Inflammatory bowel disease (IBD) is characterized by a higher incidence of colorectal carcinoma preceded by the appearance of dysplasia in the colonic epithelium. In addition, other epithelial changes in the mucosa of IBD have been described, recently grouped under the term nonconventional dysplasia. Nonconventional dysplasia includes at least 6 subtypes, including (a) hypermucinous; (b) goblet cell deficient (GCD); (c) terminal epithelial differentiation (TED; also known as crypt cell dysplasia [CCD]); (d) traditional serrated adenoma (TSA)-like; (e) sessile serrated lesion (SSL)-like; and (f) serrated lesion, not otherwise specified (NOS).1–3 Due to the presence of different grades of dysplasia and the frequent concomitance with carcinoma, they are now considered precursor to a new cancer pathway developing in IBD.2,3 This so-called third carcinogenic pathway is being explored and the data are still preliminary. The presence of mutations in the TP53 gene in early stages of the carcinogenic process stands out in contrast to what occurs in conventional colorectal carcinoma. In addition, mutations in the BRAF gene, which drives the serrated pathway initiated by sessile serrated lesions (SSL), are rare.4
We report a case which showed serrated epithelial lesions (SEL) with different grades of dysplasia that occurred with a concomitant carcinoma in the ileum affected by Crohn's disease (CD).
A 52-year-old woman consulted for abdominal pain. She referred previous episodes of abdominal pain and sub-occlusive crisis of 2 years of evolution.
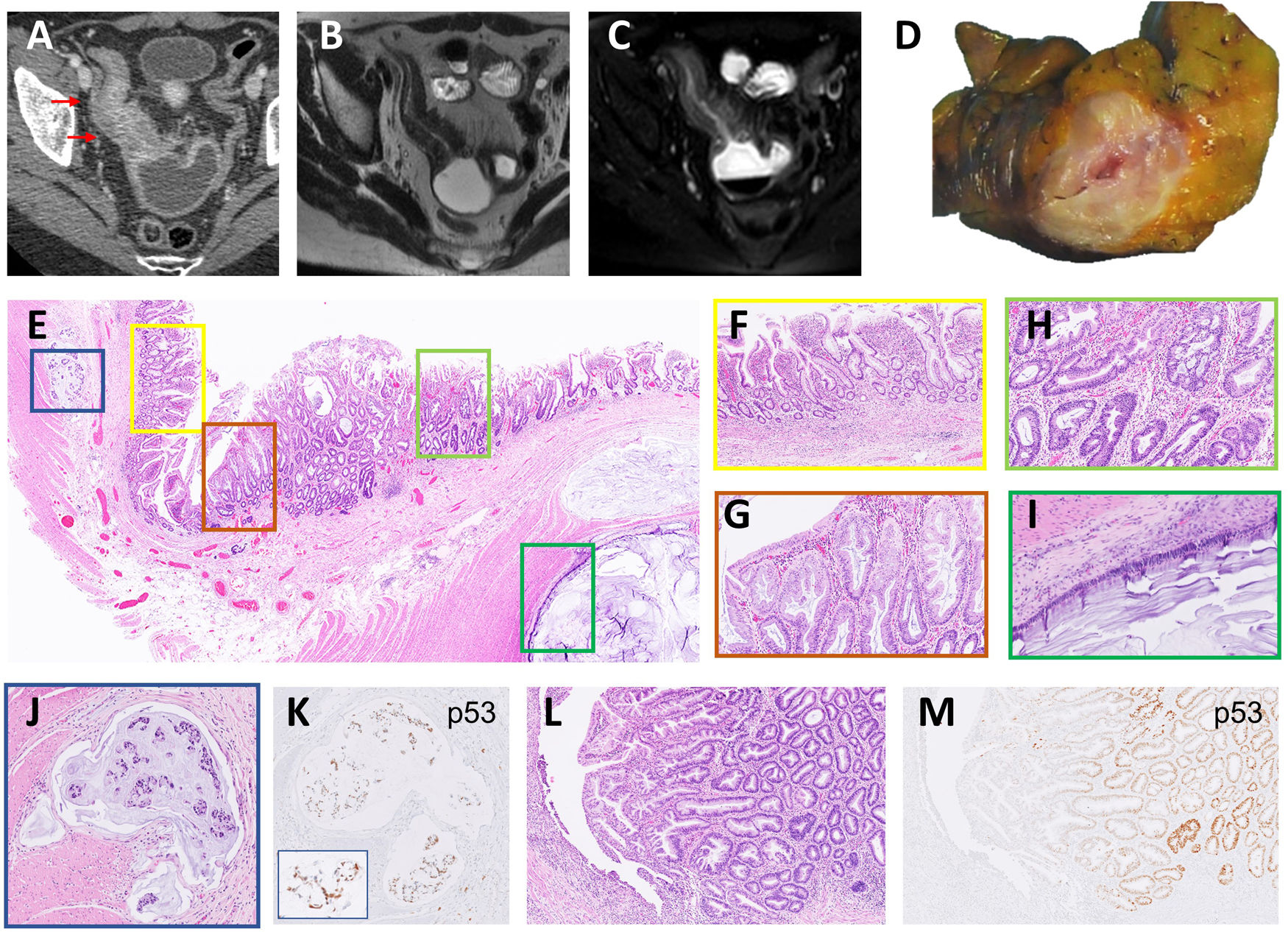
An axial computed tomography (CT) showed a severe wall thickening segment of the distal ileum which was also stenotic with an upstream dilatation (Fig. 1A). A weighted axial magnetic resonance imaging (MRI) confirmed the findings (Fig. 1B and C).
