In the last few decades we have witnessed an interesting transformation of the population pyramids throughout the world. As the population's life expectancy increases, there are more chronic diseases such as diabetes mellitus and dementias, and both of them have shown an association.
General objetive:To determine the association between Alzheimer's disease in diabetic patients and the insulin degrading enzyme in outpatients of a second level Hospital in Monterrey, Mexico.
Materials and methodsThis was a case control study in which we included outpatients from the Geriatrics Clinic of a Hospital in Northeastern Mexico. Cases were patients with a Mini Mental Score Exam (MMSE) below 24 and DSM-IV criteria for Dementia. Controls were patients who had MMSE scores greater than 24.
ResultsData from 97 patients were analyzed. Regarding physical examination and the results of laboratory tests, there were no differences between the two groups (p>0.05).
A 98% prevalence of the insulin degrading enzyme was documented in the sample studied. We found an association between a homozygous status for the CC genotype and Dementia with an estimated Odds Ratio (OR) of 2.5 (CI 95% 1.6–3.3) on the bivariate test, while, on the multivariate analysis, the OR was estimated 3.3 (CI 95% 1.3–8.2).
ConclusionsEvidence shows that cognitive impairment is more frequent among those exposed to the C allele of the rs2209972 SNP of the insulin degrading enzyme gene.
En las últimas décadas se ha producido una transformación en las pirámides poblacionales, con un aumento en la expectativa de vida, lo que conlleva un mayor número de enfermedades crónico-degenerativas, como son la diabetes mellitus y la demencia, que han mostrado tener una asociación estrecha, pero cuya etiología aún está por discernir.
El objetivo de este estudio fue determinar la asociación entre el alelo C de la enzima degradadora de insulina y la enfermedad de Alzheimer en pacientes con diabetes tipo 2.
Material y métodosEstudio de casos y controles, en el cual se incluyeron pacientes de la clínica de Geriatría del Hospital General del Noreste de México. Los casos fueron aquellos con una puntuación en el Mini-Mental State Examination (MMSE) menor de 24 y criterios para demencia de acuerdo con el DSM-IV. Los controles fueron pacientes con MMSE superior a 24.
ResultadosSe analizaron datos de 97 pacientes. No hubo diferencias respecto a las características basales clínicas y de laboratorio entre los casos y los controles (p>0,05).
Se documentó una prevalencia de 98% del alelo C de la enzima degradadora de insulina.
Encontramos una asociación entre homocigosidad para el genotipo CC de la enzima degradadora de insulina y enfermedad de Alzheimer, con una OR de 2,5 (IC 95% 1,6–3,3) en el examen bivariado, y en el examen multivariado se encontró asociación con una OR de 3,3 (IC 95% 1,3–8,2).
ConclusionesLa evidencia muestra una asociación entre deterioro cognitivo y presencia del alelo C del polimorfismo rs2209972 del gen de la enzima degradadora de insulina.
Alzheimer's disease (AD) is the most common dementia in the geriatric population and its prevalence is greater than 60% among individuals with dementia.1,2
Diabetes mellitus (DM) is very prevalent in the world and in our country.3 Certain geriatric populations have a prevalence of DM of up to 44%, with cognitive impairment in more than 60%.4 There are studies that report that cognitive impairment is more common in diabetic patients.5–7 Even after adjusting for vascular damage these individuals have twice the risk of AD compared to those of the same age without diabetes.8,9 Several theories explain this7,9,10 as well as the development of AD.11 Several genes are implicated in the genesis of late-onset AD; two of these are: the insulin degrading enzyme (IDE) and the apolipoprotein epsilon 4 allele (APOE4).12
IDE catabolizes both insulin and beta amyloid (Aβ), with higher affinity for the former, leading authors to conclude that insulin plays a central role in the development of AD.13 It has been reported that insulin can promote accumulation of Aβ due to reduced degradation by IDE.14
IDE has a chromosomal location in a region very close to that which has been linked to late-onset AD.15 It has been stated that the C allele (rs2209972) of the enzyme is linked to AD and is present in up to 17.6% of patients with AD, in contrast to only 8% in controls, with a 3-fold greater risk in carriers.15
There are even some authors who have concluded that the association between IDE and AD is not necessarily a cause-effect one, but rather a phenomenon secondary to neurodegeneration.17
However, there is currently insufficient evidence to irrefutably prove its relationship, or the lack of it, to AD in individuals with diabetes. To date there is no knowledge of the prevalence of this polymorphism, not only in Mexico but also in Latin America, and much less its relationship with AD. This is why we carried out this analytical observational case–control study to determine the risk of AD on the presence of the allele C of the insulin-degrading enzyme in patients with type 2 diabetes.
