Unfractionated heparins (UFH) and low molecular weight heparins (LMWH) are widely used drugs that have prevented and treated countless arterial and venous thrombotic problems. However, its use is also associated with a paradoxical reaction leading to a potentially life-threatening prothrombotic state, resulting in numerous amputations or fatal outcomes each year.1 In the United States, approximately 12 million people, or one-third of hospitalized patients, are exposed to heparin each year, with a frequency of heparin-induced thrombocytopenia (HIT) of 0.76% in patients receiving therapeutic doses of intravenous UFH and less than 0.1% in those receiving prophylactic doses. Although the number of amputations due to HIT is unknown, recent studies describe an incidence of 3%–4%.2
HIT is an immune adverse drug reaction in patients recently exposed to heparin, which presents with thrombocytopenia frequently associated with venous or arterial thrombosis and significant morbidity and mortality. It is currently difficult to diagnose and until recently there were no effective treatment options.
There are several guidelines that address the diagnosis and treatment of HIT taking into account the levels of evidence and grade of recommendation or following GRADE-type methodologies. The lack of randomised studies means that the level of evidence provided by these guidelines is generally low. Most recommend the use of predictive clinical scores to determine the probability of HIT, followed by laboratory tests to confirm the presence of anti-PF4/heparin antibodies.2–4
Characteristics of heparin-induced thrombocytopeniaThe true incidence of HIT is unknown due to the lack of prospective studies. It occurs in approximately 0.5%–1% of patients exposed to UFH for medical and surgical indications, varying in relation to the type of pathology and drug used. The incidence is lower (0.1%−0.5%) in patients receiving LMWH.2 HIT is more common in patients undergoing orthopaedic or cardiac surgery and in those who require extracorporeal membrane oxygenation (ECMO), than in medical patients or those with obstetric diseases. It has been suggested that postoperative inflammation and the long-term administration of heparin could be responsible for its higher incidence. The pathophysiology of HIT is characterized by the presence of antibodies, generally IgG type, that react against the heparin-platelet factor 4 (PF4) complex. These antibodies induce a prothrombotic state, causing platelet activation and aggregation, as well as endothelial and monocyte activation and generation of procoagulant microparticles, with intense thrombin formation.1 Paradoxically, HIT is one of the most powerful prothrombotic states described, although it is caused by an anticoagulant drug. Unlike other clinical situations characterized by platelet consumption or destruction, it does not usually present with haemorrhage.
Classification of heparin-induced thrombocytopeniaDepending on its origin, HIT is divided into non-immune HIT type I, which is much more common than type II, of an immune mediated nature, and which can occur from the first day of therapy. The first is a mild reaction (it is rare for the platelet count to be less than 100 × 109/L), which is not associated with any complications. It is produced by the formation of platelet microaggregates due to their activation after administration of generally high doses of intravenous heparin, and recovery typically occurs within 3–4 days despite continuing with the drug.
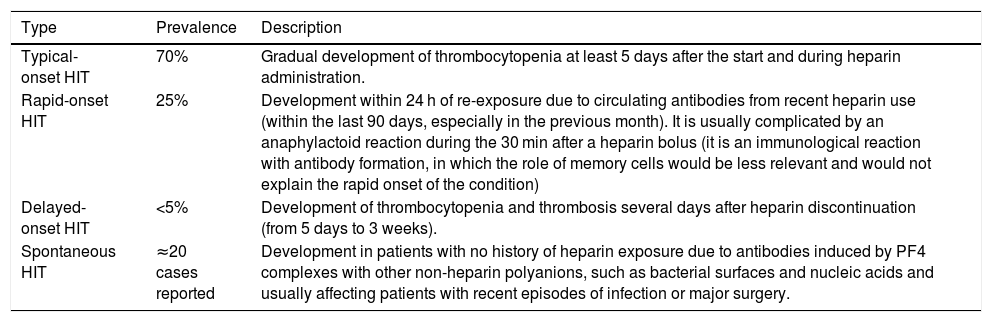
Within HIT type II, there are eight subtypes, classified according to the time of onset of thrombocytopenia and the recovery time.2–4Table 1 shows the four most common types. The other four more unusual presentations are: i) persistent HIT, defined as one in which the rise in platelets is not observed until at least one week after discontinuation of heparin; ii) fondaparinux-induced HIT; iii) heparin flush-induced HIT, only induced by exposure to the heparin used for catheter anticoagulation, and iv) HIT associated with disseminated intravascular coagulation (DIC). In any of these cases, the average antibody clearance is 50–80 days.5
Classification of the most common HIT subtypes.
| Type | Prevalence | Description |
|---|---|---|
| Typical-onset HIT | 70% | Gradual development of thrombocytopenia at least 5 days after the start and during heparin administration. |
| Rapid-onset HIT | 25% | Development within 24 h of re-exposure due to circulating antibodies from recent heparin use (within the last 90 days, especially in the previous month). It is usually complicated by an anaphylactoid reaction during the 30 min after a heparin bolus (it is an immunological reaction with antibody formation, in which the role of memory cells would be less relevant and would not explain the rapid onset of the condition) |
| Delayed-onset HIT | <5% | Development of thrombocytopenia and thrombosis several days after heparin discontinuation (from 5 days to 3 weeks). |
| Spontaneous HIT | ≈20 cases reported | Development in patients with no history of heparin exposure due to antibodies induced by PF4 complexes with other non-heparin polyanions, such as bacterial surfaces and nucleic acids and usually affecting patients with recent episodes of infection or major surgery. |
The objective of this review is to help the clinician with the correct diagnosis, prevention and treatment of patients with HIT, focusing on type II, in the context of Spanish hospitals.
