Recent evidence suggests that the number of low residue diet (LRD) days does not influence the bowel cleansing quality in non-selected patients. However, there are not data in the subgroup of patients with risk factors of inadequate bowel cleansing.
ObjectiveThe aim of this study was to assess whether a 3-day LRD improved the bowel cleansing quality in patients with risk factors of poor bowel cleansing.
Patients and methodsPost hoc analysis of a randomized controlled trial carried out between December 2017 and March 2018 in a tertiary care hospital. Patients with high risk of poor bowel cleansing were selected following a validated score. The patients were randomized to the 1-day LRD or 3-day LRD groups. All patients received a 2-L split-dose of polyethylene glycol plus ascorbic acid. Intention-to-treat (ITT) and per-protocol (PP) analyses were conducted for the main outcome.
Results135 patients (1-day LRD group=67, 3-day LRD=68) were included. The rate of adequate cleansing quality was not significantly different between the groups in the ITT analysis: 76.1%, 95% CI: [64.6–84.8] vs. 79.4%, 95% CI: [68.2–87.4]; odds ratio (OR) 1.2, 95% CI [0.54–2.73]) or in the PP analysis: 77.3%, 95% CI: [65.7–85.8] vs. 80.3%, 95% CI: [69.0–88.3]; OR 1.2, 95% CI [0.52–2.77]). Compliance with the diet or cleansing solution, satisfaction or difficulties with the LRD and the polyp/adenoma detection rates were not significantly different.
ConclusionOur results suggest that 1-day LRD is not inferior to 3-day LRD in patients with risk factors of inadequate bowel cleansing.
La evidencia reciente sugiere que el número de días de dieta baja en residuos (DBR) no influye en la calidad de la limpieza intestinal en pacientes no seleccionados. Sin embargo, no hay datos en el subgrupo de pacientes con factores de riesgo de una limpieza intestinal insuficiente.
ObjetivoEl objetivo de este estudio fue evaluar si una DBR de 3 días mejoraba o no la calidad de la limpieza intestinal en pacientes con factores de riesgo de limpieza intestinal deficiente.
Pacientes y métodosAnálisis post-hoc de un ensayo controlado aleatorizado realizado entre diciembre de 2017 y marzo de 2018 en un hospital de atención terciaria. Los pacientes con alto riesgo de limpieza intestinal deficiente se seleccionaron mediante una puntuación validada. Los pacientes se aleatorizaron a los grupos de DBR de un día o DBR de 3 días. Todos los pacientes recibieron una dosis dividida de 2l de polietilenglicol más ácido ascórbico. Se realizaron análisis por intención de tratar (IdT) y por protocolo (PP) para el criterio principal de valoración.
ResultadosSe incluyeron 135 pacientes (grupo DBR de un día=67, DBR de 3 días=68). No se observaron diferencias significativas en la tasa de calidad de limpieza suficiente entre los grupos en el análisis por IdT (76,1%; IC del 95%: [64,6-84,8] frente al 79,4 7%, IC del 95%: [68,2-87,4]; razón de posibilidades (OR): 1,2; IC del 95%: [0,54-2,73]) o en el análisis PP: (77,3%; IC del 95 %: [65,7-85,8] frente al 80,3%, IC del 95%: [69,0-88,3]; OR: 1,2; IC del 95% [0,52 -2,77]). No se observaron diferencias significativas en el cumplimiento de la dieta o con la solución limpiadora, en la satisfacción o las dificultades con la DBR y en las tasas de detección de pólipos/adenomas.
ConclusiónNuestros resultados sugieren que la DBR de un día no es inferior a la DBR de 3 días en pacientes con factores de riesgo de limpieza intestinal insuficiente.