MethodsWith prior approval by the local Ethics Committee, the sample size was calculated for a case–control study, using the statistical package STATA v11.0, with an alpha of 0.05 and a power of 0.8 with a difference in proportions of 0.7 to 0.4 for 49 participants per group; we included an extra patient per group.
We carried out a case–control, observational, retrospective and analytical study, including diabetic patients 65 years or older from a geriatric clinic of a secondary care hospital in northeast Mexico who underwent a comprehensive geriatric evaluation as outpatients and gave informed consent. Patients were matched by age and gender. We excluded those with a Mini Mental Status Examination (MMSE)18 score less than 10, a score greater than or equal to 5 on the 15-item Yesavage Geriatric Depression Scale19 uncontrolled hypothyroidism or hyperthyroidism, acute confusional state, chronic liver disease Child B or C, epilepsy or use of antiepileptics, antipsychotics, serum creatinine level greater than 1.5, positive VDRL, anemia, computed tomography showing images of infarct, hemorrhage or tumor. Cases were participants with cognitive impairment (MMSE<25) and with a diagnosis of dementia made using DSM IV criteria. Controls were those with a normal neuropsychological evaluation (MMSE ≥ 25).
VariablesThe variables analyzed were age, gender, years of schooling, history of smoking, family history of dementia, a history of hypertension, years with diagnosis of diabetes mellitus, number of drugs being taken, fasten glucose, total cholesterol, HDL, LDL and triglyceride levels, Barthel functional index,20 and the MMSE score.
General study procedureA capillary blood sample was obtained and the first drop of blood was used to determine the capillary blood glucose. In individuals with blood glucose under 200mg/dL, a second drop was obtained from which a volume of 10μL was taken and placed in a 250μL Ependorff tube.
Determination of genetic polymorphismsGenotyping was performed from the blood obtained, which was subjected to alkaline lysis with NaOH, followed by amplification of the polymorphic fragment by conventional end-point PCR using GoTaq Master Mix (Promega, Inc., Madison, WI). The reaction mixture had a volume of 15μL with the following composition: GoTaq Master Mix, 7.5μL, water, 5.7μL; sense primer, 0.3μL, antisense primer, 0.3μL, blood alkaline lysate, 1.2μL. The primers used at a 10μM concentration were ACCCCTCACAGTGTGCTCTGAA (sense) and TCCACCAAAAGTGTCTGCTATGC (antisense).21 A Bio-Rad C-1000 thermal cycler (Bio-Rad Laboratories, Inc. Hercules, CA) was used. The amplification program was 55 cycles of denaturation at 95°C/45s, annealing at 60°C/45s, and extension at 72°C/45s, for a total of 55 cycles. After amplification, the products were digested with the restriction enzyme BsaHI, which cuts the C wild allele. Thus, the homozygous CC generates two fragments; the TT homozygote is not cut and is observed as a single fragment; and the heterozygous CT results in three fragments. Digestion products were analyzed by submarine electrophoresis in 2% agarose gel, stained with ethidium bromide, and visualized on an ultraviolet transilluminator and recorded by digital photography using an orange filter. The genotypes were determined by two independent observers.
The APOE genotype was determined in the same way as the CT polymorphism of IDE, except that the annealing temperature was 58°C and digestion was performed with the HhaI enzyme; the restriction pattern with this enzyme allows differentiation of the six possible genotypes (APOE 22, 23, 24, 33, 34 and 44). The primers used were those described by Hixson and Vernier,22 except that the antisense primer was four bases longer.23 They were: TAAGCTTGGCACGGCTGTCCAAGGA (sense) and ACAGAATTCGCCCCGGCCTGGTACACTGCC (antisense).
Statistical analysisWe used descriptive statistics with measures of central tendency and dispersion to characterize the participants. For quantitative variables, the mean±standard deviation was used, and for qualitative variables frequencies and percentages were used, comparing these with Student's t test and Chi square, respectively. We measured the frequency of genetic variants in cases and controls. We also measured their association with odds ratios and their respective 95% confidence interval. A multivariate analysis of the variables associated with cognitive impairment was done, as it was a logistic regression model to the different alleles.