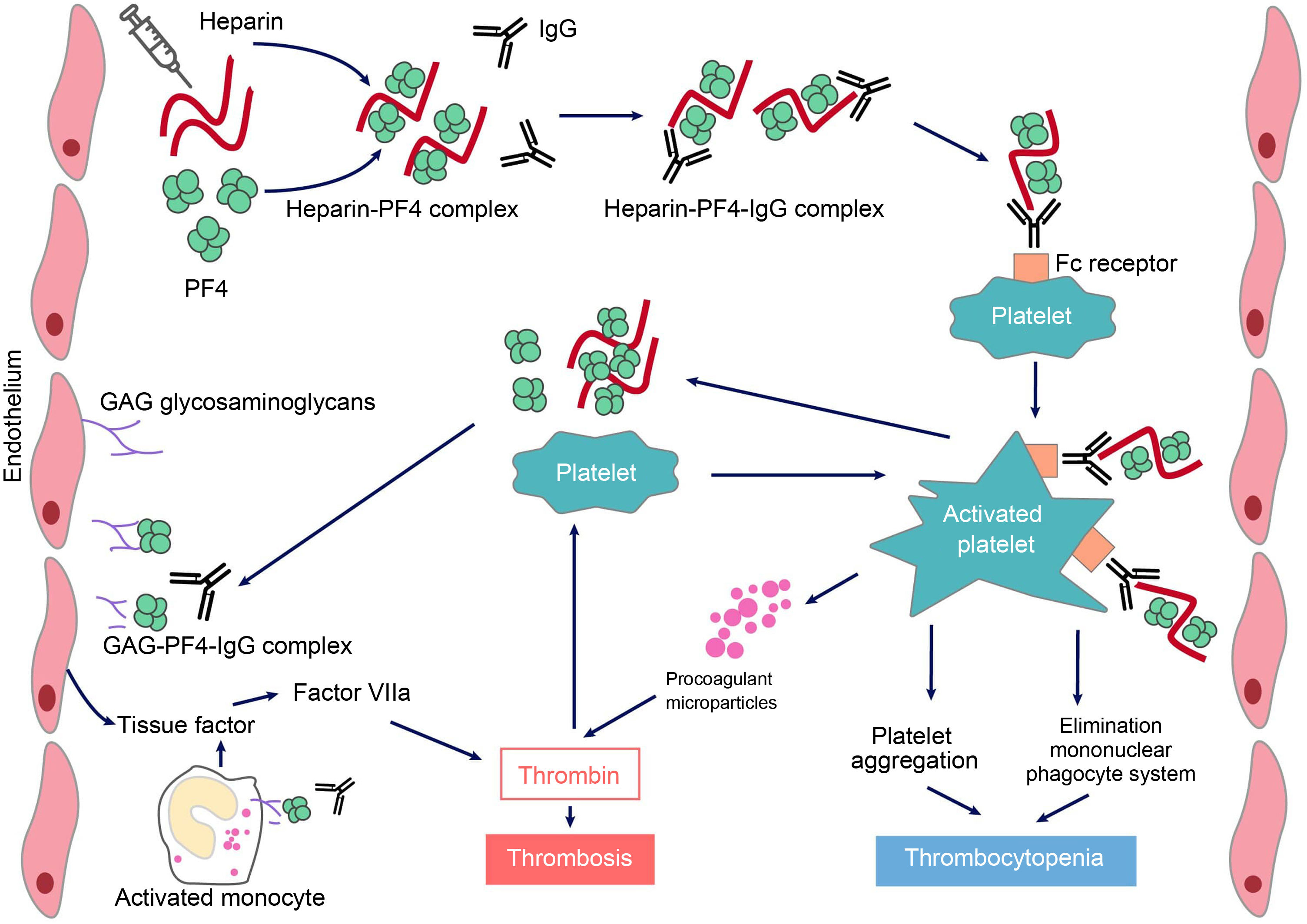
Pathogenesis and pathophysiology of heparin-induced thrombocytopeniaUnder normal conditions, PF4 is stored in α platelet granules and is released following activation. It is positively charged and therefore can bind to negatively charged heparan sulfate (a substance on the surface of endothelial cells similar to heparin); PF4 can also bind to exogenous heparin with a much higher affinity than to heparan sulfate, which can initiate an immune response.