A high level of cleansing quality is mandatory for increasing colonoscopy efficiency either in a diagnostic or a screening setting. Current guidelines recommend a bowel cleansing adequate rate ranging from 85% to 95% of the outpatient colonoscopies in an endoscopy unit.1,2
Several factors have been associated with bowel cleansing quality including host related factors, preparation solution related factors and depending on the cleansing process.3 Whereas some of these factors are potentially modifiable by the implementation of educational strategies or modifications of the medication, others are not, such as comorbidities specially those involved in gastrointestinal motility (i.e. Diabetes Mellitus, cirrhosis, stroke, or chronic kidney disease). In patients with non-modifiable factors an adequate bowel preparation is difficult to achieve and when non-compliance has been ruled out, enhanced strategies are warranted.
A low residue diet (LRD) rather than a clear liquid diet has been recommended for US and European societies.1,4 It seems to increase tolerance, compliance with the bowel preparation protocol and satisfaction of the patient without undermining bowel cleansing quality. The European Society of Gastrointestinal Endoscopy (ESGE) guideline stated that there was no evidence to recommend a LRD for more than 24h.4 Recently, a randomized controlled trial carried out by our group compared 1 day structured LRD with 3 day structured LRD in 404 consecutive outpatients scheduled for colonoscopy.5 In this study there was no statistically significant differences between both groups in cleansing quality, supporting the recommendations of the ESGE guideline. However, the fact that the number of LRD days were not associated with a greater cleansing quality in non-selected outpatients do not necessarily imply that it is not an effective strategy in hard to prepare patients that accounts for a minority of the total patients scheduled for an outpatient colonoscopy.