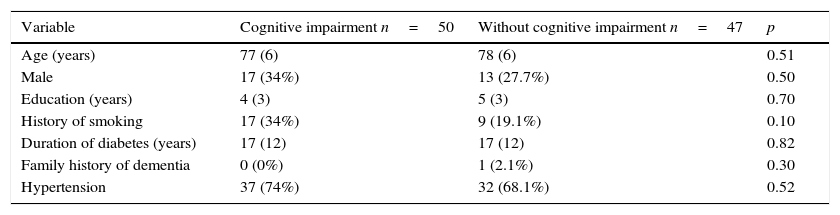
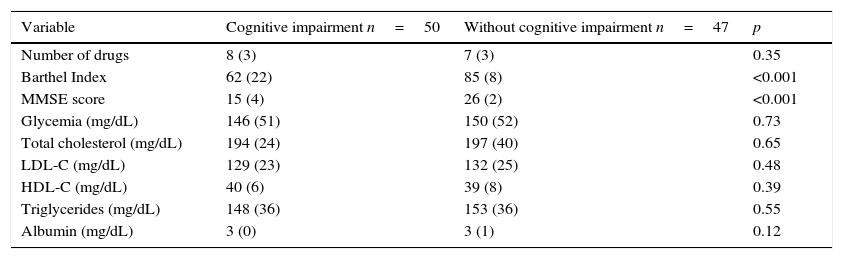
ResultsOriginally 50 control patients and 50 case patients were included; however, the data from three of the controls were discarded due to an inadequate blood sample for genotyping, and these three were not considered for the final analysis. The general characteristics that included age, gender, years of education, smoking status, duration of DM, hypertension, and family history of dementia of both groups were homogeneous in their distribution (Table 1), p>0.05. Clinical and laboratory characteristics, including serum albumin, total cholesterol and its fractions, triglycerides, and fasting glycemia, were similar, p>0.05, except for the cognitive and functional assessment, p<0.001(Table 2). The multivariate analysis to the above variables showed no differences regarding cognitive impairment.
Comparative analysis of the general characteristics of participants with and without cognitive impairment.
| Variable | Cognitive impairment n=50 | Without cognitive impairment n=47 | p |
|---|---|---|---|
| Age (years) | 77 (6) | 78 (6) | 0.51 |
| Male | 17 (34%) | 13 (27.7%) | 0.50 |
| Education (years) | 4 (3) | 5 (3) | 0.70 |
| History of smoking | 17 (34%) | 9 (19.1%) | 0.10 |
| Duration of diabetes (years) | 17 (12) | 17 (12) | 0.82 |
| Family history of dementia | 0 (0%) | 1 (2.1%) | 0.30 |
| Hypertension | 37 (74%) | 32 (68.1%) | 0.52 |
Data are presented as mean (standard deviation) and absolute frequencies and percentages, being compared with Student's t test and Chi square, respectively.
Comparative analysis of the clinical and laboratory characteristics of participants with and without cognitive impairment.
| Variable | Cognitive impairment n=50 | Without cognitive impairment n=47 | p |
|---|---|---|---|
| Number of drugs | 8 (3) | 7 (3) | 0.35 |
| Barthel Index | 62 (22) | 85 (8) | <0.001 |
| MMSE score | 15 (4) | 26 (2) | <0.001 |
| Glycemia (mg/dL) | 146 (51) | 150 (52) | 0.73 |
| Total cholesterol (mg/dL) | 194 (24) | 197 (40) | 0.65 |
| LDL-C (mg/dL) | 129 (23) | 132 (25) | 0.48 |
| HDL-C (mg/dL) | 40 (6) | 39 (8) | 0.39 |
| Triglycerides (mg/dL) | 148 (36) | 153 (36) | 0.55 |
| Albumin (mg/dL) | 3 (0) | 3 (1) | 0.12 |
MMSE, Mini Mental Status Examination. Data represent mean (standard deviation), compared with Student’ t test.
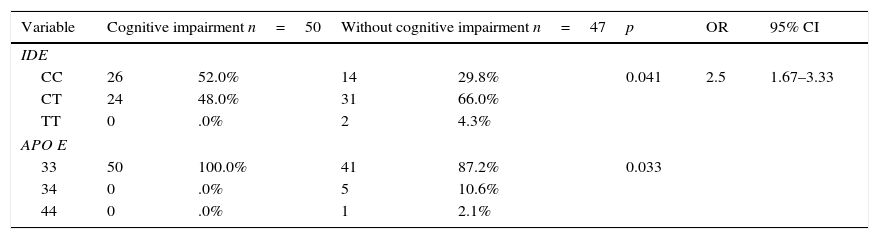
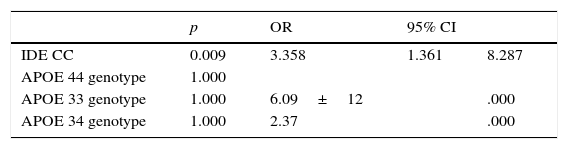
The distribution of the APOE and IDE alleles showed that only one sample of APOE4 was found in the control group and none in the case group. The prevalence of the C allele of IDE was 98% in any of its forms. Homozygosity was found in 41.2%, 26 in the case group and 14 in the control group, p=0.041 and OR 2.5 (95% CI 1.67–3.33) (Table 3). The difference increased after performing logistic regression p=0.009, OR 3.35(95% CI 1.36–8.28), without finding any association for the APOE allele (Table 4).