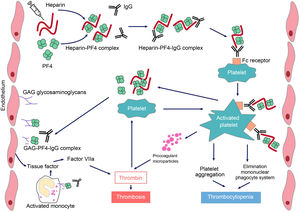
HIT is induced by IgG-type antibodies (by IgA or IgM is not so common) produced by plasma cells, which recognise neoepitopes of heparin/PF4 complexes. Heparin interacts with PF4 forming a neoantigen, in an optimal stoichiometric ratio (1:1). The resulting complex binds to the Fcɤ receptors in platelets and monocytes, inducing their activation and the generation of procoagulant microparticles. Activated platelets produce prothrombotic substances (such as thrombin) and PF4, creating a state of severe hypercoagulability and a vicious circle that can only be broken by discontinuing heparin and initiating appropriate treatment. PF4 can also bind to the von Willebrand factor (VWF) strings released after endothelial activation, and can form antigenic complexes recognized by HIT antibodies, which promote platelet adhesion and the spread of thrombosis.6 In addition, antibodies generated via endothelial cell binding induce the development of tissue factor. Finally, immune complexes activate neutrophils, favouring the formation of neutrophil extracellular traps (NET) providing another procoagulant stimulus.7 Platelet counts fall because the IgG-coated platelets are removed by the macrophages and, at the same time, as platelets are activated, they are consumed intravascularly. As a consequence, large amounts of thrombin will be generated, favouring thrombosis (Fig. 1).
Pathophysiology of heparin-induced thrombopenia.
Treatment with the polyanionic anticoagulant heparin promotes the formation of complexes with positively charged platelet factor 4 (PF4). These complexes express neoepitopes that induce the formation of antibodies by plasma cells. The resulting immune complexes activate platelets and promote the formation of procoagulant microparticles and the generation of thrombin. Pathogenic antibodies also recognize PF4 bound to heparan sulfate and other glycosaminoglycans, inducing activation of the endothelium and monocytes and promoting the generation of tissue factor. The consequence will be the occurrence of thrombosis in patients with HIT.
The most common complications are deep vein thrombosis (DVT), pulmonary thromboembolism (PTE), or skin necrosis, particularly important if vitamins K antagonists (VKA) are administered in the acute phase. The risk of these complications is higher within the first 10 days after initiating heparin but persists for up to 30 days after discontinuation of heparin.
DiagnosisHIT should be suspected if platelet counts drop by more than 50%. The presence of thrombosis 5−10 days after heparin administration would support this suspicion, which should initially be confirmed by determination of PF4/anti-heparin antibodies. Platelet counts are usually not less than 20 × 109/L, although it has not been precisely established whether the reduction could be related to greater aggregation rather than platelet destruction.1–4
The diagnosis of HIT is based on three criteria:
- 1)
The patient is receiving or has been exposed to UFH or LMWH.
- 2)
There is at least one clinical or laboratory finding (significant decrease in platelet count, new-onset venous or arterial thrombosis).
- 3)
There is laboratory evidence of specific HIT antibodies.
The signs and symptoms of HIT include those that suggest thrombotic complications. In addition, erythema or pain or even skin necrosis may occur where the subcutaneous heparin was injected. Patients receiving intravenous heparin may develop fever, hypertension, tachycardia, dyspnoea, and chest pain, as well as skin rashes.
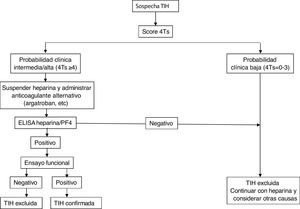
The first action to take when HIT is suspected is to estimate the likelihood of occurrence using the 4T score, based on four parameters: (i) drop in platelet count (thrombocytopenia), (ii) time in which the drop occurs, (iii) presence or absence of thrombosis and (iv) another possible alternative diagnosis (Table 2). A score below 4 points has a high negative predictive value (97%–99%), but a low positive predictive value. Disease is confirmed in 10%–20% with a value of 4–5 points and in up to 40%–80% for scores of 6.1,8 The probability with the 4Ts should serve as a guide, but not substitute for clinical assessment (Table 2).
HIT Clinical Probability Score (4T).
| 4T | Score |
|---|---|
| Thrombocytopenia | |
| Platelet decrease >50% in the last 3 days and nadir ≥20 × 109/L | 2 |
| Platelet decrease 30%−50% or nadir 10−19 × 109/L | 1 |
| Decrease <30% or platelets nadir <10 × 109/L | 0 |
| Platelet count drop time | |
| Start of platelet decrease after 5−10 days (or from day 1 after the start with previous exposure to heparin in the last 30 days) | 2 |
| Decrease from day 1 after the start and previous exposure in the last 30−100 days. Start of platelet decrease from day 14 | 1 |
| Platelet decrease <4 days without exposure to heparin in the last 100 days | 0 |
| Thrombosis or other sequelae | |
| New thrombosis (confirmed); injection site skin necrosis, anaphylactic reaction after heparin bolus; adrenal haemorrhage | 2 |
| Progressive or recurrent thrombosis; non-necrotic skin lesions; suspected thrombosis (not confirmed) | 1 |
| None | 0 |
| Other causes of thrombocytopenia | |
| No other obvious cause | 2 |
| Possible | 1 |
| Other cause present | 0 |
Clinical probability: According to the 4T rule, the clinical probability is defined as: High = 6–8 points; Intermediate = 4–5 points; Low ≤ 3 points.
