Diffuse lepromatous leprosy (DLL) is a severe clinical outcome of lepromatous leprosy (LL). The aetiologic cause is believed to be different from Mycobacterium leprae. A new species, Mycobacterium lepromatosis, was identified from a group of Mexican patients with DLL, and severe leprosy reactional state type 3 (Lucio's phenomenon). However, a total sequencing of its genome is necessary to prove the existence of this new species. This is a report on a non-typical Colombian case of leprosy – HIV coinfection, associated with an immune reconstitution inflammatory syndrome clinically compatible with a leprosy reaction type 3 or Lucio's phenomenon.
La lepra difusa (LLD) es una variedad de la lepra lepromatosa (LL), frecuente en México. El agente etiológico se cree que es diferente a Mycobacterium leprae y se considera una especie nueva denominada Mycobacterium lepromatosis, hecho que no se ha comprobado. El reporte de este caso se realiza para dar a conocer el cuadro clínico atípico que presentó una paciente colombiana con coinfección VIH–LL variedad difusa (LLD), asociado a síndrome de reconstitución inmunológica, compatible clínicamente con una leprorreacción tipo 3 o fenómeno de Lucio.
Diffuse lepromatous leprosy (DLL) is a severe clinical outcome of lepromatous leprosy. A new species, Mycobacterium lepromatosis, was identified from a group of Mexican patients with DLL, and severe leprosy reactional state type 3 (phenomenon of Lucio), however total sequence of its genome is necessary to probe the existence of this new species.1
The phenomenon of Lucio was described for Lucio and Alvarado in 1852 and it was redefined by Latapí in 1948, until now this reactional outcome is object of debate for clinicians and scientists due to its confuse pathogenesis. Clinically, the immune hypersensitivity triggered by bacterial antigens is associated with constitutional symptoms, necrotizing vasculitis, sepsis, and in some cases death.2–4
In addition, the advent of human immunodeficiency virus (HIV) and routine use of highly effective antiretroviral therapy (HAART) in patients with Hansen's disease may relate to other events as immune reconstitution inflammatory syndrome (IRIS), which may occur in 40% of patients with HIV and HAART.5,6 This syndrome characterizes by a paradoxical inflammatory condition because of immune restoration generated by antiretrovirals. In patients with HIV-leprosy, IRIS has been generally associated with inflammatory processes such leprosy-reactions type1, in contrast current clinical case shows a leprosy reaction type 3 or phenomenon of Lucio.3,4,7,8
Case descriptionColombian female patient, 37 years old, on July 2013 consulted by one month of recurrent febrile episodes and multiple skin ulcers in lower limbs since one year ago. On physical examination patient has signs as pinna oedema, loss of the bilateral external third of eyebrows, chronic indurated lesions in abdomen, pigmented scarring lesions in lower limbs that patient refers as an episode of ulcers during the pregnancy in 2006. Patient relates that since 3 years ago, her skin began to become smooth and shiny, associated with occasional nosebleeds, headache and tenderness infiltration in hands and feet. In addition, patient has skin ulcers with burning pain associated with local oedema and serous-hematic and purulent discharge. Patient refers weight loss of 4kg in 6 months. She relates that seven days ago her spouse died by AIDS. Patient was hospitalized with study diagnosis of ecthyma gangrenosum, vasculitis by HIV, anaemia, sepsis, and leprosy.
Laboratory exams: haemoglobin 6,9; red cells morphology microcytic hypochromic, leukocytes 3.300, 70% neutrophils, platelets 230.000. Functional hepatic tests in normal ranges. Skin biopsy reported bacillary index (BI)=3 plus. Patient diagnosed as DLL and Lucio's phenomenon. Multidrug therapy (MDT-MB) initiated with dapsone+rifampicin+clofazimine. HIV viral load was 480.474copies/ml; CD4/CD8=0.8%, total CD4T lymphocytes (helper): 170cells/μl, total CD8T cells (cytotoxic suppressor): 202cells/μl; CD3T lymphocyte subpopulations: 377cells/μl). Besides MDT, patient received abacavir/lamivudine plus lopinavir/ritonavir.
