Hypertension has been associated with worse outcomes in patients with COVID-19 infection, so concerns have been raised about the possibility that inhibitors of the renin–angiotensin system (RAS) could influence the prognosis of these patients.
MethodsThis is an observational study of 921 consecutive patients admitted with COVID-19 respiratory infection to Hospital General Universitario Ciudad Real from March 1 to April 30, 2020. Following data were collected including patient demographic information, medical history, clinical characteristics, laboratory data, therapeutic interventions during the hospitalization and clinical outcomes.
ResultsThe mean age was 78 years, and 59.2% of patients had a history of hypertension. Patients with previous treatment with RAS inhibitor (42.4%) showed lower risk of the primary composite endpoint (mortality or need for invasive mechanical ventilation). Treatment with RAS inhibitor (both outpatient treatment and during hospitalization) had neither effect on mortality nor need for invasive ventilation. There were no differences in time-to-event analysis between groups.
ConclusionsRAS inhibitor treatment prior to admission in patients with COVID-19 respiratory infection was associated with lower risk of the primary composite endpoint and did not show neither impact on mortality nor need for invasive mechanical ventilation, even if these drugs were prescribed during hospitalization.
La presencia de hipertensión arterial se asocia con peor pronóstico en pacientes con COVID-19, y se ha sugerido que el uso de inhibidores del eje renina-angiotensina puede influir en el pronóstico de los pacientes.
MétodosRegistro observacional de 921 pacientes consecutivos ingresados por infección respiratoria COVID-19 entre el 1 de marzo y el 30 abril de 2020 en el Hospital General Universitario de Ciudad Real. Se registraron datos clínicos y analíticos, intervenciones terapéuticas y desarrollo de eventos durante el ingreso hospitalario.
ResultadosLa mediana de edad fue de 78años y el 59,2% tenían hipertensión arterial. Aunque el perfil clínico fue más desfavorable en el grupo de pacientes con prescripción previa de IECA o ARA2 respecto al resto, los primeros presentaron menor riesgo de desarrollo del evento primario combinado (mortalidad total o necesidad de soporte ventilatorio invasivo). Asimismo, el empleo previo al ingreso o durante el mismo de estos fármacos mostró un efecto neutro sobre la mortalidad total y sobre la necesidad de ventilación mecánica invasiva. En el análisis de supervivencia no se observó mayor riesgo de presentar más precozmente ninguno de los eventos registrados.
ConclusionesLa prescripción previa al ingreso por infección respiratoria COVID-19 de inhibidores del eje renina-angiotensina se asoció a un menor riesgo de desarrollo del evento primario combinado y a un efecto neutro sobre la mortalidad total y sobre la necesidad de ventilación mecánica invasiva.
The international community is witnessing a health alert situation due to the pandemic caused by a new type of coronavirus, discovered and isolated for the first time in December 2019 in Wuhan (China): the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).1 The clinical condition caused by this germ has been given the name COVID-19. The most common symptoms are fever, cough, dyspnea, and myalgia. The most serious complications that can motivate admission to critical care hospital units include acute respiratory distress syndrome (ARDS), cardiac injury and secondary superinfection.1,2
The pathophysiology of this virus is still unknown. One of the mechanisms that arouses the most interest and controversy is the special relationship between SARS-CoV-2 and the renin–angiotensin–aldosterone system (RAAS), and specifically with the angiotensin-converting enzyme 2 (ACE2), one of its regulatory enzymes. This protein, present on the surface of epithelial cells in the lungs (among other locations),3 is postulated as a route of entry for the virus infection, with the ACE2 cellular expression being reduced once the said cells are infected.4–6 Since conflicting functions have been proposed between ACE2 and the original ACE (or ACE1),2,6,7 the inhibition of the latter (either by direct drugs – ACE inhibitors [ACEI] – or by drugs that block the effects of its metabolite, angiotensin 2 – angiotensin 2 receptor antagonists [ARBs] –) would lead to an increase in the expression of ACE2 in the respiratory epithelium, favoring and maybe worsening the infection by SARS-CoV-2.8–11 Since both ACEIs and ARBs are first-line drugs in the treatment of arterial hypertension,2 the results of studies carried out in an Asian population could be explained, where the adverse prognostic effect of arterial hypertension in patients with COVID-19 stands out.2,12–14
However, based on the results of previous studies in animals in which the protective effect of ACE2 on acute lung damage is demonstrated,15–17 other publications postulate the possible beneficial effect (or, at least, not harmful) of ACEIs or ARBs.4,5,18,19 Thus, as the expression of ACE2 is reduced after the SARS-CoV-2 infection, the angiotensin 2 would remain unopposed, exerting pro-inflammatory effects on various tissues (including the lung)6; on the contrary, by increasing the expression of ACE2 with the use of ACEIs or ARBs, the vasodilator and anti-inflammatory effect of the metabolites resulting from the action of this enzyme would be favored.4,6,20 This argument, together with the scant scientific evidence available to date, has led different international societies to recommend not suspending treatment with these drugs in patients with COVID-19.21,22
Spain is one of the countries with the highest rates of infection and mortality from COVID-19, with more than 243,000 confirmed cases and 27,136 deaths having been reported (as of June 12, 2020), and the province of Ciudad Real has presented the highest mortality rate in the country (200.9 deaths per 100,000 inhabitants).23 Although a prevalence of HT is estimated at around 43% (approximately 16.5 million people),24 there is little evidence of the effect of this comorbidity and the use of drugs such as ACEIs or ARBs in the prognosis of the Spanish population admitted for COVID-19.
The objective of this study is to evaluate the influence of ACEIs and ARBs (prescribed prior to admission and/or administered during hospitalization) on the prognosis of patients admitted for COVID-19 with respiratory involvement at the General University Hospital of Ciudad Real (HGUCR), Spain, a provincial reference center.
MethodsStudy design and populationObservational single-center cohort study of a consecutive series of patients admitted for COVID-19 at the HGUCR. The study protocol was approved by the health center's clinical research ethics committee. Informed consent was obtained for the use of the data, and confidentiality was guaranteed according to the Law on Protection of Personal Data and guarantee of digital rights by creating an anonymized and dissociated database.
Patients aged 18 years or over were included who were hospitalised on the ward or in intensive care unit of the HGUCR between March 1 and April 30, 2020 with a positive result of the polymerase chain reaction (PCR) test for SARS-CoV-2 and clinical and/or radiological semiology of respiratory involvement. Patients admitted to this center who had a negative PCR result for SARS-CoV-2 or who had a positive result but without respiratory involvement upon admission were excluded.