This is a post hoc analysis of a randomized controlled trial and the purpose was to evaluate the effectiveness of a structured and reproducible 3-day LRD compared to a 1-day LRD on colon cleansing of hard to prepare outpatients scheduled for colonoscopy.
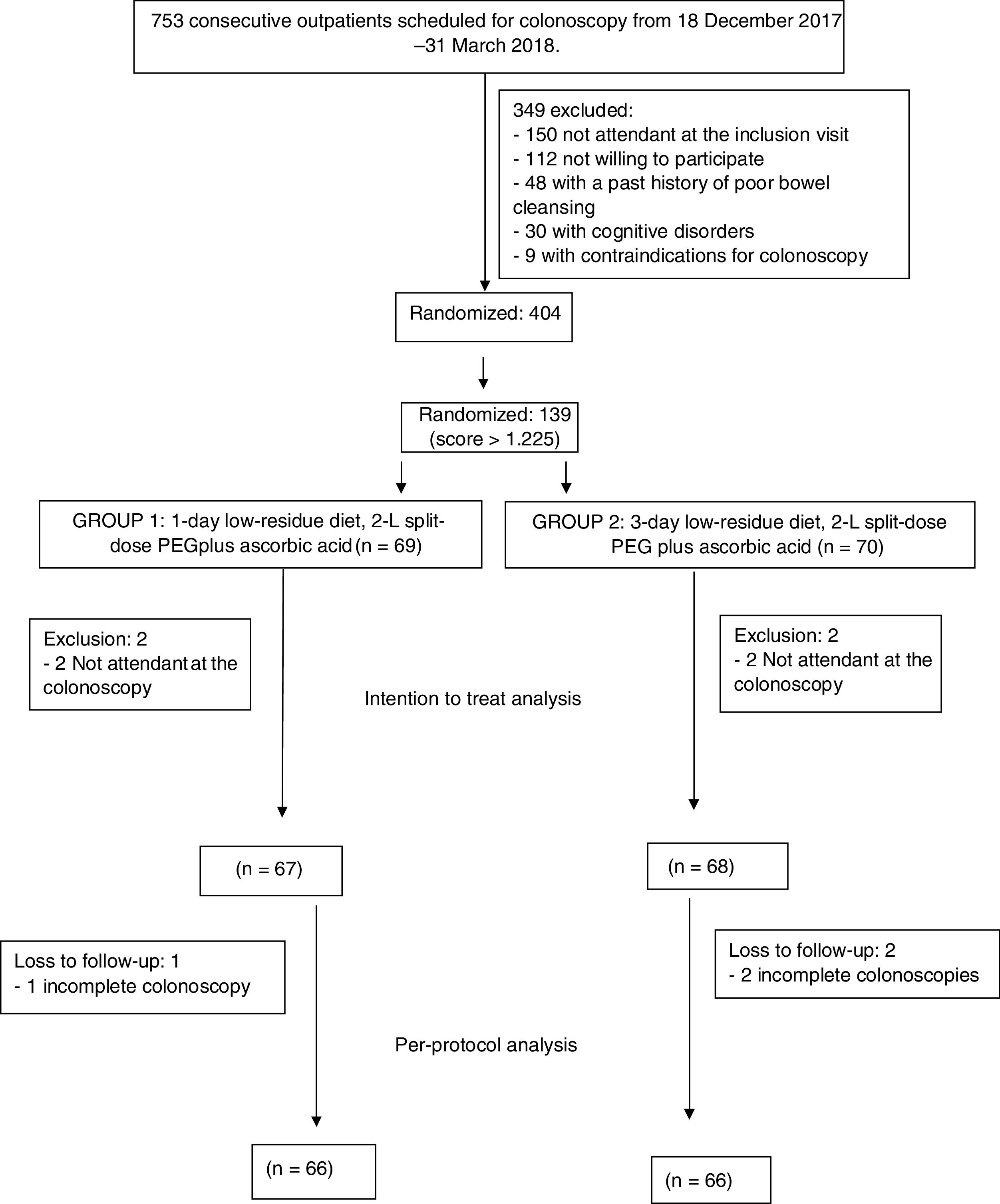
MethodsSettingThis is a study nested within a prospective randomized controlled study conducted at the Open Access Endoscopy Unit of the University Hospital of Canary Islands between December 2017 and March 2018. About 3000 colonoscopies are performed a year in our unit in the morning. Our hospital is a tertiary referral centre with a population area of 400,000 inhabitants. In this randomized controlled trial, 404 consecutive outpatients of the North area of the island of Tenerife scheduled for a colonoscopy were included. The subgroup of patients with high risk of inadequate bowel cleansing was chosen for the present study.
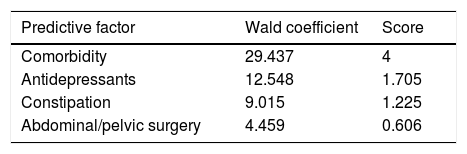
PatientsPatients older than 18 years scheduled for outpatient colonoscopy in the morning shift were considered for inclusion. Patients with contraindications for colonoscopy, a past history of inadequate bowel preparation, and refusal to participate were excluded. The full details can be consulted.5 The original protocol was approved by the Local Ethics Committee of Hospital Universitario de Canarias (NCT03247452). For the purpose of the present study, only patients with a high risk of inadequate bowel cleansing were chosen. A validated predictive score was used to select these patients.6 This score includes well-known factors related to poor bowel cleansing, and can be easily calculated (Table 1): antidepressants, comorbidity, constipation and abdominal/pelvic surgery. In a validation cohort this model showed an area under the curve (AUC) of 0.70. The optimal cut-off was 1.225 and predicted inadequate bowel preparation with a negative predictive value of 88% and a positive predictive value of 36% suggesting that the score was acceptable for predicting an adequate bowel cleansing.