- •
Thrombocytopenia: is usually intermediate (≥20 × 109/L). A decrease >30% in platelets, 5−10 days after exposure to UFH or LMWH is suggestive of HIT, although there may be an earlier decrease (24 h) in patients exposed to heparin in the previous 3 months. It does not usually present with bleeding.
- •
Time: typically, the symptoms appear within 5−10 days in patients treated with UFH or LMWH or much more rapidly if there has been previous exposure (last 30−100 days).
- •
Thrombosis: it is the characteristic clinical finding and develops in 30%−50% of confirmed HITs. It can be venous (as in the lower extremities, splanchnic or venous catheter) or arterial (cerebral, lower limb gangrene, among others). It may be the initial clinical manifestation, although it usually occurs immediately after the onset of thrombocytopenia.
- •
Other causes of thrombocytopenia: many diseases that present with isolated thrombocytopenia (mainly secondary to drugs, of immune-ITP- or infectious aetiology, post-transfusion purpura) or associated with thrombotic phenomena (see Differential diagnosis).
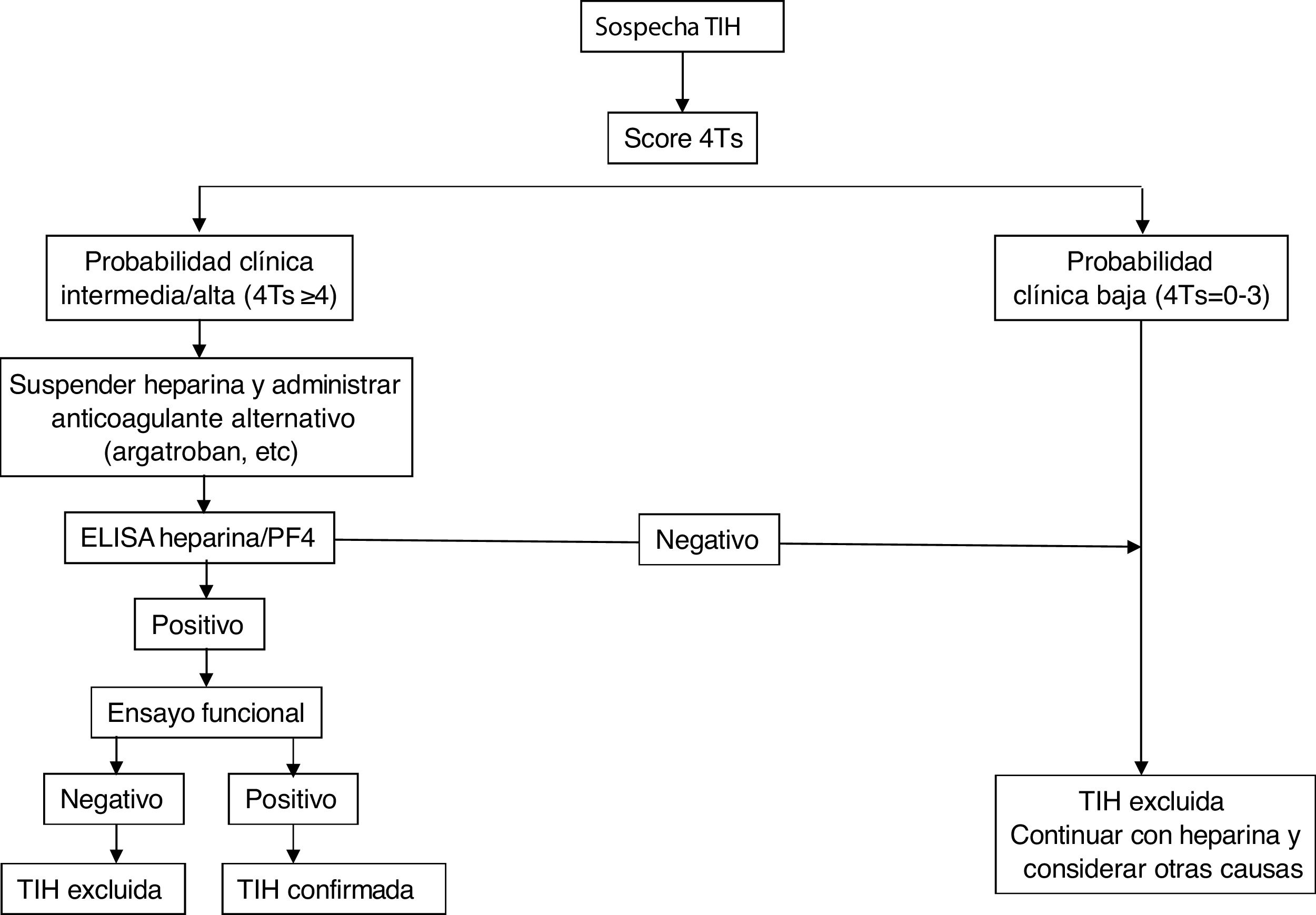
In general, if the calculated score is ≥4, antibody determination should be performed using the techniques detailed below. For this purpose, serum or plasma samples (depending on the assay) should be collected at least 4 h after the last heparin administration and should be analysed or stored frozen for further study within 4 h after collection.