Data collectionVariables were collected (demographic, clinical, comorbidities, lab tests, radiological and therapeutic) of the patients admitted during the period described, and the clinical events that developed during hospitalization were analyzed. Demographic variables (age, sex), clinical variables (main symptomatology, respiratory rate, blood pressure, impaired level of consciousness) and comorbidities (hypertension, diabetes mellitus, active or previous smoker, obesity, obstructive sleep apnea syndrome, chronic obstructive pulmonary disease, chronic kidney disease, ischemic heart disease, heart failure, atrial fibrillation, autoimmune disease, solid organ transplantation) were collected from the clinical history, as were the clinical events that occurred during hospitalization (death from any cause, need for invasive mechanical ventilation). Home treatment prior to admission was consulted in the computerized prescription system of the Castilla-La Mancha Health System. Radiological findings (presence of infiltrate or consolidation, and laterality – unilateral or bilateral –) and lab test variables (leukocyte count, D-dimer, fibrinogen, protein C-reactive, lactate dehydrogenase, urea, creatinine, high-sensitive troponin I, ferritin and interleukin-6 levels) were obtained from the center's computerized record of additional tests. The list of hospital medicinal products administered was obtained from the electronic hospital prescription registry.
DefinitionsThe primary endpoint was defined as all-cause mortality and/or the need for invasive mechanical ventilation support during hospital admission. The admission date was set as the start date of follow-up for each patient, and the end date of follow-up was established as the first event that occurred during hospitalization (need for invasive mechanical ventilation or death from any cause) or, if no event occurred, then the date of hospital discharge.
A clinical management protocol was developed in which the decision to hospitalise was based on criteria published by the Spanish Ministry of Health, which included: respiratory failure (defined as oxygen saturation <90% or blood pressure oxygen <60mmHg); breathing frequency >30 breaths per minute, breathing room air; radiological abnormalities (by chest x-ray or computed tomography) compatible with pneumonia (bilateral pneumonia, or unilateral pneumonia with involvement of different lung lobes) associated with COVID-19; or relevant clinical involvement of other systems.
The following were registered as antihypertensive drugs: ACEIs, ARBs, mineralocorticoid receptor antagonists, calcium antagonists, diuretics, beta-blockers and alpha-blockers with cardiovascular indication. Previous exposure to antihypertensive drugs was defined on the basis of active drug prescription data up to one month prior to the start of follow-up. For the primary analysis, a variable with mutually exclusive categories was established: use of ACEIs or ARBs and non-use of ACEIs or ARBs. Also, the effect of each pharmacological group was analyzed separately. Finally, a difference was made between whether the ACEI or ARB prescription was given prior to or during admission.
Statistical analysisQuantitative variables are presented using central tendency statistics (mean for variables with normal distribution and median for continuous variables with non-Gaussian distribution) and dispersion statistics (standard deviation accompanying the mean for variables with normal distribution, and interquartile range accompanying the median for the rest of the quantitative variables). Qualitative variables are presented as frequencies and percentages. The normality of the distributions was contrasted using the Kolmogorov–Smirnov test. For the hypothesis contrast, when the comparison was made on a quantitative variable, the Student's t-test was used as a parametric test for independent samples, and the Mann–Whitney U test as a non-parametric test. For the comparison of categorical variables, the chi-square test was used, and if the number of effectives was less than 5, then the Fisher's exact test was used. The relationship between multiple variables was studied by applying logistic regression models to evaluate dichotomous qualitative dependent variables, introducing the independent variables that showed statistical significance in the univariate analysis, into the equation. The time to events were analyzed followed a Kaplan–Meier model, and the groups were compared using the log-rank test. For all contrasts, a 5% alpha risk was selected (assuming statistical significance if p<0.05). All the intervals were shown with a 95% confidence level. All the statistical analyses were performed with Statistics software (version 25.0, IBM, New York, United States).
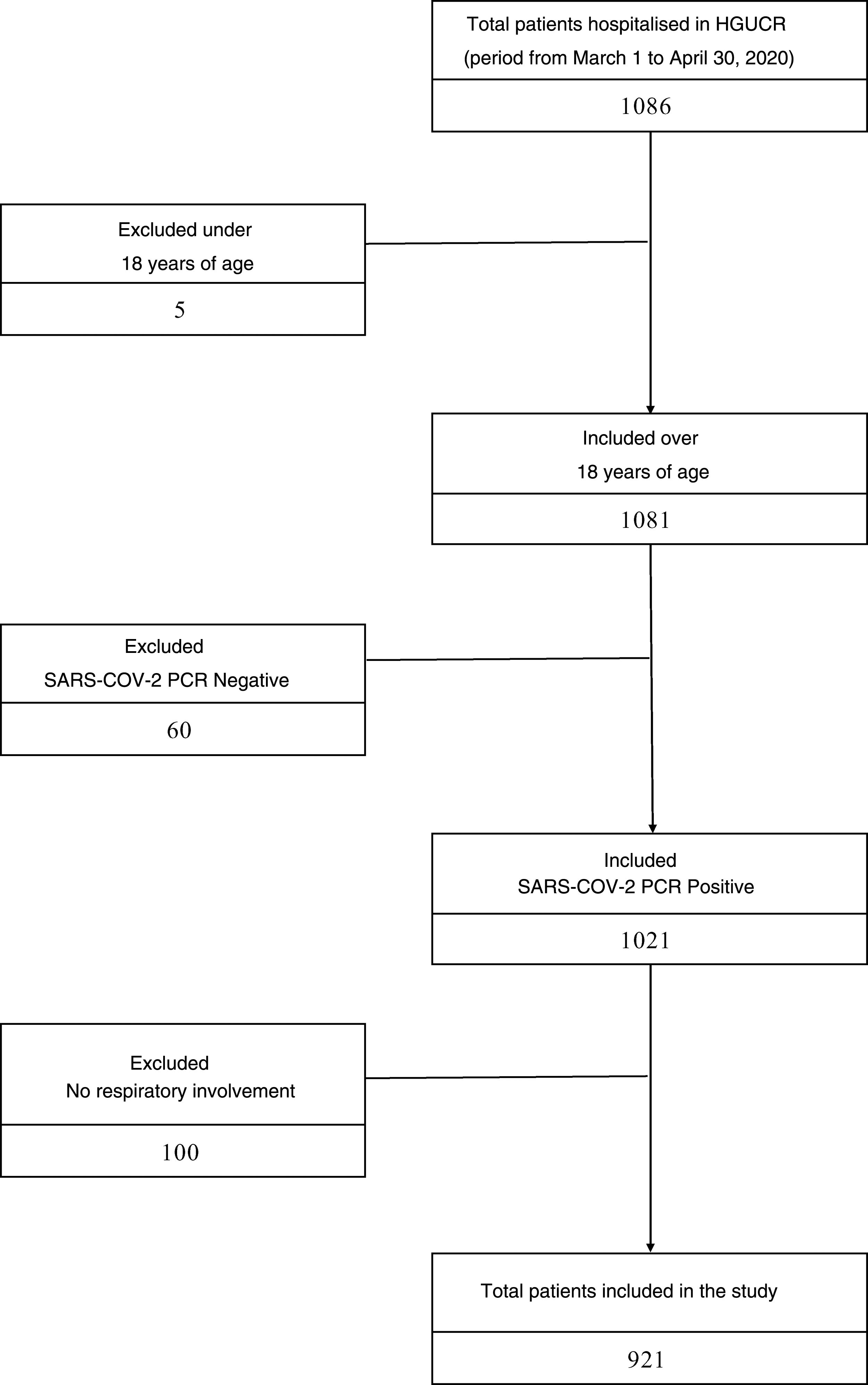
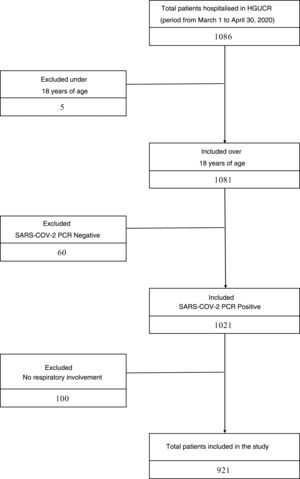
ResultsStudy populationBetween March 1 and April 30, 2020, 1086 patients were hospitalised in our center with COVID-19 infection. Excluded from the study were 5 patients under 18 years of age, 60 patients with a negative PCR result for SARS-CoV-2 and 100 patients who, although they had a positive PCR result for SARS-CoV-2, did not present clinical or radiological respiratory involvement. The sample that was finally analyzed consisted of 921 patients, of whom 400 had prescribed ACEI or ARB prior to admission, and 521 patients did not (Fig. 1). The median age of the study cohort was 78 years (interquartile range 68–85years). The most prevalent cardiovascular risk factor was arterial hypertension (545patients, 59.2%), the majority receiving treatment with an ACEI or ARB (400patients, 42.2%). The mean follow-up period was 8.8 (6.5) days. Other clinical characteristics of the study cohort are listed in Table A.1.