Predictive score of poor bowel preparation.6
| Predictive factor | Wald coefficient | Score |
|---|---|---|
| Comorbidity | 29.437 | 4 |
| Antidepressants | 12.548 | 1.705 |
| Constipation | 9.015 | 1.225 |
| Abdominal/pelvic surgery | 4.459 | 0.606 |
The procedures carried out in this study were previously described in detail. In brief, patients were randomized using a computer generated in a 1:1 sequence generated by a statistician of the Research Unit of our hospital. Comorbidities (diabetic patients on treatment, cirrhosis diagnosed by clinical, imaging or analytical criteria, stroke, or chronic kidney disease defined as renal glomerular filtration <60ml/min), history of abdominal or pelvic surgery, bowel habit (<3 bowel movements/week and at least one of the following: straining, hard stools defined as Bristol scale 1 or 2 and incomplete evacuation)7 and medication (treatment with tricyclic antidepressants, opioids or calcium antagonists) were collected. They were given written information about the 1-day LRD or 3-day LRD patients were advised to complete a food record sheet 1 or 3 days before colonoscopy depending on their group assignment. The diet recommendations were designed by an endocrinologist specialized in nutrition. Both groups were prepared with split-dose polyethylene glycol plus ascorbic acid (PEG+Asc)
Day of colonoscopyTwo nurses blinded to the allocation group collected the information regarding tolerance, satisfaction, difficulties, willingness to follow the same LRD in the future, and the volume of the bowel preparation ingested. The colonoscopies were performed by 4 experienced endoscopists blinded to the allocation group. Cleansing quality was assessed by the Boston Bowel Preparation Scale (BBPS).8 The endoscopists passed the BBPS Educational Program by obtaining a score ≥3 before the study commenced. Cleansing quality variables, colonoscopy findings and variables related were collected during the procedure. Adverse effects and incidents of the preparation protocol (solution and diet) were assessed according to the American Society of Gastrointestinal endoscopy (ASGE) lexicon.9 The withdrawal time from the caecum was collected using a stopwatch; the watch was stopped in cases requiring biopsies or polyp resection and then resumed.
OutcomesThe main outcome of this study was the bowel cleansing quality assessed by the BBPS.8
BBPS in complete colonoscopies:
- -
When the three colon segments were assessed and each one was scored ≥2 points, bowel cleansing was considered satisfactory.
- -
When the colonoscopy was complete in a patient with a segmental colon resection and the assessed segments scored ≥2 points, bowel cleansing was also considered satisfactory.
- -
The worst case was when at least one of the three segments was scored <2 points. Bowel cleansing was considered unsatisfactory.
BBPS in incomplete colonoscopies:
- -
When a segment was not assessed, bowel cleansing was considered unsatisfactory.
Rates of patients with adequate bowel cleansing were compared.
Secondary outcomesCompliance with the LRD was assessed by a modification of the validated Fat and Fibre Behaviour Questionnaire10 and by a personal food record. In case of non-compliance with the diet, was considered non-compliant.
The level of satisfaction with the LRD and the difficulty following the dietary recommendations were assessed using a 5-point subjective scale.11 Willingness to repeat the same LRD in the future was assessed as a dichotomous variable (yes/no).12
Adverse effects and incidents were assessed by asking the patients about events potentially related to the bowel preparation, such as nausea, vomiting, bloating and abdominal pain.