Laboratory diagnosticsIt is based on the identification and/or quantification of PF4/anti-heparin antibodies with immunological and functional techniques and should be used exclusively in patients with intermediate or high probability. For the former, immunoassays (ELISA) are used that detect antibodies against the heparin/PF4 complex and show an excellent negative predictive value (98%–99%), but low positive predictive value for the detection of clinically relevant antibodies. The source of PF4 differs between the assays: Recombinant PF4 bound to heparin, purified PF4 bound to polyvinyl sulfonate, platelet lysates bound to heparin immobilized on the surface of microplates or particles containing heparin/PF4 in solid phase immunoassays based on their agglutination. Despite its excellent negative predictive power, several studies have shown the limitation of these methods for the diagnosis of HIT, since the disease was confirmed in only 2%–15% of patients with positive antibodies by ELISA.8,9 Strategies have been used to increase the sensitivity of the method, such as combining tests, restricting the assays to IgG subtypes, or modifying various study conditions to increase the predictive capacity of the assay.10
Diagnostic confirmation requires the performance of functional studies that detect platelet activation. The functional serotonin release assay (SRA) is the reference against which the other methods must be validated, although its determination requires a facility authorized to work with radioactive samples. Others are heparin-induced platelet activation (HIPA) assay, which use aggregometry and flow cytometry techniques. All of them are more specific for clinically relevant antibodies than immunological assays, so that a negative test would exclude the diagnosis of HIT (especially with a low 4T score). Diagnostic exclusion would be done through screening tests, while functional assays would confirm the diagnosis of HIT when positive, but are restricted to specialized laboratories.11,12 The combination of the 4T score and antibody titre has been shown to predict the result of the functional study.13
Fig. 2 shows a diagnostic/therapeutic algorithm for HIT.
Other diagnostic studiesFor patients with clinical suspicion or confirmation of HIT, an ultrasound is indicated doppler to rule out DVT, regardless of the absence of symptoms, since these findings will have implications related to the duration of treatment. If there is abdominal pain with hypotension, adrenal haemorrhage associated with adrenal vein thrombosis should be suspected. Cavernous sinus thrombosis should be considered in patients with severe headache.2–4
Differential diagnosisThe differential diagnosis of HIT should include other clinical scenarios that present with thrombocytopenia, such as immune thrombocytopenia, post-transfusion or drug-induced purpura, or additionally associated with thrombotic complications, such as DIC, thrombotic microangiopathies, and antiphospholipid syndrome. Previous exposure to heparin and detection of PF4/anti-heparin antibodies can aid in the diagnosis of HIT.2–4
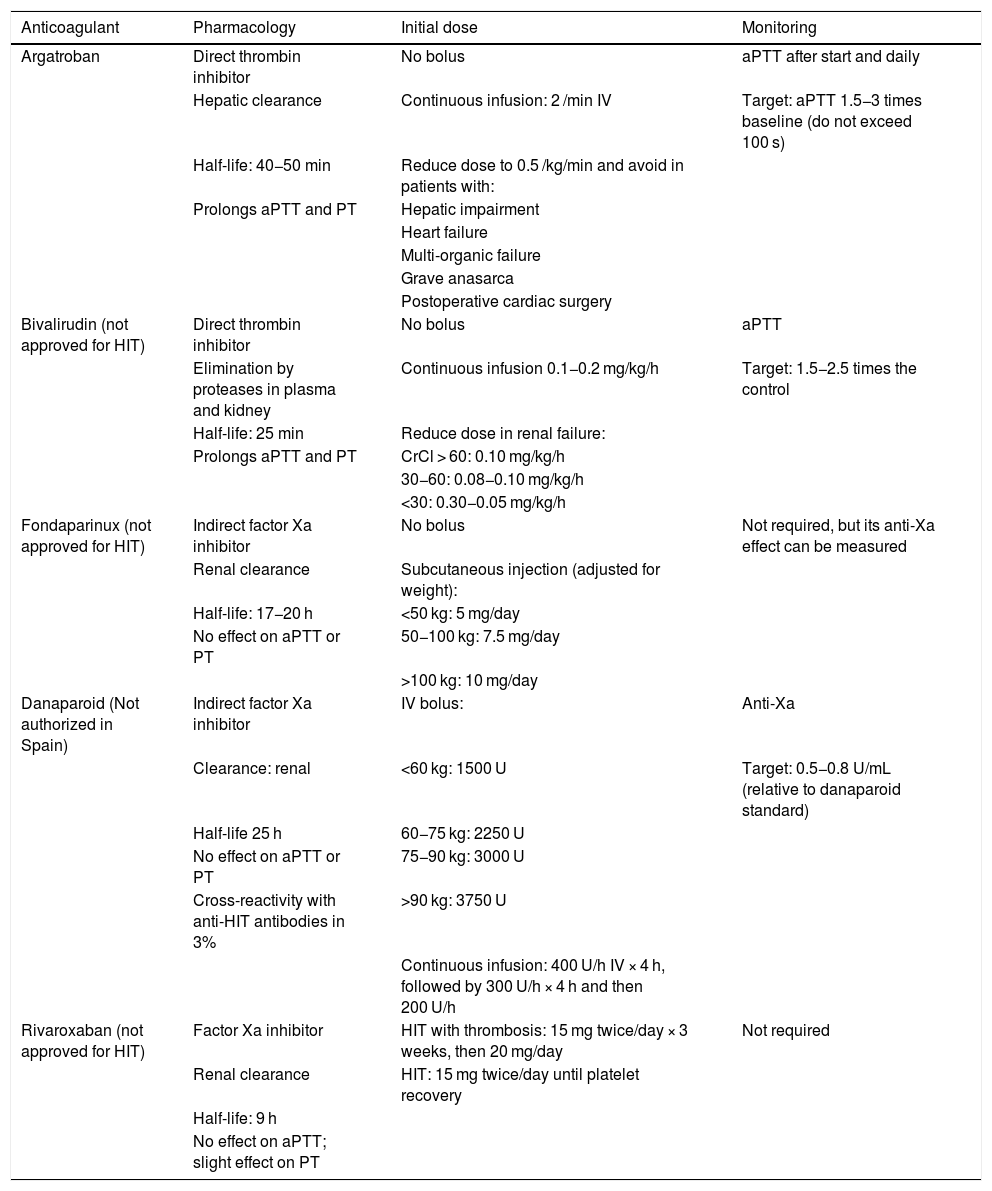