Association of the IDE and APOE polymorphism with and without cognitive impairment.
| Variable | Cognitive impairment n=50 | Without cognitive impairment n=47 | p | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| IDE | |||||||
| CC | 26 | 52.0% | 14 | 29.8% | 0.041 | 2.5 | 1.67–3.33 |
| CT | 24 | 48.0% | 31 | 66.0% | |||
| TT | 0 | .0% | 2 | 4.3% | |||
| APO E | |||||||
| 33 | 50 | 100.0% | 41 | 87.2% | 0.033 | ||
| 34 | 0 | .0% | 5 | 10.6% | |||
| 44 | 0 | .0% | 1 | 2.1% | |||
The data represent absolute frequencies and percentages, with comparisons made using Chi square.
It is positively striking that the groups were homogeneous in their general, clinical, and laboratory characteristics, except in the case of cognitive impairment, since without this difference this work would not have been carried out. As was expected, patients with cognitive impairment had a worst performance on basic activities of daily living, as described elsewhere16; this has future clinical implications, since complications occur after ADL dependence is present, as revealed by the same authors.
The fact that there was a slightly greater presence of women (not statistically significant) probably does not represent a bias in the results; this fact has also been seen in the Baltimore Longitudinal Study.24
The evidence showed that the C allele of IDE is present in the studied Mexican population in a very high proportion, much more than previously reported in other populations by different authors.15,25 It is important to keep in mind that we only worked with patients with diabetes and that this distribution could be different in a healthy population without DM and even in diabetic patients in different areas.
The degradation of Aβ has been measured up to 80% by the effect of IDE. It has been found that it degrades both soluble and insoluble forms.8 This elimination of Aβ decreases in the presence of hyperinsulinemia to levels similar to the total deletion of the IDE gene. On the other hand, the insulin level is up to 2.8 times higher in IDE negative mice.8,26 This may explain why the homozygous C allele was found more frequently in our patients with diabetes and cognitive impairment. This reinforces the findings described by Caccamo27 that the degradation of the amyloid plaque plays a central role in the development of AD; it has been described recently that even plasma levels of beta amyloid are related to some IDE polymorphisms in Caribbean Hispanics,28 yet these authors did not include the rs2209972, which is the one described in our article.
Even though the presence of some SNP of the IDE has been associated with an earlier age at onset of AD,29 we found no differences in the age of both groups, but the allele described in that article does not correspond to the one we studied, which might explain this difference.
Contrary to the previously known risk of APOE4 with regard to AD, this allele was barely found in this study, without any relation to patients with dementia, contrary to its very high prevalence in other populations.30
Moreover, a recent Chinese study revealed that the IDE is linked to the development of dementia with some SNP's and that some other had some protective effect in the absence of the APOE431; the former finding coincides with ours.
Some authors have concluded that the association between IDE and AD is not necessarily a cause-effect phenomenon, but rather a phenomenon secondary to neurodegeneration.32 Unfortunately, we were not able to explore this hypothesis in our work.
This highlights the importance of the C allele of IDE in Mexican population as a risk factor for Alzheimer disease in patients with diabetes mellitus.
The findings of this study are not without bias. The limitations of this study are those related to case–control studies,33 which include selection bias, recall bias, due to the retrospective nature. Other limitation is that cognitive function was not evaluated with neuropsychological tests,34 which are only used in case of doubt (not the case of the patients evaluated in our work).
In favor of the validity of the work we can say that it was done with an appropriate methodology including, of course, the calculation of the sample size for a case–control study and, even with multivariate analysis and a logistic regression analysis, the differences with regard to the C allele of IDE and APOE4, continued. This can be seen as a strong point of the work, which is the first in the Mexican population describing not only the association between the allele and the AD, but the presence of the allele itself in this population. Also, the data of this study may be important not only from the standpoint of Alzheimer disease, because the studied allele may have other manifestations, which have been associated with other chronic degenerative diseases of the CNS such as Parkinson's disease.35
Even though there are some recent articles about IDE and AD, recently published reports have not included the allele studied by us.17,28,29,31
Additional studies will be needed to determine if these findings are reproducible in other populations including those without diabetes.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We thank Sergio Lozano-Rodriguez, MD, Scientific Publications Support Coordinator and Hector E. Taméz Perez MD of the Jose E. Gonzalez University Hospital of the Universidad Autónoma de Nuevo León, for his help in translating this manuscript.