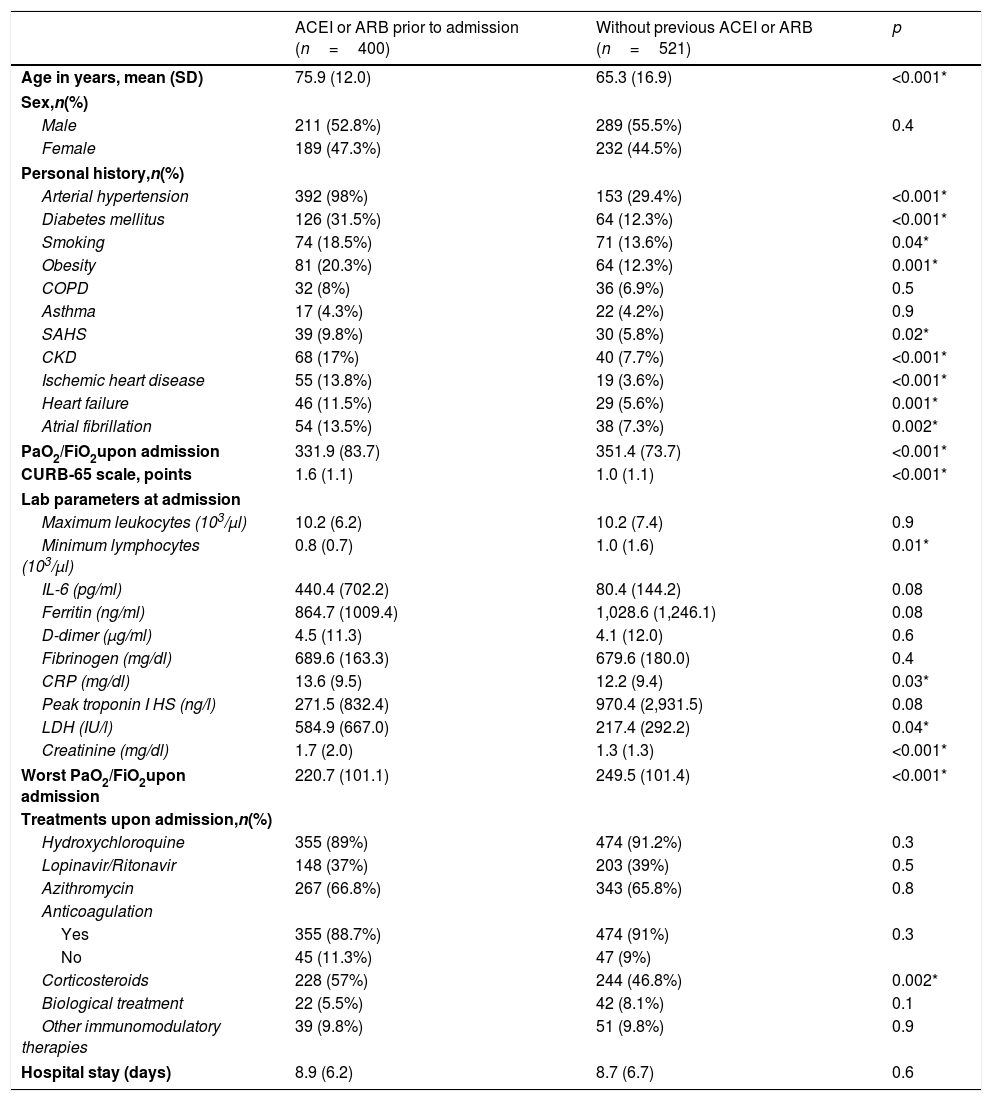
Patients with a previous prescription of ACEI or ARB had a higher mean age compared to the cohort of patients without prior use of these drugs (75.9 [12.0] vs. 65.3 [16.9] years, p<0.001). Likewise, the prevalence of risk factors and established cardiovascular disease was higher in the group with previous use of ACEI or ARB. Furthermore, this group of patients presented a worse Kirby index (relationship between arterial oxygen pressure and inspired fraction of oxygen [PaO2/FiO2]), both on admission and during the course of the condition, as well as more marked lymphopenia and increased inflammatory laboratory parameters, compared to the group without previous treatment with ACEI or ARB. Regarding in-hospital treatment, no differences were observed between the two groups in relation to antiviral treatment, although the prescription of systemic corticosteroids was more frequent in patients with previous prescription of ACEI or ARB (Table 1).
Baseline clinical characteristics and in-hospital management.
