative: CRP<6.7mg/L; CRP positive: CRP>6.7mg/L.'/>
155mcg/g; CRP negative: CRP<6.7mg/L; CRP positive: CRP>6.7mg/L.' title='Follow-up decision algorithm of patients with Crohn's disease considering the pretest probability of clinical disease activity. PPV: positive predictive value; NPV: negative predictive value; CRP: C-reactive protein; FC: fecal calprotectin; FC+CRP: combined tests in series FC and CRP. FC negative: FC<155mcg/g; FC positive: FC>155mcg/g; CRP negative: CRP<6.7mg/L; CRP positive: CRP>6.7mg/L.'/>