Statistical analysis and sample sizePatients with a bowel cleansing prediction score>1.225 were selected.
1-Day LRD group and 3-day LRD were compared using the Chi-square statistic for categorical variables and Student's t-test for continuous variables. Intention-to-treat (ITT) and per-protocol (PP) analyses were conducted.
An univariate and multivariate analysis were carried out to assess variables associated with poor bowel cleansing (BBPS<2 points in one or more segments). Variables that achieved at least P<0.10 were included in the final multiple logistic regression model. The results are expressed as odds rations (OR) with 95% confidence intervals (CI). P-values <0.05 were considered statistically significant. Statistical Package for Social Sciences v. 21.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
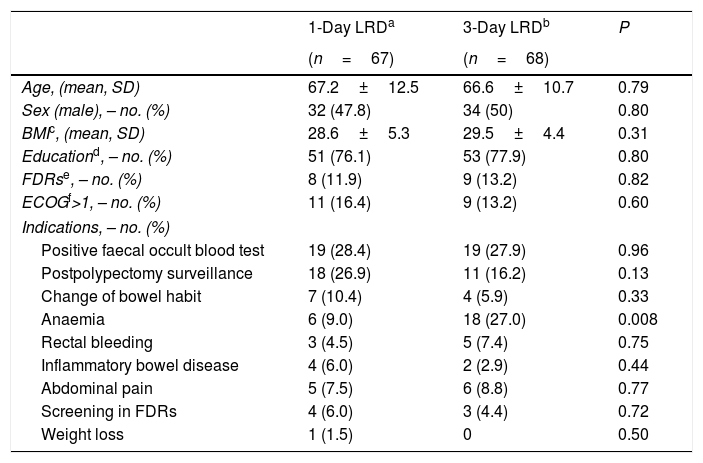
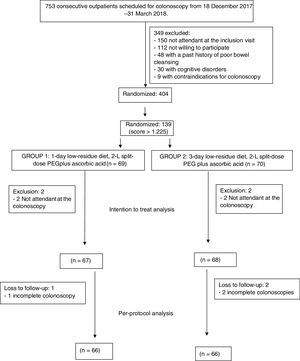
ResultsOverall, 404 consecutive patients were included in the study period. However, 139 patients had a score>1.225 and were included in this study. Four patients did not attend to the colonoscopy and were finally excluded after inclusion in the ITT analysis (2 in 1-day LRD and 2 in 3-day LRD). Finally, 135 patients were analyzed in the ITT analysis, 67 patients in the 1-day LRD and 68 patients in the 3-day LRD (Fig. 1). Both groups were comparable regarding baseline characteristics (Table 2, supplementary Table 1). There were not significant differences either in the time elapsed between the completion of the bowel preparation and the colonoscopy (1-day LRD mean 4.3h, 95% CI [4.0–4.4] vs. 3-day LRD mean 4.2h, 95% CI [4.1–4.5]). No patient took<75% of the bowel preparation.
Baseline characteristics of the patients.
| 1-Day LRDa | 3-Day LRDb | P | |
|---|---|---|---|
| (n=67) | (n=68) | ||
| Age, (mean, SD) | 67.2±12.5 | 66.6±10.7 | 0.79 |
| Sex (male), – no. (%) | 32 (47.8) | 34 (50) | 0.80 |
| BMIc, (mean, SD) | 28.6±5.3 | 29.5±4.4 | 0.31 |
| Educationd, – no. (%) | 51 (76.1) | 53 (77.9) | 0.80 |
| FDRse, – no. (%) | 8 (11.9) | 9 (13.2) | 0.82 |
| ECOGf>1, – no. (%) | 11 (16.4) | 9 (13.2) | 0.60 |
| Indications, – no. (%) | |||
| Positive faecal occult blood test | 19 (28.4) | 19 (27.9) | 0.96 |
| Postpolypectomy surveillance | 18 (26.9) | 11 (16.2) | 0.13 |
| Change of bowel habit | 7 (10.4) | 4 (5.9) | 0.33 |
| Anaemia | 6 (9.0) | 18 (27.0) | 0.008 |
| Rectal bleeding | 3 (4.5) | 5 (7.4) | 0.75 |
| Inflammatory bowel disease | 4 (6.0) | 2 (2.9) | 0.44 |
| Abdominal pain | 5 (7.5) | 6 (8.8) | 0.77 |
| Screening in FDRs | 4 (6.0) | 3 (4.4) | 0.72 |
| Weight loss | 1 (1.5) | 0 | 0.50 |
As expected, cleansing quality was better in patients with a score≤1.225 (N=255) compared with those with a score > 1.225 (N=135) either by the ITT analysis (86.7% vs. 77.8%, P=0.024, OR 1.86, 95% CI 1.08–3.19) or PP (89.2% vs. 78.8%, P=0.007, OR 2.22, 95% CI 1.24–3.97).
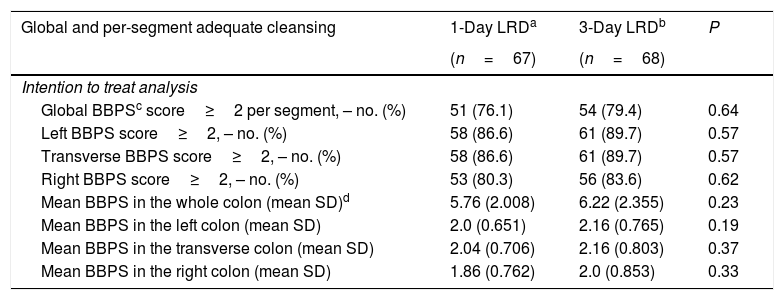
When only the patients with a score>1.225 were considered, quality of bowel cleansing according to the BBPS was comparable between those assigned to 1-day LRD and to 3-day LRD, either overall or per colon segments (Table 3). In the ITT analysis bowel cleansing was adequate in 76.1%, 95% CI: [64.6–84.8] of the patients assigned to the 1-day LRD and 79.4%, 95% CI: [68.2–87.4] of those to the 3-day LRD (odds ratio (OR) 1.2, 95% CI [0.54–2.73]). Percentages corresponding to the PP analysis were 77.3%, 95% CI: 65.7–85.8] for the 1-day LRD and 80.3%, 95% CI: [69.0–88.3] for the 3-day LRD (OR 1.2, 95% CI [0.52–2.77]).