| ACEI or ARB prior to admission (n=400) | Without previous ACEI or ARB (n=521) | p | |
|---|---|---|---|
| Age in years, mean (SD) | 75.9 (12.0) | 65.3 (16.9) | <0.001* |
| Sex,n(%) | |||
| Male | 211 (52.8%) | 289 (55.5%) | 0.4 |
| Female | 189 (47.3%) | 232 (44.5%) | |
| Personal history,n(%) | |||
| Arterial hypertension | 392 (98%) | 153 (29.4%) | <0.001* |
| Diabetes mellitus | 126 (31.5%) | 64 (12.3%) | <0.001* |
| Smoking | 74 (18.5%) | 71 (13.6%) | 0.04* |
| Obesity | 81 (20.3%) | 64 (12.3%) | 0.001* |
| COPD | 32 (8%) | 36 (6.9%) | 0.5 |
| Asthma | 17 (4.3%) | 22 (4.2%) | 0.9 |
| SAHS | 39 (9.8%) | 30 (5.8%) | 0.02* |
| CKD | 68 (17%) | 40 (7.7%) | <0.001* |
| Ischemic heart disease | 55 (13.8%) | 19 (3.6%) | <0.001* |
| Heart failure | 46 (11.5%) | 29 (5.6%) | 0.001* |
| Atrial fibrillation | 54 (13.5%) | 38 (7.3%) | 0.002* |
| PaO2/FiO2upon admission | 331.9 (83.7) | 351.4 (73.7) | <0.001* |
| CURB-65 scale, points | 1.6 (1.1) | 1.0 (1.1) | <0.001* |
| Lab parameters at admission | |||
| Maximum leukocytes (103/μl) | 10.2 (6.2) | 10.2 (7.4) | 0.9 |
| Minimum lymphocytes (103/μl) | 0.8 (0.7) | 1.0 (1.6) | 0.01* |
| IL-6 (pg/ml) | 440.4 (702.2) | 80.4 (144.2) | 0.08 |
| Ferritin (ng/ml) | 864.7 (1009.4) | 1,028.6 (1,246.1) | 0.08 |
| D-dimer (μg/ml) | 4.5 (11.3) | 4.1 (12.0) | 0.6 |
| Fibrinogen (mg/dl) | 689.6 (163.3) | 679.6 (180.0) | 0.4 |
| CRP (mg/dl) | 13.6 (9.5) | 12.2 (9.4) | 0.03* |
| Peak troponin I HS (ng/l) | 271.5 (832.4) | 970.4 (2,931.5) | 0.08 |
| LDH (IU/l) | 584.9 (667.0) | 217.4 (292.2) | 0.04* |
| Creatinine (mg/dl) | 1.7 (2.0) | 1.3 (1.3) | <0.001* |
| Worst PaO2/FiO2upon admission | 220.7 (101.1) | 249.5 (101.4) | <0.001* |
| Treatments upon admission,n(%) | |||
| Hydroxychloroquine | 355 (89%) | 474 (91.2%) | 0.3 |
| Lopinavir/Ritonavir | 148 (37%) | 203 (39%) | 0.5 |
| Azithromycin | 267 (66.8%) | 343 (65.8%) | 0.8 |
| Anticoagulation | |||
| Yes | 355 (88.7%) | 474 (91%) | 0.3 |
| No | 45 (11.3%) | 47 (9%) | |
| Corticosteroids | 228 (57%) | 244 (46.8%) | 0.002* |
| Biological treatment | 22 (5.5%) | 42 (8.1%) | 0.1 |
| Other immunomodulatory therapies | 39 (9.8%) | 51 (9.8%) | 0.9 |
| Hospital stay (days) | 8.9 (6.2) | 8.7 (6.7) | 0.6 |
ARB: angiotensin receptor antagonist2; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; FiO2: fraction of inspired oxygen; ACEI: angiotensin converting enzyme inhibitor; IL-6: interleukin 6; LDH: lactate dehydrogenase; PaO2: partial pressure of oxygen; CRP: C-reactive protein; SAHS: sleep apnea-hypopnea syndrome; HS: highly sensitive.
Data expressed as an absolute number (percentage) or mean (standard deviation).
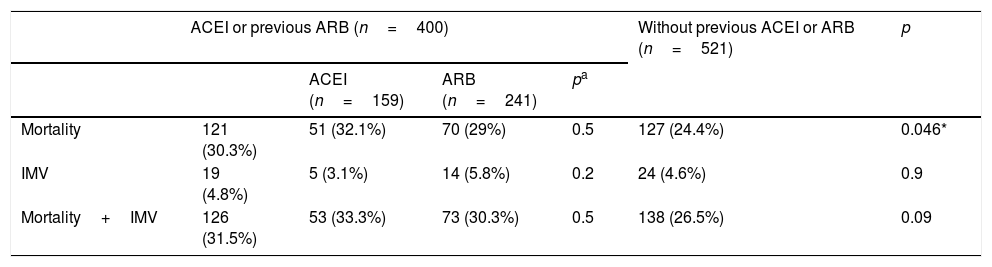
During the follow-up period, 264 individuals (28.7%) presented the primary composite endpoint (all-cause mortality or need for invasive mechanical ventilation). A total of 248 (26.9%) patients died during hospital admission, while 43 (4.7%) patients required invasive ventilation support. Table 2 shows the incidents and their differences with respect to each of the events recorded in relation to the prescription of ACEI or ARB.
Incidence of in-hospital events.
| ACEI or previous ARB (n=400) | Without previous ACEI or ARB (n=521) | p | ||||
|---|---|---|---|---|---|---|
| ACEI (n=159) | ARB (n=241) | pa | ||||
| Mortality | 121 (30.3%) | 51 (32.1%) | 70 (29%) | 0.5 | 127 (24.4%) | 0.046* |
| IMV | 19 (4.8%) | 5 (3.1%) | 14 (5.8%) | 0.2 | 24 (4.6%) | 0.9 |
| Mortality+IMV | 126 (31.5%) | 53 (33.3%) | 73 (30.3%) | 0.5 | 138 (26.5%) | 0.09 |
| ACEI or ARB in hospitalization (n=145) | Without ACEI or ARB in hospitalization (n=776) | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ACEI or ARB maintained (n=121) | Withdrawal of the drug (n=279) | pb | ACEI (n=60) | ARB (n=85) | pc | ||||
| Mortality | 39 (26.9%) | 32 (26.4%) | 89 (31.9%) | 0.3 | 14 (23.3%) | 25 (29.4%) | 0.4 | 209 (26.9%) | 0.9 |
| IMV | 10 (6.9%) | 7 (5.8%) | 12 (4.3%) | 0.5 | 2 (3.3%) | 8 (9.4%) | 0.2 | 33 (4.3%) | 0.2 |
| Mortality+IMV | 44 (30.3%) | 35 (28.9%) | 91 (32.6%) | 0.5 | 15 (25%) | 29 (34.1%) | 0.2 | 220 (28.4%) | 0.6 |
ARB: angiotensin receptor antagonists 2; ACEI: angiotensin converting enzyme inhibitors; IMV: invasive mechanical ventilation.
Data expressed as an absolute number (percentage).