TreatmentTreatment of HIT should begin as soon as a 4T score of 4 or higher is obtained. Two immediate steps are crucial: discontinuing heparin and initiating a non-heparin anticoagulant1–4 (Table 3). VKA should be discontinued in patients on VKA and phytomenadione (vitamin K) should be administered to replenish protein C and S stores. Anticoagulation with alternative drugs should be administered at therapeutic doses in most cases, including any patient with: high probability 4T score, intermediate probability 4T score and other indication for therapeutic anticoagulation, or intermediate probability 4T score and no indication for therapeutic anticoagulation who is not at high risk of bleeding. Alternatively, a prophylactic dose of another anticoagulant can be initiated in a small group of patients considered to be at high risk for bleeding, provided that they do not require therapeutic anticoagulation for another reason and have an intermediate probability 4T score. If prophylactic anticoagulation is started and the immunoassay is positive, the patient should be switched to therapeutic anticoagulation pending the results of the functional study.
Non-heparin anticoagulants for the treatment of HIT.
| Anticoagulant | Pharmacology | Initial dose | Monitoring |
|---|---|---|---|
| Argatroban | Direct thrombin inhibitor | No bolus | aPTT after start and daily |
| Hepatic clearance | Continuous infusion: 2 /min IV | Target: aPTT 1.5−3 times baseline (do not exceed 100 s) | |
| Half-life: 40−50 min | Reduce dose to 0.5 /kg/min and avoid in patients with: | ||
| Prolongs aPTT and PT | Hepatic impairment | ||
| Heart failure | |||
| Multi-organic failure | |||
| Grave anasarca | |||
| Postoperative cardiac surgery | |||
| Bivalirudin (not approved for HIT) | Direct thrombin inhibitor | No bolus | aPTT |
| Elimination by proteases in plasma and kidney | Continuous infusion 0.1−0.2 mg/kg/h | Target: 1.5−2.5 times the control | |
| Half-life: 25 min | Reduce dose in renal failure: | ||
| Prolongs aPTT and PT | CrCl > 60: 0.10 mg/kg/h | ||
| 30−60: 0.08−0.10 mg/kg/h | |||
| <30: 0.30−0.05 mg/kg/h | |||
| Fondaparinux (not approved for HIT) | Indirect factor Xa inhibitor | No bolus | Not required, but its anti-Xa effect can be measured |
| Renal clearance | Subcutaneous injection (adjusted for weight): | ||
| Half-life: 17−20 h | <50 kg: 5 mg/day | ||
| No effect on aPTT or PT | 50−100 kg: 7.5 mg/day | ||
| >100 kg: 10 mg/day | |||
| Danaparoid (Not authorized in Spain) | Indirect factor Xa inhibitor | IV bolus: | Anti-Xa |
| Clearance: renal | <60 kg: 1500 U | Target: 0.5−0.8 U/mL (relative to danaparoid standard) | |
| Half-life 25 h | 60−75 kg: 2250 U | ||
| No effect on aPTT or PT | 75−90 kg: 3000 U | ||
| Cross-reactivity with anti-HIT antibodies in 3% | >90 kg: 3750 U | ||
| Continuous infusion: 400 U/h IV × 4 h, followed by 300 U/h × 4 h and then 200 U/h | |||
| Rivaroxaban (not approved for HIT) | Factor Xa inhibitor | HIT with thrombosis: 15 mg twice/day × 3 weeks, then 20 mg/day | Not required |
| Renal clearance | HIT: 15 mg twice/day until platelet recovery | ||
| Half-life: 9 h | |||
| No effect on aPTT; slight effect on PT |
The treatments are summarized in Table 3:
- •
Argatroban: direct thrombin inhibitor, it is the only drug in Spain with the indication for treatment of HIT approved since 2011. The Food and Drug Administration (FDA), after a first authorization in 2000 as prophylaxis or treatment of HIT in adults, also authorized its use as an anticoagulant in patients undergoing percutaneous coronary intervention (PCI) in 2002.