Comparison of adequate bowel cleansing between study groups.
| Global and per-segment adequate cleansing | 1-Day LRDa | 3-Day LRDb | P |
|---|---|---|---|
| (n=67) | (n=68) | ||
| Intention to treat analysis | |||
| Global BBPSc score≥2 per segment, – no. (%) | 51 (76.1) | 54 (79.4) | 0.64 |
| Left BBPS score≥2, – no. (%) | 58 (86.6) | 61 (89.7) | 0.57 |
| Transverse BBPS score≥2, – no. (%) | 58 (86.6) | 61 (89.7) | 0.57 |
| Right BBPS score≥2, – no. (%) | 53 (80.3) | 56 (83.6) | 0.62 |
| Mean BBPS in the whole colon (mean SD)d | 5.76 (2.008) | 6.22 (2.355) | 0.23 |
| Mean BBPS in the left colon (mean SD) | 2.0 (0.651) | 2.16 (0.765) | 0.19 |
| Mean BBPS in the transverse colon (mean SD) | 2.04 (0.706) | 2.16 (0.803) | 0.37 |
| Mean BBPS in the right colon (mean SD) | 1.86 (0.762) | 2.0 (0.853) | 0.33 |
| (n=66) | (n=66) | ||
|---|---|---|---|
| Per-protocol analysis | |||
| Global BBPS score≥2 per segment, – no. (%) | 51 (77.3) | 53 (80.3) | 0.67 |
| Left BBPS score≥2, – no. (%) | 57 (86.4) | 59 (89.4) | 0.59 |
| Transverse BBPS score≥2, – no. (%) | 58 (87.9) | 60 (90.9) | 0.57 |
| Right BBPS score≥2, – no. (%) | 53 (81.5) | 56 (84.8) | 0.61 |
| Mean BBPS in the whole colon (mean, SD) | 5.85 (1.891) | 6.32 (2.261) | 0.20 |
| Mean BBPS in the left colon (mean, SD) | 2.0 (0.656) | 2.15 (0.769) | 0.23 |
| Mean BBPS in the transverse colon (mean SD) | 2.08 (0.664) | 2.18 (0.763) | 0.40 |
| Mean BBPS in the right colon (mean SD) | 1.89 (0.732) | 2.03 (0.822) | 0.31 |
No statistically significant differences between groups were found per colon segment either (Table 3).
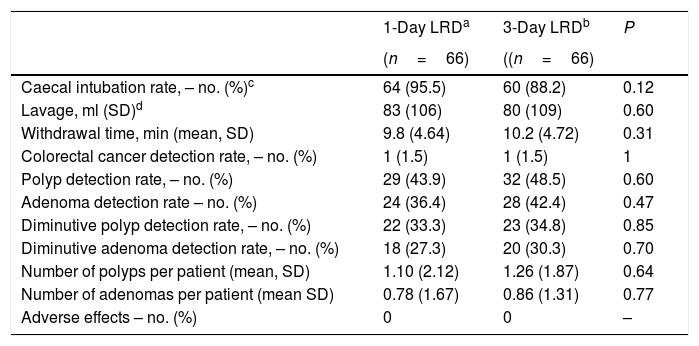
Colonoscopy findingsTable 4 shows colonoscopy findings. Cecum was not reached in 10 patients, being poor bowel preparation the main cause (5 patients in the 3-day LRD group and 2 in the 1-day LRD group). However, there were not statistical differences in the caecal intubation rate (95.5% vs. 88.3% in 1-day LRD and 3-day LRD, respectively), in the volume of liquid used and withdrawal time (Table 4). There were not statistically significant differences in the ADR (adenoma detection rate), PDR (polyp detection rate), diminutive ADR, diminutive PDR and number of polyps or adenomas per patient (Table 4). No adverse effects were derived from the colonoscopy procedures.