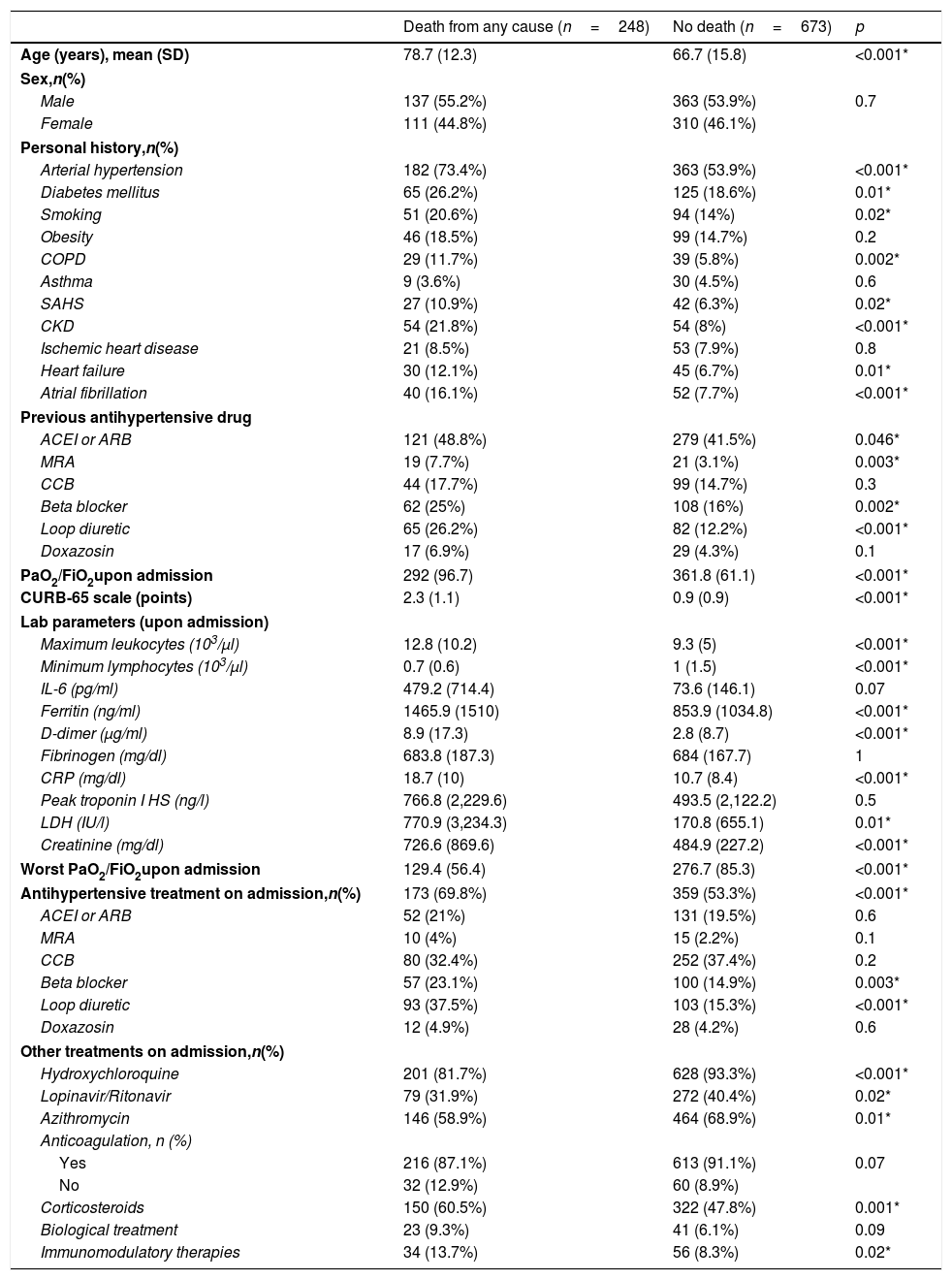
Patients who died during hospitalization were older and had a higher prevalence of hypertension, diabetes mellitus, smoking, chronic obstructive pulmonary disease, chronic kidney disease, heart failure, and atrial fibrillation, compared to patients who were alive at discharge. They also presented worse PaO2/FiO2 (both on admission and during the course of the condition), as well as a higher analytical inflammation parameters. Regarding treatment directed at COVID-19 infection, higher rates of prescription of hydroxychloroquine, the lopinavir/ritonavir combination, and azithromycin were observed in patients who did not die; however, these results should be interpreted with caution, since the study design was not aimed at studying the differences in the administration of treatments directed against COVID-19 infection, and it may be possible that these drugs were not administered to patients who died as a measure of limitation of therapeutic effort, given the aforementioned differences in terms of age between both groups (Table 3).
Comparison of baseline clinical, analytical and therapeutic characteristics based on hospital mortality.
| Death from any cause (n=248) | No death (n=673) | p | |
|---|---|---|---|
| Age (years), mean (SD) | 78.7 (12.3) | 66.7 (15.8) | <0.001* |
| Sex,n(%) | |||
| Male | 137 (55.2%) | 363 (53.9%) | 0.7 |
| Female | 111 (44.8%) | 310 (46.1%) | |
| Personal history,n(%) | |||
| Arterial hypertension | 182 (73.4%) | 363 (53.9%) | <0.001* |
| Diabetes mellitus | 65 (26.2%) | 125 (18.6%) | 0.01* |
| Smoking | 51 (20.6%) | 94 (14%) | 0.02* |
| Obesity | 46 (18.5%) | 99 (14.7%) | 0.2 |
| COPD | 29 (11.7%) | 39 (5.8%) | 0.002* |
| Asthma | 9 (3.6%) | 30 (4.5%) | 0.6 |
| SAHS | 27 (10.9%) | 42 (6.3%) | 0.02* |
| CKD | 54 (21.8%) | 54 (8%) | <0.001* |
| Ischemic heart disease | 21 (8.5%) | 53 (7.9%) | 0.8 |
| Heart failure | 30 (12.1%) | 45 (6.7%) | 0.01* |
| Atrial fibrillation | 40 (16.1%) | 52 (7.7%) | <0.001* |
| Previous antihypertensive drug | |||
| ACEI or ARB | 121 (48.8%) | 279 (41.5%) | 0.046* |
| MRA | 19 (7.7%) | 21 (3.1%) | 0.003* |
| CCB | 44 (17.7%) | 99 (14.7%) | 0.3 |
| Beta blocker | 62 (25%) | 108 (16%) | 0.002* |
| Loop diuretic | 65 (26.2%) | 82 (12.2%) | <0.001* |
| Doxazosin | 17 (6.9%) | 29 (4.3%) | 0.1 |
| PaO2/FiO2upon admission | 292 (96.7) | 361.8 (61.1) | <0.001* |
| CURB-65 scale (points) | 2.3 (1.1) | 0.9 (0.9) | <0.001* |
| Lab parameters (upon admission) | |||
| Maximum leukocytes (103/μl) | 12.8 (10.2) | 9.3 (5) | <0.001* |
| Minimum lymphocytes (103/μl) | 0.7 (0.6) | 1 (1.5) | <0.001* |
| IL-6 (pg/ml) | 479.2 (714.4) | 73.6 (146.1) | 0.07 |
| Ferritin (ng/ml) | 1465.9 (1510) | 853.9 (1034.8) | <0.001* |
| D-dimer (μg/ml) | 8.9 (17.3) | 2.8 (8.7) | <0.001* |
| Fibrinogen (mg/dl) | 683.8 (187.3) | 684 (167.7) | 1 |
| CRP (mg/dl) | 18.7 (10) | 10.7 (8.4) | <0.001* |
| Peak troponin I HS (ng/l) | 766.8 (2,229.6) | 493.5 (2,122.2) | 0.5 |
| LDH (IU/l) | 770.9 (3,234.3) | 170.8 (655.1) | 0.01* |
| Creatinine (mg/dl) | 726.6 (869.6) | 484.9 (227.2) | <0.001* |
| Worst PaO2/FiO2upon admission | 129.4 (56.4) | 276.7 (85.3) | <0.001* |
| Antihypertensive treatment on admission,n(%) | 173 (69.8%) | 359 (53.3%) | <0.001* |
| ACEI or ARB | 52 (21%) | 131 (19.5%) | 0.6 |
| MRA | 10 (4%) | 15 (2.2%) | 0.1 |
| CCB | 80 (32.4%) | 252 (37.4%) | 0.2 |
| Beta blocker | 57 (23.1%) | 100 (14.9%) | 0.003* |
| Loop diuretic | 93 (37.5%) | 103 (15.3%) | <0.001* |
| Doxazosin | 12 (4.9%) | 28 (4.2%) | 0.6 |
| Other treatments on admission,n(%) | |||
| Hydroxychloroquine | 201 (81.7%) | 628 (93.3%) | <0.001* |
| Lopinavir/Ritonavir | 79 (31.9%) | 272 (40.4%) | 0.02* |
| Azithromycin | 146 (58.9%) | 464 (68.9%) | 0.01* |
| Anticoagulation, n (%) | |||
| Yes | 216 (87.1%) | 613 (91.1%) | 0.07 |
| No | 32 (12.9%) | 60 (8.9%) | |
| Corticosteroids | 150 (60.5%) | 322 (47.8%) | 0.001* |
| Biological treatment | 23 (9.3%) | 41 (6.1%) | 0.09 |
| Immunomodulatory therapies | 34 (13.7%) | 56 (8.3%) | 0.02* |
CCB: calcium channel blockers; ARB: angiotensin receptor antagonist 2; MRA: mineralocorticoid receptor antagonist; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; FiO2: fraction of inspired oxygen; ACEI: angiotensin converting enzyme inhibitor; IL-6: interleukin 6; LDH: lactate dehydrogenase; PaO2: partial pressure of oxygen; CRP: C-reactive protein; SAHS: sleep apnea-hypopnea syndrome; HS: highly sensitive.