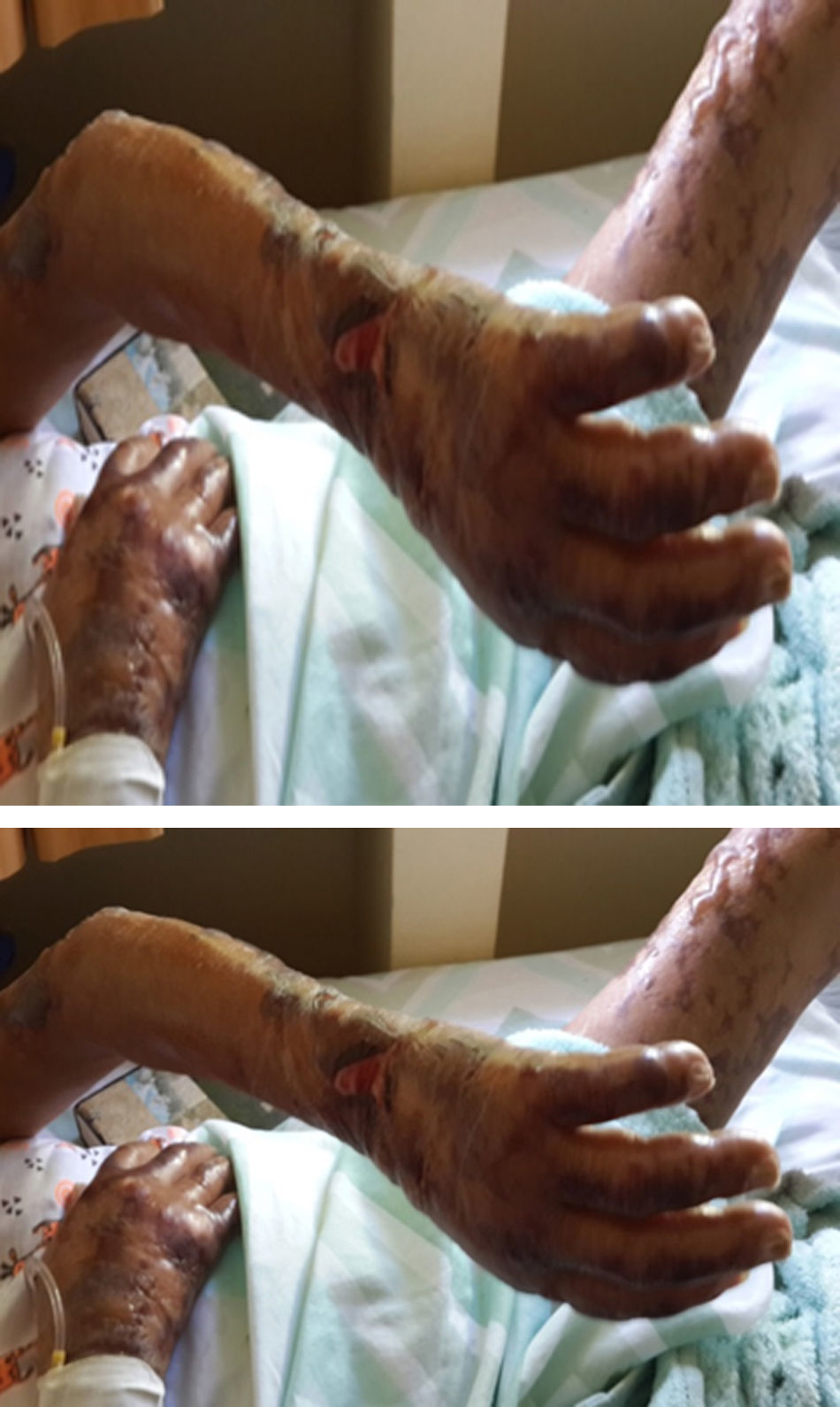
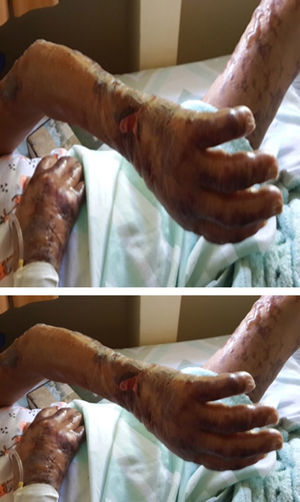
After three weeks of treatment for HIV and leprosy, the patient consulted for fever associated with multiple bullous lesions with reticular pattern in upper limbs and proximal third of the lower extremities. In addition, lesions ulcerated are evident with erythematous, dirty background, serum-hematic secretion, some with necrotic aspect, and signs of inflammation and infection. Also, refers numbness and paresthesias in the hands and feet (Figs. 1 and 2).