Colonoscopy findings.
| 1-Day LRDa | 3-Day LRDb | P | |
|---|---|---|---|
| (n=66) | ((n=66) | ||
| Caecal intubation rate, – no. (%)c | 64 (95.5) | 60 (88.2) | 0.12 |
| Lavage, ml (SD)d | 83 (106) | 80 (109) | 0.60 |
| Withdrawal time, min (mean, SD) | 9.8 (4.64) | 10.2 (4.72) | 0.31 |
| Colorectal cancer detection rate, – no. (%) | 1 (1.5) | 1 (1.5) | 1 |
| Polyp detection rate, – no. (%) | 29 (43.9) | 32 (48.5) | 0.60 |
| Adenoma detection rate – no. (%) | 24 (36.4) | 28 (42.4) | 0.47 |
| Diminutive polyp detection rate, – no. (%) | 22 (33.3) | 23 (34.8) | 0.85 |
| Diminutive adenoma detection rate, – no. (%) | 18 (27.3) | 20 (30.3) | 0.70 |
| Number of polyps per patient (mean, SD) | 1.10 (2.12) | 1.26 (1.87) | 0.64 |
| Number of adenomas per patient (mean SD) | 0.78 (1.67) | 0.86 (1.31) | 0.77 |
| Adverse effects – no. (%) | 0 | 0 | – |
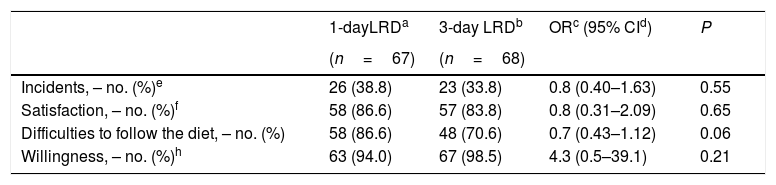
All patients were compliant with the diet recommendations. Although no adverse effects were reported, 38.8% and 33.8% patients in the 1-day and 3-day LRD groups reported incidents (P=0.55) (Table 5). There was a trend in favour of tolerance of the 1-day LRD but without statistically significant differences (Table 5). Satisfaction levels and willingness to repeat the same diet in the future were also high in both groups (Table 5).
Tolerance, acceptance and willingness to take the same diet.
| 1-dayLRDa | 3-day LRDb | ORc (95% CId) | P | |
|---|---|---|---|---|
| (n=67) | (n=68) | |||
| Incidents, – no. (%)e | 26 (38.8) | 23 (33.8) | 0.8 (0.40–1.63) | 0.55 |
| Satisfaction, – no. (%)f | 58 (86.6) | 57 (83.8) | 0.8 (0.31–2.09) | 0.65 |
| Difficulties to follow the diet, – no. (%) | 58 (86.6) | 48 (70.6) | 0.7 (0.43–1.12) | 0.06 |
| Willingness, – no. (%)h | 63 (94.0) | 67 (98.5) | 4.3 (0.5–39.1) | 0.21 |
In the univariate analysis, constipation, suffering from a stroke, low education, anaemia and dissatisfaction with the low fibre diet were significantly associated with a poor bowel cleansing (supplementary Table 2). In the multivariate analysis significant variables were included but also not having first degree relatives affected of colorectal cancer and an indication for colonoscopy different to adenoma surveillance because P value was lower than 0.1. In the multivariate analysis only anaemia (OR 3.1, 95% CI [1.11–8.70], constipation (OR 3.8, 95% CI [1.48–10]) and suffering from a stroke (OR 5.6, 95% CI [1.42–21.74], were the only variables independently associated with poor bowel cleansing (supplementary Table 3).