Data expressed as an absolute number (percentage) or mean (standard deviation).
The primary event was presented during admission by those patients who were older and those who presented a higher prevalence of risk factors and cardiovascular disease. It should be noted that greater respiratory involvement (both at admission and during disease progression) and worse laboratory parameters (greater lymphopenia, higher levels of inflammatory parameters and deterioration of renal function) were observed in these patients, compared to those who did not develop the primary event. These last differences were similar to those observed in the comparison between those who required invasive ventilation support and those who did not. The comparative analysis of the baseline clinical characteristics, laboratory parameters and therapies received during hospitalization based on the development of the combined primary event or the need for invasive mechanical ventilation is shown in Tables A.2 and A.3, respectively.
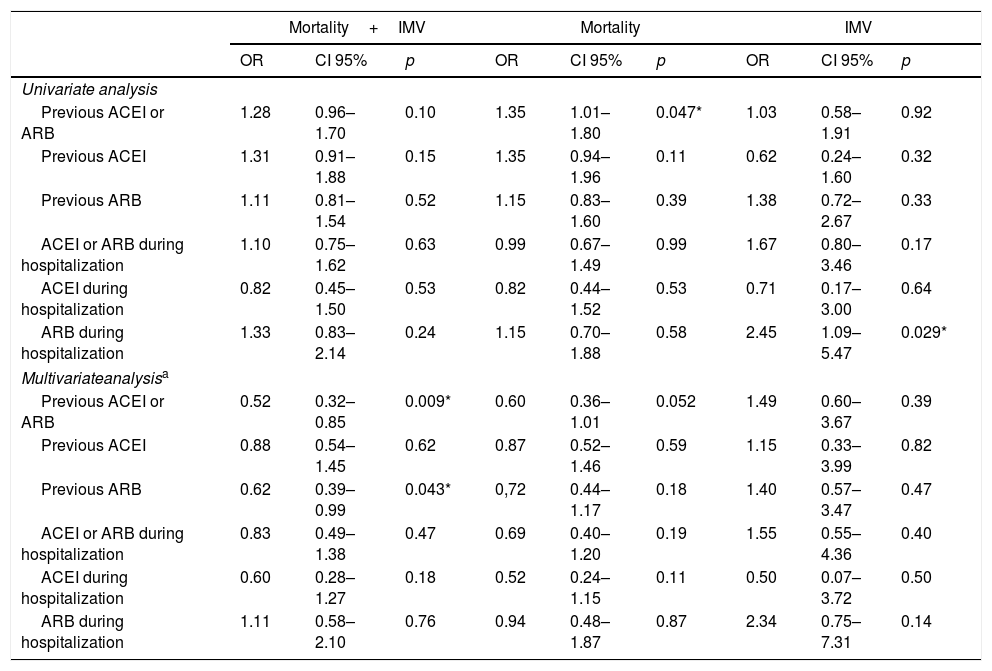
Inhibition of the renin–angiotensin system and events during disease progressionPrevious treatment with ACEI or ARB, in combination or separately, showed a neutral effect on the principle endpoint in the univariate analysis (Table 4). However, in the logistic regression model adjusted to those variables that were associated with the combined event (Table A.4) the previous use of ACEIs or ARBs was associated with a lower risk of developing the same, as was the case with the exclusive prior prescription of ARBs (Table 4).
Relationship between ACEI/ARB and in-hospital events.
| Mortality+IMV | Mortality | IMV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | CI 95% | p | OR | CI 95% | p | OR | CI 95% | p | |
| Univariate analysis | |||||||||
| Previous ACEI or ARB | 1.28 | 0.96–1.70 | 0.10 | 1.35 | 1.01–1.80 | 0.047* | 1.03 | 0.58–1.91 | 0.92 |
| Previous ACEI | 1.31 | 0.91–1.88 | 0.15 | 1.35 | 0.94–1.96 | 0.11 | 0.62 | 0.24–1.60 | 0.32 |
| Previous ARB | 1.11 | 0.81–1.54 | 0.52 | 1.15 | 0.83–1.60 | 0.39 | 1.38 | 0.72–2.67 | 0.33 |
| ACEI or ARB during hospitalization | 1.10 | 0.75–1.62 | 0.63 | 0.99 | 0.67–1.49 | 0.99 | 1.67 | 0.80–3.46 | 0.17 |
| ACEI during hospitalization | 0.82 | 0.45–1.50 | 0.53 | 0.82 | 0.44–1.52 | 0.53 | 0.71 | 0.17–3.00 | 0.64 |
| ARB during hospitalization | 1.33 | 0.83–2.14 | 0.24 | 1.15 | 0.70–1.88 | 0.58 | 2.45 | 1.09–5.47 | 0.029* |
| Multivariateanalysisa | |||||||||
| Previous ACEI or ARB | 0.52 | 0.32–0.85 | 0.009* | 0.60 | 0.36–1.01 | 0.052 | 1.49 | 0.60–3.67 | 0.39 |
| Previous ACEI | 0.88 | 0.54–1.45 | 0.62 | 0.87 | 0.52–1.46 | 0.59 | 1.15 | 0.33–3.99 | 0.82 |
| Previous ARB | 0.62 | 0.39–0.99 | 0.043* | 0,72 | 0.44–1.17 | 0.18 | 1.40 | 0.57–3.47 | 0.47 |
| ACEI or ARB during hospitalization | 0.83 | 0.49–1.38 | 0.47 | 0.69 | 0.40–1.20 | 0.19 | 1.55 | 0.55–4.36 | 0.40 |
| ACEI during hospitalization | 0.60 | 0.28–1.27 | 0.18 | 0.52 | 0.24–1.15 | 0.11 | 0.50 | 0.07–3.72 | 0.50 |
| ARB during hospitalization | 1.11 | 0.58–2.10 | 0.76 | 0.94 | 0.48–1.87 | 0.87 | 2.34 | 0.75–7.31 | 0.14 |
ARB: angiotensin receptor antagonists 2; CI 95%: 95% confidence interval; ACEI: angiotensin converting enzyme inhibitors; OR: odds ratio; IMV: invasive mechanical ventilation.