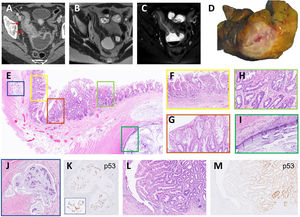
(A) Contrast enhanced CT in venous phase showed severe mural oedema and hyperenhancement of a long segment of the distal ileum that was also stenotic. Fat stranding of the perienteric fat and the comb sign were also visible. Upstream dilatation of the small bowel was present. (B, C) Axial MRI T2 weighted images without (B) and with fat saturation (C) confirmed the CT findings and indicate intramural oedema. (D) Cross section of the intestinal wall showed a narrow lumen and wall thickening. (E) Panoramic section of the thickened bowel wall showing various lesions. (F) Flattened villi and complex architectural glands as signs of chronicity of CD. (G) Detail of serrated glands with LGD in a SEL. (H) Serrated glands with HGD in the other side of the same SEL. (I) Mucinous adenocarcinoma infiltrating the bowel wall as pools of mucin covered by a monolayer of atypical cells. (J) Lymphovascular invasion by mucin with signet-ring cells. (K) The same signet-ring cells showed nuclear p53 expression. (L) Linear ulcer in an area of active CD and adjacent another polypoid SEL. (M) p53 staining was observed in glands with HGD.
A colonoscopy was performed with visualization of the terminal ileum without showing any lesions. Two months later and given the persistence of the lesions in a new CT scan, a resection of the pathological bowel and an ileum-ileal anastomosis preserving the ileum-cecal valve was performed.
Macroscopic findings: the resected terminal ileum was angled into two fragments, one with a thickened wall and narrowed lumen and the other with a dilated lumen without notable alterations (Fig. 1D).
Microscopic findings: a section of the thickened wall of the ileum presented different alterations (Fig. 1E). The mucosa presented characteristic features of long-standing CD with flattened villi and irregularly shaped glands (Fig. 1F). The adjacent mucosa showed a SEL with eosinophilic cells with low-grade (LG) and high-grade dysplasia (HGD) (Fig. 1G and H). A high-grade adenocarcinoma with mucinous pools infiltrated the wall bowel (Fig. 1I) with lymphovascular invasion by signet-ring cells (Fig. 1J). In a separate section a linear ulceration was adjacent to a SEL (Fig. 1L) with HGD. The dysplastic epithelium and the carcinoma showed p53 expression (Fig. 1K and M). Proteins of the mismatch repair genes (MLH1, MSH2, MSH6, and PMS2) retained the nuclear expression. No mutations were found in KRAS, NRAS, and BRAF genes in the carcinoma by sequencing.
SEL in IBD are reported in the colonic mucosa and it may be easily missed by endoscopists and pathologists, especially if the lesions are flat. When considered, they should be differentiated from SSL. In our case, the SEL occurred in the mucosa of the small bowel where serrated adenomas are rarely described.5 The p53 expression and the lack of BRAF mutations corroborated the findings described in this carcinogenesis pathway.
In conclusion, SEL is a precursor lesion of IBD-associated carcinoma that may appear in the small intestine. When dysplasia is present, p53 expression may be useful to differentiate SEL from SSL.
FundingNo funding was obtained for this study.
Conflicts of interestThe authors declare no conflicts of interest.
We thanks to the Department of Pathology of Hospital Universitari General de Catalunya-Grupo Quirónsalud for technical support.