- •
Danaparoid: an antithrombin-dependent factor Xa inhibitor, it has been authorised since 1996 in most European countries, but not in Spain, and was approved by the FDA in 2001.
- •
Bivalirudin: a highly selective direct thrombin inhibitor, analogous to hirudin. The FDA authorized its use in 2000 as an anticoagulant in patients undergoing PCI. In Europe, the EMA approved the indication in 2009 and in Spain it has been authorized since 2016 as an anticoagulant in adult patients who undergo PCI or with unstable angina/non-ST segment elevation myocardial infarction who will undergo an emergency intervention or one that can be postponed. In 2005, the FDA also approved its use in HIT, an indication currently not authorized in Europe.
Due to the nature of the disease, the agent licensed for this indication in Spain (argatroban) should be readily and easily accessible in the hospital setting for administration to patients with a presumptive diagnosis of HIT.
Argatroban is a synthetic anticoagulant with a rapid onset of action, a half-life of 1 h and continuous IV administration, achieving stable levels in 1−3 h. In acute HIT the dose is 2 μg/kg/min. In moderate hepatic impairment, the dose should be reduced to 0.5 μg/kg/min, also in individuals with multi-organ failure, heart failure or post-cardiac surgery. Because it is not eliminated by the kidney, it is preferred in patients with kidney failure. A prospective study showed that it significantly reduced the incidence of thrombosis, death from thrombosis or thrombosis-related amputation compared to historical controls (HR 0.33, 95% CI 0.20−0.54),14 results later confirmed in a multicentre study in France.15 Anticoagulant levels are monitored with the activated partial thromboplastin time (aPTT), aiming for a range of 1.5−3 times the baseline value, not exceeding 100 s. Although there may be differences in the therapeutic range of aPTT between the different agents, the objective is to maintain a level approximately twice that of the control. Artificially elevated results may be observed when administered to patients with coagulopathies (e.g., liver disease) or to those who have previously received VKA, and in these cases additional tests such as the ecarin assay have been suggested to monitor the effect of the drug. The efficacy of argatroban in relation to other alternative anticoagulants has been widely demonstrated in the context of HIT. In clinical trials leading to the drug's approval, argatroban reduced the risk of the combined outcome (new thrombosis, death from thrombosis, amputation from thrombosis) compared to historical controls.16–18
Danaparoid is a heparinoid that contains heparan, dermatan, and chondroitin sulfate. It has anti-Xa activity, is eliminated via the kidneys and does not prolong clotting times. Its half-life is approximately 25 h. It is administered subcutaneously or intravenously, preceded by a bolus, with doses that vary depending on the medical or surgical context. It has also shown a beneficial effect on HIT in small series of patients with improved platelet count and reduced thrombosis.19 Its levels are monitored with functional assays of anti-Xa activity. Monitoring may be required in patients with renal failure, given its long half-life. It is contraindicated in patients with creatinine clearance <30 mL/min. It is not authorised in Spain.
Bivalirudin is another direct thrombin inhibitor that can be used in these patients. However, its indication in Spain does not include HIT.17
Another anticoagulant that can be used in HIT is fondaparinux. Although not approved for this indication, it is considered safe and effective.20 An important consideration is that it is eliminated via the kidneys and is contraindicated in the event of creatinine clearance <30 mL/min. In some patients treated with fondaparinux there is cross-reactivity with PF4/anti-heparin antibodies18 and, although rare, this could have clinical implications (Table 3).
Although the evidence is limited, theoretically all direct oral anticoagulants (DOACs) can be used in the treatment of HIT, although more cases have been published with rivaroxaban.21 As an advantage, this strategy would allow patients to be treated on an outpatient basis. However, DOACs are not approved for the treatment of HIT. Its use can be considered in patients who have recovered their platelet levels with any of the previous agents, to make the transition from parenteral to oral drugs, mainly in patients who require anticoagulation for months to avoid VKA.
With discontinuation of heparin and the use of an alternative anticoagulant, HIT usually resolves. However, for cases of refractory thrombocytopenia/thrombosis after five days of treatment with non-heparinoid anticoagulants, it has been suggested that a regimen with IV immunoglobulins (2 g/kg cumulative dose) would be safe and effective, achieving up to 77% response.22
Duration of anticoagulant treatmentAs a general rule, therapeutic anticoagulation should be maintained until platelet count stabilization is reached (>150 × 109/L or the patient's baseline figure) in patients with HIT without thrombosis. Switching to an alternative anticoagulant, especially argatroban, which increases the INR, from a VKA, is a challenge for the physician. There should be a 5-day overlap period between the VKA and the chosen parenteral anticoagulant. The duration of anticoagulant treatment will be at least 3 months (preferably 3–6 months) if the patient has experienced thrombotic episodes.
