Visceral angioedema is a rare complication of therapy with angiotensin-converting enzyme (ACE) inhibitors. Clinical presentation includes nausea, vomiting, abdominal pain and diarrhea. Early detection of this entity can prevent recurrent episodes and unnecessary invasive procedures, including surgery.
This article describes a 46-year-old-woman who presented to the emergency department with abdominal pain, associated with nausea and vomiting. She had been taking ramipril for 15 days.
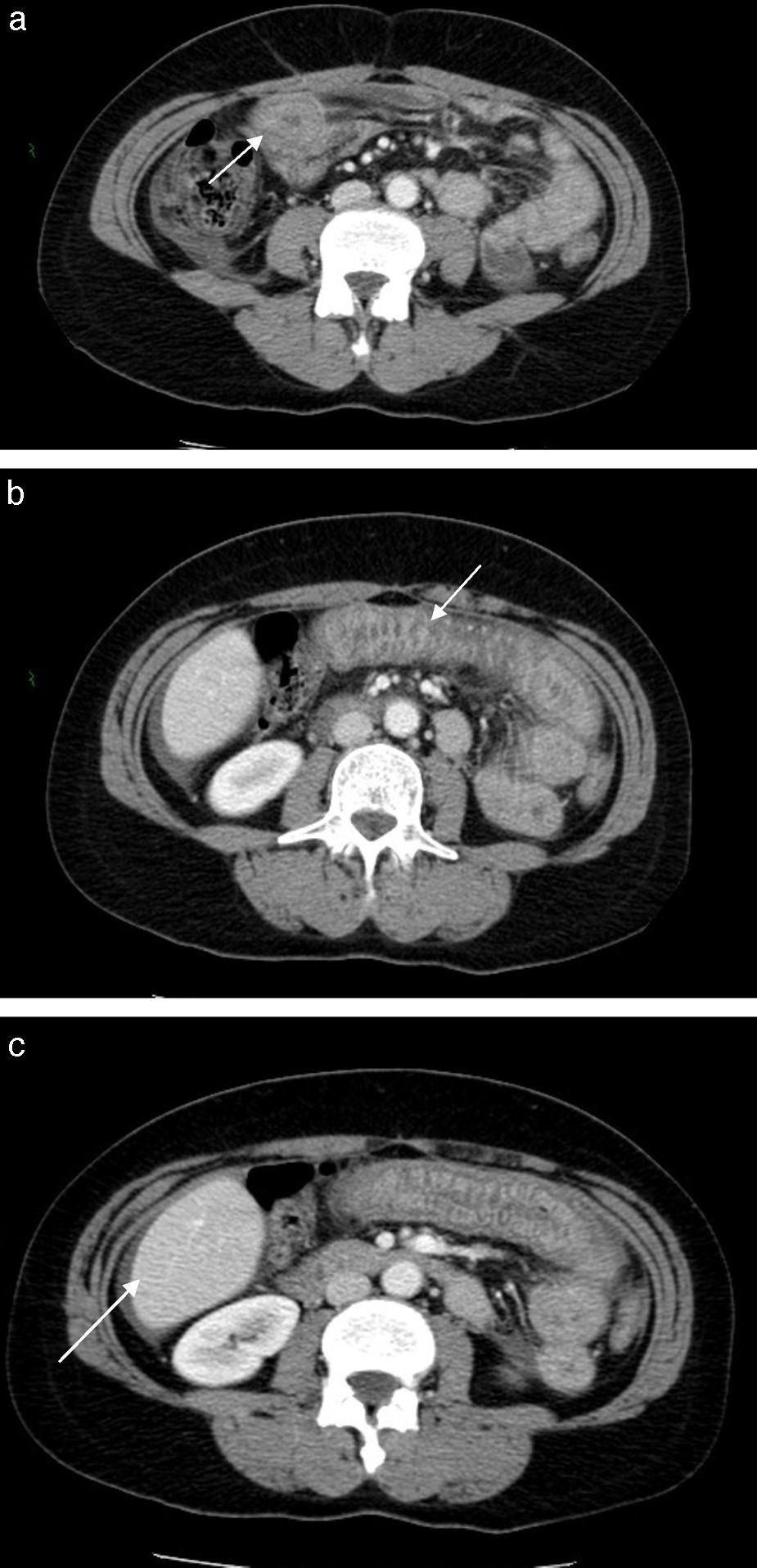
A computed tomography was performed which revealed thickening of a jejunal segment, with submucosal edema.
ACE inhibitor-associated angioedema was suspected and the medication was discontinued, with resolution of symptoms in 48h.
After 7 months of follow-up, the patient is asymptomatic.
Despite of its rarity, ACE inhibitor-induced small-bowel angioedema should be included in the differential diagnosis when patients receiving ACE inhibitor therapy present with abdominal complaints.
O angioedema visceral é uma complicação rara da terapêutica com inibidores da enzima de conversão da angiotensina (IECA).
O quadro clínico inclui náuseas, vómitos, dor abdominal e diarreia.
O reconhecimento precoce desta entidade pode evitar episódios recorrentes e procedimentos invasivos desnecessários, incluindo cirurgia.
Descrevemos o caso clínico de uma mulher de 46 anos, que recorreu ao serviço de urgência por dor abdominal, acompanhada de náuseas e vómitos. Estava medicada desde há quinze dias com ramipril.