Physical examination reported a malnourished patient, in poor general conditions, bilateral infiltration in auricular lobes, thinning of the nasal septum, and some skin oedematous lesions in abdomen. Simplified neurological examination showed thickening and pain on palpation of the auricular, ulnar, median, radial, posterior tibial, and common fibular nerves, with anaesthesia in glove and sock pattern (hands and feet), also mild resorption of the distal phalanx of the fifth finger of left hand. The rest of the physical exam described a patient with well cardiopulmonary conditions, no evidence of masses or organ enlargement in abdomen.
Complementary tests included serology for detection of IgM antibodies to phenolic glycolipid 1 (PGL-1), exhibited high positive titters. Furthermore, DNA extracted from skin biopsy tested by Sanger sequencing technique was performed in Professor Stewart Cole's laboratory (Global Health Institute, Ecole Polytechnique Fédérale de Lausanne, Switzerland); ruling out the infection caused by M. lepromatosis. Results reported M. leprae European genotype 3-I as the cause of DLL.
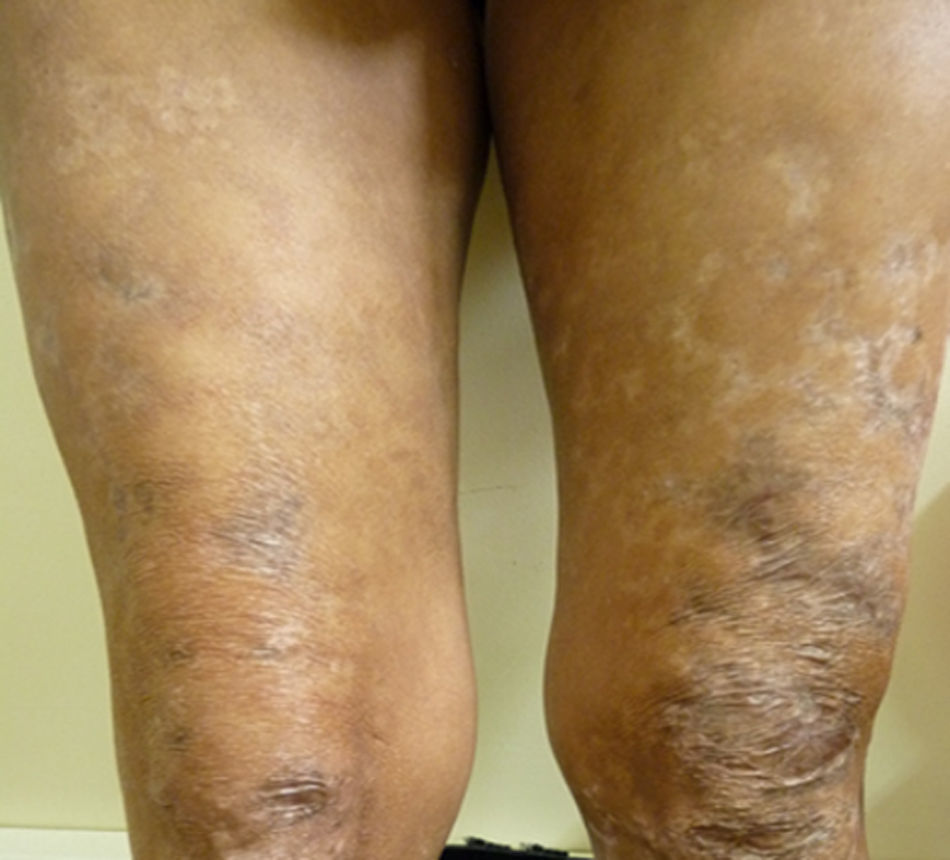
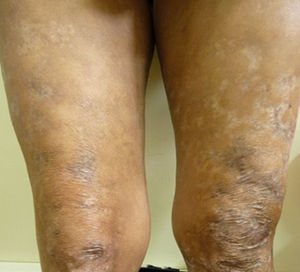
After two months of hospitalization and medical management with antiretrovirals, MDT-MB, trimethoprim sulfamethoxazole 160/800mg and rifampin 600mg per day, the patient was discharged due to favourable clinical evolution, showing healing of skin lesions in upper and lower limbs (Fig. 3), and improvement of HIV viral load.