Regarding mortality while hospitalised, the univariate analysis showed a higher risk associated with the previous use of ACEI or ARB (OR 1.35; CI 95%: 1.01–1.80; p=0.047), although this relationship disappeared after the adjusted analysis (Table 4).
The use of ACEIs or ARBs during hospitalization did not show an impact on the risk of developing the principle endpoint or death from any cause, neither in the univariate analysis nor after adjusting for the predictor variables of each of the events. However, the prescription of ARBs during admission was associated with an increased risk of the need for invasive ventilation support (OR 2.45; CI 95%: 1.09–5.47; p=0.029), although, finally, in the adjusted logistic regression model it showed a neutral effect on said event (Table 4).
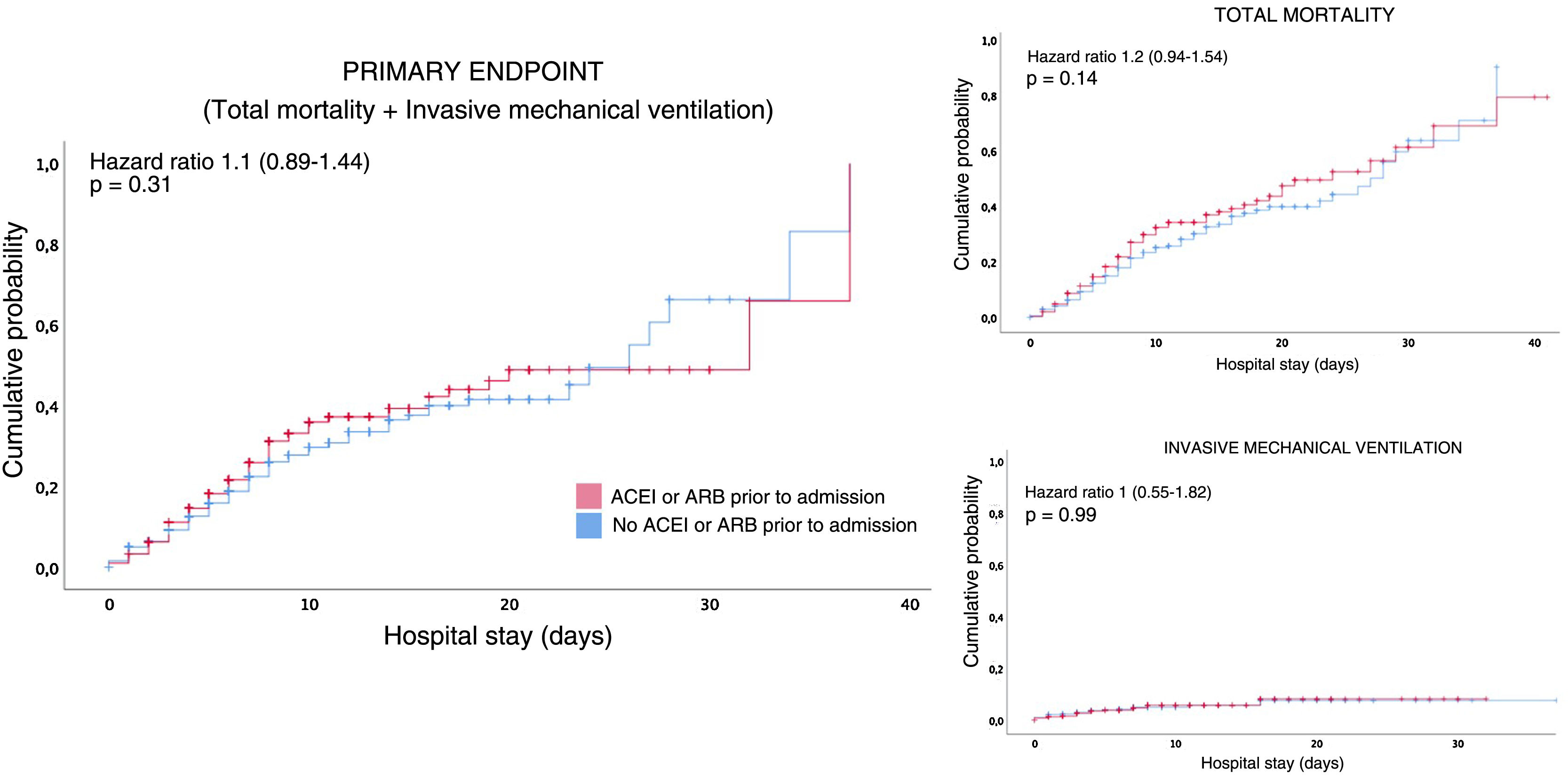
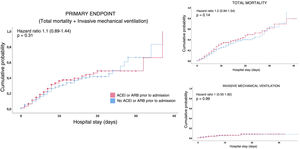
Survival analysisIn the survival analysis, a comparison of the patients who were under outpatient treatment with ACEI or ARB versus those who were not prescribed ACEIs or ARBs showed no greater risk of presenting an earlier combined primary event (Fig. 2A), or death from any cause (Fig. 2B), nor the need for invasive mechanical ventilation (Fig. 2C).
DiscussionIn the present study, the influence of prescribing ACEI or ARB drugs prior to admission and during hospitalization on the in-hospital prognosis of patients with respiratory infection caused by SARS-CoV-2 has been evaluated. It demonstrated a neutral effect on the risk of mortality due to any cause or the need for invasive mechanical ventilation. Likewise, the use of these drugs prior to hospitalization for respiratory infection due to SARS-CoV-2 was associated with a lower risk of developing the combined primary event in the adjusted analysis. This result supports the recommendations made by different scientific societies to maintain treatment with these medicinal products.21,22
No differences were observed in the survival analysis, although this result could be influenced by the short follow-up period (only 38% had hospital stays longer than 8days). This fact may be justified by early hospital discharges due to the saturation of the health system.