A tomografia computorizada identificou espessamento parietal de um segmento jejunal, com edema submucoso.
Foi colocada a hipótese diagnóstica de angioedema visceral induzido por IECA.
Suspendeu-se a terapêutica com ramipril, com remissão completa da sintomatologia em 48horas.
Após 7 meses de follow-up, encontra-se assintomática.
Apesar da sua raridade, o angioedema visceral induzido por IECA deve ser incluído no diagnóstico diferencial de dor abdominal nos doentes medicados com IECA.
A 46-year-old-woman presented to the emergency department with a one-day crampy abdominal pain, associated with nausea and vomiting. The patient's past medical history was relevant for hypertension, for which she was taking ramipril for 15 days.
She denied additional medication besides ramipril. She also denied smoking. She had no known allergies to drugs or environmental agents, and her travel history was negative.
There was no family history for any diseases.
On physical examination, she was afebrile, with a heart rate of 67 beats per minute and a blood pressure of 123/76mmHg. She had no stridor or facial swelling. Breath sounds were normal. Abdominal exam disclosed tenderness in the periumbilical area.
Laboratory investigations revealed a white blood cell count of 11.100microliter (μL) (9.400neutrophils/μL; 100eosinophils/μL; 1.400lymphocytes/μL) and C-reactive protein of 2.5miligrams/deciliter (mg/dL) (normal value<0.3mg/dL); serum electrolytes, liver enzymes, serum amylase and renal function tests were in the normal range. Serologies for celiac disease and Crohn's disease were negative.
Chest X-ray did not reveal any changes and upright abdominal X-ray revealed air-fluid levels.
An abdominal computed tomography (CT) was performed which revealed thickening of a jejunal segment, with submucosal edema giving a “target-sign” appearance. There was also moderate ascites (Fig. 1).
Push enteroscopy was performed 3 days after onset of symptoms and did not reveal any changes (the estimated depth of insertion was proximal jejunum). Biopsies were performed which revealed normal results.
She was diagnosed with visceral angioedema secondary to angiotensin-converting enzyme (ACE) inhibitor therapy. Ramipril was discontinued, and symptoms resolved completely in 48h. Upright abdominal X-ray was repeated and did not reveal air-fluid levels.
She was discharged home after 6 days on calcium channel blocker.
After 7 months of follow-up, the patient is asymptomatic.
2DiscussionAngiotensin-converting enzyme (ACE) inhibitors have achieved widespread usage in the treatment of cardiovascular and renal disease; they have proved effectiveness in the treatment of hypertension, they decrease mortality in congestive heart failure and left ventricular dysfunction after myocardial infarction and they delay the progression of diabetic nephropathy.1
The most common adverse reactions are cough and skin rash.2 Peripheral angioedema has been reported in 0.1–0.2% of patients, and visceral angioedema has been reported to occur even less commonly.3–6
These drugs inhibit competitively the activity of ACE to prevent formation of angiotensin II from angiotensin I. Inhibition of this enzyme also leads to accumulation of kinins including bradykinin.1
Visceral angioedema pathogenesis is not clear. The most plausible mechanism is an increase in the levels of bradykinin and its metabolite.7 Bradykinin increases vascular permeability1 and promotes vasodilation4,8,9 by stimulating the production of arachidonic acid metabolites, nitric oxide, and endothelium-derived hyperpolarizing factor in vascular endothelium, thereby leading to angioedema.10
In fact, during an acute attack of angioedema secondary to ACE inhibition, the bradykinin concentration can increase to more than 10 times the normal level9 and fall to normal levels during remission after withdrawal of the drug.11
ACE-inhibitor-induced angioedema of the intestine is more common in women12 in the fifth decade of life.11,13
Clinical presentation includes nausea, vomiting, abdominal pain and diarrhea.14–17 Symptoms typically present within 24–48h after initiation of ACE inhibitor,18 but there are case reports between 2 weeks and 18 months after initiation of therapy13 and there is one case report 9 years after initiation of therapy.6
Although the CT features are not pathognomonic, they provide valuable clues to the diagnosis; a previous case series suggested CT findings of ascites, small bowel wall thickening, dilation without obstruction, and straightening should prompt inclusion of the diagnosis in the differential while taking an ACE-inhibitors.19
Inflammatory bowel disease, infection, vasculitis, ischemic bowel and mechanical obstruction all need to be considered in the differential.20,21
Supportive care and cessation of ACE-inhibitor usage are the cornerstones of treatment.17 Symptoms usually resolve within 48h13,20,22,23 as illustrated by the presented case. Nevertheless, in a previous study,11 a median of 10 months (range from 1 day to 10 years) elapsed between the onset of angioedema and withdrawal of the ACE inhibitor. In the presented case, due to the prompt diagnosis, ramipril therapy was withdrawn in the same day as angioedema presentation.
Recurrent angioedema has been reported to occur in 1.5–10% of patients after changing from ACE-inhibitor to angiotensin II receptor blockers. However, it is believed that this may actually represent residual effects of ACE-inhibitor angioedema.24
It is important to recognize this entity as many of the patients discussed in the literature underwent unnecessary invasive procedures such as exploratory laparotomy, intestinal biopsy and resection.16,18,20,21
In conclusion, ACE inhibitors-induced small-bowel angioedema should be included in the differential diagnosis in any patient with unexplained abdominal pain while on ACE inhibitors.16,18
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.