DiscussionIn the present study, we found that patients with risk factors of poor bowel cleansing do not benefit from following more than one LRD days before the colonoscopy. Although, currently the main European and US societies recommend a LRD at least with the same evidence level that of the traditional liquid diet before colonoscopy,1,4 there is scarce evidence about the proper number of LRD days before the examination. Although, it would make sense thinking that the more number of LRD days the better cleansing quality would be, there is a gap of evidence on this topic. If the number of LRD days have a benefit on cleansing quality would be specially interesting to recommend more LRD days in hard to prepare patients, it means, those patients with risk factors of poor bowel cleansing in order to guide enhanced bowel cleansing protocols. In this study, we used a validated score designed by our group to choose patients with high risk of poor bowel cleansing.6 This score is composed of 4 well-known variables associated in several studies with poor bowel cleansing, such as, abdominal-pelvic surgery, tricyclic antidepressants, comorbidities (diabetes mellitus, stroke, cirrhosis and renal failure) and constipation. The model built with these variables had a fair accuracy with an area under the curve of 0.70 and the optimal cut-off (1.225) achieved a negative predictive value of 88% suggesting that this model could be used to identify patients that will be well prepared with a standard cleansing protocol. However, the positive predictive value was only 36% suggesting that this score would need refinement to identify patients who will have a poor bowel cleansing. In other words, most patients with a high score (64%) will have an adequate bowel cleansing despite the result of the score. This fact could be the reason why we did not find statistically significant differences between 1-day LRD and 3-day LRD in this study, because in most of these patients (regardless the number of days with a LRD) the standard cleansing protocol would be enough. Another explanation, as some authors have pointed out, is that LRD could play a minor role in bowel cleansing, being the most important factor the timing, it says, the time elapsed between the ingestion of the bowel solution and the examination, the 5h rule.13
The findings of this study are in line with our randomized controlled trial comparing 1-day LRD with 3-day LRD in consecutive outpatients scheduled for colonoscopy.5 In this well powered study, there were no significant differences in cleansing quality, tolerance, satisfaction with the LRD and willingness to follow the same LRD in the future.
This study has a number of strengths. First, this study was nested in a well-designed and powered randomized controlled trial. Second, although, variables associated with poor bowel preparation are diverse and it could constitute a source of heterogeneity, this is the first study that used a validated score to select patients with a high risk of inadequate bowel preparation. Finally, structured and reproducible LRD, designed by an endocrinologist specialized in nutrition, was recommended for the patients included.
However, this study has also some limitations. First, only patients prepared with PEG+Asc were included and therefore the results may not be extrapolated to other bowel preparations. Second, this is a single centre study and the procedures were carried out during the morning-shift colonoscopies; these results should be replicated by other groups and in the afternoon-shift colonoscopies. Finally, this is a post Hoc study and sample size was not calculated for this aim. Ideally this study should be replicated with a broader cohort of subjects with an adequate sample size calculation. In a recent randomized study carried out by our group comparing a conventional bowel preparation identical to that of the present study (2l PEG+ascorbic+1 day low-residue diet) versus an enhanced one (bisacodyl+4l PEG+3 days low residue diet) in patients with a high risk of poor cleansing (unpublished data), the group that received conventional preparation had adequate cleaning in 78% of cases. Taking this group as a reference and considering a power of 80%, a confidence level of 95% and an expected improvement on cleansing quality of 10% in the 3-day low-residue diet group, the number of patients to include would be 218 per group (436 patients). It means that 31% of the total sample has been included in the present study. Although this percentage may seem small, it is not negligible and suggests that there is no great difference between the two groups.
In conclusion, this study suggests that a well structured 3-day LRD is not superior to the ESGE recommended 1-day LRD in terms of colon cleansing quality in patients with risk factors of poor bowel cleansing and thereby the number of LRD days before colonoscopy should not be modified depending of the patient risks factors of poor bowel cleansing. Other strategies should be explored in this subgroup of patients.
Source of fundingThis research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflicts of interestNone to declare.