The observed results could be explained from the pathophysiological hypotheses about the SARS-CoV-2 infection. The higher percentage of deceased patients in the group of previous prescription of ACEI or ARB agrees with the hypothesis that ACEI and ARB promote the expression of ACE2 at the level of the respiratory epithelium, and therein can increase the susceptibility of infection among patients with use of said medicinal products.4–6,8–11 Likewise, in this study, a higher prevalence of cardiovascular risk factors and cardiovascular disease was observed among patients who develop the primary event. This is in harmony with previous observational studies in which a worse progression of COVID-19 infection was associated with these conditions, in which ACEIs and ARBs are drugs that are widely used.2,12–14 However, in our study, the previous use of ACEIs or ARBs did not show a negative impact on the risk of death from any cause or the need for invasive mechanical ventilation, and it was even associated with a lower risk of developing the combined event. This fact could be explained by the increasingly accepted idea of the dual role that ACE2 plays in the COVID-19 infection: although its overexpression could increase the risk of infection, the reduction of the expression of ACE2 associated with aging and cardiovascular comorbidities and potentiated by SARS-CoV-2 infection,17,25 would leave angiotensin 2 unopposed, inducing pro-inflammatory effects on various tissues. The enzyme ACE2 works by inhibiting angiotensin 2 and increasing the production of angiotensin 1–7, a peptide with anti-inflammatory and vasodilator effects.4,6 Previous studies have shown that patients who survive acute respiratory distress have higher levels of said peptide compared to the deceased.26 This hypothesis agrees with the results of our study which evidenced not only a lower risk of the primary event combined with the indistinct prior use of ACEI or ARB, but also with those whose sole prescription was an ARB drug.
The great amount of interest in the effect of the inhibition of the renin–angiotensin axis in patients with COVID-19 infection has promoted the publication of various observational studies. The majority of these studies evaluate the risk associated with the consumption of ACEIs or ARBs when suffering from COVID infection or being hospitalised for it. Two of the most relevant works are those recently published by Abajo et al.27 and by Mancia et al.28 Both are case–control studies (conducted in a Spanish and Italian cohort, respectively) in which the use of ACEI or ARB prior to admission did not increase the risk of hospitalization due to COVID-19.27,28 This agrees with the results obtained in our study. However, our study highlights a higher percentage of deceased patients among those who previously took ACEIs or ARBs compared to the rest, and these differences were not observed in the aforementioned studies by Abajo et al.27 and Mancia et al.28 In this sense, it should be noted that approximately half of the COVID-19 cases in the Italian cohort considered “mild-moderate” (those that did not require admission to intensive care units nor died) did not require hospitalisation,28 while our study analyses the possible effect of ACEI or ARB on the prognosis only of hospitalised patients. Another possible justification is the difference between the characteristics of the study cohorts. Despite the similarity between the mean ages of the study populations, the mean age of the group that died in our study is higher than that of the patients with a worse progression in both studies (78.7 [12.3] vs. 75.3 [12.3] vs. 75.0 [10.0] years). Additionally, the current study population with COVID infection had a higher prevalence of various comorbid disorders, highlighting the differences in rates of chronic kidney disease (11.8% vs. 7.8% vs. 2.9%) and heart failure (8.2% vs. 7% vs. 5.1%). Likewise, compared to the study by Mancia et al.,28 the cohort of our study presented a higher frequency of chronic obstructive pulmonary disease (7.4% vs. 3%) or asthma (4.2% vs. 0.3%).28
Publications about the effect of ACEIs or ARBs on the in-hospital progress of patients with COVID-19 infection are harder to find. In this sense, one of the most outstanding studies is that of Gao et al.,29 in which the use of ACEIs or ARBs during hospitalization in hypertensive patients was associated with a lower risk of overall mortality compared to patients who were not treated with these renin–angiotensin axis inhibitors. In our study, the effect of these drugs on total mortality was neutral. However, the differences in terms of death figures between the two studies stand out. Specifically, the mortality rate in the group treated with ACEI or ARB of the Chinese cohort was 3.7%, a figure that contrasts with the mortality of 26.9% observed in the patients in our study who received ACEI or ARB during hospitalization. These differences can be justified for several reasons. First, the difference in the sample sizes of both cohorts: while our study included 400 patients with previous prescriptions of ACEIs or ARBs, the study cohort by Gao et al.29 only registered 188 patients who were being administered these drugs. Furthermore, this disparity in mortality figures could be explained by taking into account the differences in the baseline clinical characteristics of both cohorts: older age in our cohort of patients taking ACEIs or ARBs (data expressed in median and interquartile range: 78 [68–85] vs. 64 [55–68] years), higher prevalence of diabetes mellitus (31.5% vs. 23.4%), chronic obstructive pulmonary disease (8% vs. 0.5%) or chronic kidney disease (17% vs. 3.7%).
In summary, this work constitutes one of the first studies carried out in a Spanish cohort analysing the effect of both home-use and use during hospitalization of ACEIs or ARBs on hospital mortality and the need for invasive ventilation support in patients with COVID-19. The results described correspond with those of other recent publications and support the recommendations of the different international societies regarding not stopping these drugs. However, the observational nature of all these studies limits their interpretation, requiring the development of randomized clinical trials that provide evidence in this regard.
LimitationsThis study has several limitations. First, although the drug prescription for the month prior to hospitalization of the included patients was reviewed, it was not possible to guarantee correct therapeutic adherence. Furthermore, the effects according to different doses of the drugs were not evaluated. Another limitation was the unavailability of a control group without established COVID-19 infection that would allow us to compare the results of our cohort of cases. Likewise, the saturation of the critical care units could influence the selection of patients who are candidates for invasive ventilation support measures and, therefore, the incidence of the events analyzed. Additionally, the patients’ follow-up period was limited to their hospital stay, which was a short time interval. Finally, due to the observational and single-center nature of this study, the residual effect of possible unknown or unstudied confounding factors cannot be ruled out.
ConclusionsPatients with respiratory infection caused by SARS-CoV-2 who were admitted to the HGUCR and with a previous prescription of ACEIs or ARBs had a lower risk of developing the combined primary event. Likewise, the use of these drugs prior to admission or during the same showed a neutral effect on total mortality and on the need for invasive mechanical ventilation.
FundingNo type of funding has been received for the preparation of this work.
Conflict of interestsThe authors of this document declare the absence of any conflict of interest related to the publication of this manuscript.
We want to thank Dr. Lourdes Porras Leal and Dr. Francisco Javier González Gasca, heads of the Infectious Diseases department in our center. In addition, we want to thank the management, effort and work of the Medical Directorate, the Nursing Directorate and the Directorate of the Comprehensive Care Management of Ciudad Real. Finally, we thank the colleagues from Internal Medicine, Intensive Medicine and, in general, to all those who participated in the care of patients with COVID-19 infection.
Please cite this article as: Martínez-del Río J, Piqueras-Flores J, Nieto-Sandoval Martín de la Sierra P, Negreira-Caamaño M, Águila-Gordo D, Mateo-Gómez C, et al. Análisis de la relación entre los inhibidores del sistema renina-angiotensina y la evolución de pacientes hospitalizados por infección respiratoria COVID-19. Med Clin (Barc). 2020. https://doi.org/10.1016/j.medcli.2020.07.004