The patient did not receive treatment with thalidomide and/or steroids as it is recommended for some authors,6 the patient received 600mg/day of rifampicin as the only measure to treat Lucio reaction.9
DiscussionThis case reports an atypical type 3 reaction in a LL–HIV coinfection after HAART. Patient had a diagnosis of phenomenon of Lucio based in clinical symptoms (cutaneous blisters, bullae, skin sloughing and necrotizing vasculitis involving upper and lower limb areas),10,11 and she was treated for Lucio reaction with rifampicin 600mg per day. However, it is noteworthy that IRIS in patients with Hansen's disease is often associated to reaction type 1, usually within the first six months after initiation of HAART, or in patients with advanced stages of HIV can occur in an earlier time.6–8
Another non-typical case was reported in India by Arakkal et al,12 in which described a patient with HIV and IRIS, associated with erythema nodosum leprosum (ENL), which allowed unmask silent lepromatous infection after four weeks of starting HAART. In addition, Cusini et al.13 described a case of a Brazilian man living in Switzerland infected with HIV and manifestations of ENL and LL after starting HAART, which was classified as a rare form of IRIS. Therefore, different states reactional to LR1 as ENL or Lucio phenomenon could be generated as a result of IRIS expression in patients with HIV co-infection leprosy, shortly after starting HAART.12,13
The pathophysiological mechanisms responsible for IRIS are known incompletely. This syndrome is believed to be the result of immune reconstitution unbalanced way of effector and regulatory T cells in patients receiving antiretroviral therapy. Immune reconstitution during HAART generates the expression of regulatory T cells that may be defective in its function and in number, thus inhibiting their ability to suppress the expression of pro-inflammatory cytokines, which would be responsible for the appearance of focal and systemic signs and symptoms of inflammation.14,15
One deduction that arises for this atypical clinical case is that due to the high bacillary load, added to an improvement in immune response caused by HAART allowed the formation of immune complexes (IC). These IC deposited in the walls of capillaries and arterioles triggering the migration of phagocytic and inflammatory cells thereby causing clinical symptoms of necrotizing vasculitis that could resemble a phenomenon of Lucio. The atypical clinical manifestations described after starting HAART could be explained by the activation of TH1 response, which generates increased activity of macrophages, activation of complement and generation of inflammatory cytokines (IL6, IL10, IL22). This response of immune hypersensitivity type 3, allowed the emergence of ulcerative lesions resulting from thrombosis in the deep and superficial vessels, which generated the sloughing of large areas of skin, associated with vesicles, blisters and bullae.2–4,8,10
M. lepromatosis has been postulated as the causal agent of DLL and phenomenon of Lucio,1 however, although it was suspected that M. lepromatosis was causing this clinical case, it was ruled out, confirming M. leprae.
Therefore, this shows how far we know about clinical and immunological aspects of leprosy,11 which arises the need for further research to understand and visualize the complexity of these phenomena of immune hypersensitivity affecting patients with Hansen's disease, limiting their quality of life or sometimes leading them to death by our lack of knowledge of these special cases.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no data that enables identification of the patient appears in this article.
FundingColciencias Grant code: 325656933516.
Conflict of interestThe authors have no conflict of interest to declare.