Although HIT does not usually recur, patients should avoid re-exposure to heparin and the doctor should consider it as an allergenic drug in their medical records. Thrombotic sequelae will be related to the severity of the initial episode. A high percentage of patients suffer amputations if a specific alternative anticoagulant treatment has not been established early. For this reason, it is recommended to use alternatives such as fondaparinux, DOAC or argatroban in situations such as perioperative, diagnosis of thrombosis.
Other measuresTransfusing platelets to treat thrombocytopenia is not recommended in the absence of severe haemorrhagic symptoms, as platelets may bind IgG, become activated and release PF4, worsening the hypercoagulable state. Where possible, surgical procedures should be delayed, but an alternative anticoagulant can be administered pre- and post-operatively.
IgG antibodies can remain in the body for up to 3 months after an episode of HIT; if the patient receives any form of heparin during this period, the platelet count can drop within just 12 h. After 3 months of HIT, the anticoagulant can be used with caution, if necessary, under strict monitoring. Ideally, a functional assay should be performed to ensure that it is negative before administration; if this is not possible, the use of a direct thrombin inhibitor or fondaparinux should be considered. Bivalirudin would be the preferred drug in patients undergoing a coronary intervention.
In patients with a history of HIT, the unjustified use of any form of heparin, including that used in catheter flushes, should be avoided.
Special populationsKidney diseaseArgatroban is recommended in patients with acute HIT and creatinine clearance <30 mL/min. If the patient is on dialysis, citrate can be used for anticoagulation of the system.16
Heart surgeryThere are isolated data on the use of alternative anticoagulants in cardiac surgery, but UFH is preferred. In patients with a history of HIT or positive PF4/anti-heparin antibodies, it is recommended to delay surgery, if possible, wait for the antibodies to become negative (3 months), and perform extracorporeal circulation with heparin as usual. Non-heparin anticoagulants are recommended in the postoperative period. Bivalirudin can be used in patients undergoing coronary intervention.23,24 Plasma exchange alone or in combination with intravenous immunoglobulins has also been shown to be a safe and effective strategy to rapidly eliminate anti-PF4 antibodies and perform heart surgery with extracorporeal circulation using heparin sodium as anticoagulant.25,26
PaediatricsHIT can be a complication in paediatric patients, particularly those exposed to heparin as a consequence of cardiac surgery (e.g., cardiopulmonary bypass) or extracorporeal devices (e.g., ECMO), with argatroban and bivalirudin being the most widely used drugs.27 Although the incidence of HIT is lower than in adults, the diagnosis and treatment are similar, so the advice of paediatricians with experience in the management of children with problems of thromboembolism in combination with the haematologist is recommended.
PregnancyHIT is rare during pregnancy. Danaparoid, which does not cross the placental barrier, may be the anticoagulant of choice. The use of fondaparinux is an option, although the drug crosses the placenta, and the experience is still limited. DOACs should not be used in pregnant women with HIT.28
COVID-19There have been isolated cases of thrombocytopenia associated with anti-PF4 antibodies in patients with COVID-19 and respiratory distress syndrome associated with thrombotic events, which makes it necessary to closely monitor the platelet count, mainly in patients receiving heparin. Argatroban may represent an effective and safe alternative in patients with COVID-19 and HIT.29 Interestingly, recent studies describe isolated cases of immune thrombotic thrombocytopenia (called VITT, vaccine-induced immune thrombotic thrombocytopenia) induced by the ChAdOx1 vaccine in COVID-19 with a mechanism similar to HIT; that is, mediated by antibodies against PF4, and responsible for thrombotic events, mainly cerebral venous thrombosis and splanchnic thrombosis.30
ConclusionHIT is a serious complication of treatment with UFH and, less commonly, after LMWH. It is caused by PF4/anti-heparin antibodies and is characterized by a decrease in platelets and a severe prothrombotic state 5−10 days after exposure, which facilitates the development of venous or arterial thrombosis. The 4T score is recommended for diagnosis to establish clinical probability. This must be confirmed by immunological assays, determining antibodies against the heparin/PF4 complex, or functional assays. A negative result of the former usually rules out the diagnosis, while the positivity of the latter usually confirms the suspicion. Treatment consists of discontinuing heparin and using a non-heparin anticoagulant at therapeutic doses (argatroban, danaparoid, bivalirudin, fondaparinux), as suggested in the above-mentioned guidelines.2–6 DOACs may be used off-label instead of VKAs for long-term treatment when platelet counts have stabilised. Patients with HIT should avoid subsequent exposures to heparin.
FundingThis manuscript is sponsored by the Spanish Society of Thrombosis and Haemostasis (SETH) and has the scientific endorsement of the Spanish Society of Haematology and Haemotherapy (SEHH).
Conflict of interestsThe authors received fees from Aguettant Ibérica (Barcelona, Spain) for writing the article.
Please cite this article as: Páramo JA, Lozano ML, González-Porras JR, Mateo J. Estado actual del diagnóstico y tratamiento de la trombocitopenia inducida por heparina (TIH). Med Clin (Barc). 2022;158:82–89.