Hepatocellular carcinoma (HCC) is the most common primary liver neoplasm and one of the most common causes of death in patients with cirrhosis of the liver. In parallel with recognition of the clinical relevance of this cancer, major new developments have recently appeared in its diagnosis, prognostic assessment and in particular, in its treatment. Therefore, the Spanish Association for the Study of the Liver (AEEH) has driven the need to update the clinical practice guidelines, once again inviting all the societies involved in the diagnosis and treatment of this disease to participate in the drafting and approval of the document (Spanish Society for Liver Transplantation (SETH), the Spanish Society of Diagnostic Radiology (SERAM), the Spanish Society of Vascular and Interventional Radiology (SERVEI), the Spanish Association of Surgeons (AEC) and the Spanish Society of Medical Oncology (SEOM)). The clinical practice guidelines published in 2016 and accepted as National Health System Clinical Practice Guidelines were taken as the reference documents, incorporating the most important recent advances. The scientific evidence and the strength of the recommendation is based on the GRADE system.
El carcinoma hepatocelular (CHC) es la neoplasia primaria de hígado más frecuente y una de las causas de muerte más frecuente en los pacientes afectos de cirrosis hepática. Simultáneamente al reconocimiento de la relevancia clínica de esta neoplasia, en los últimos años han aparecido novedades importantes en el diagnóstico, evaluación pronóstica y especialmente, en el tratamiento del carcinoma hepatocelular. Por tal motivo, desde la Asociación Española para el Estudio del Hígado (AEEH) se ha impulsado la necesidad de actualizar las guías de práctica clínica, invitando de nuevo a todas las Sociedades involucradas en el diagnóstico y tratamiento de esta enfermedad a participar en la redacción y aprobación del documento (Sociedad Española de Trasplante Hepático (SETH), la Sociedad Española de Radiología Médica (SERAM), la Sociedad Española de Radiología Vascular e Intervencionista (SERVEI), la Asociación Española de Cirujanos (AEC) y la Sociedad Española de Oncología Médica (SEOM)). Se han tomado como documentos de referencia las guías de práctica clínica publicadas en 2016 aceptadas como Guía de Práctica Clínica del Sistema Nacional de Salud, incorporando los avances más importantes que se han obtenido en los últimos años. La evidencia científica y la fuerza de la recomendación se basa en el sistema GRADE.
Since the publication of the guidelines for hepatocellular carcinoma (HCC) management in 2016,1 epidemiological changes related to the cure of the hepatitis C virus and the increase in other risk factors, such as the association of metabolic syndrome with fatty liver disease, have identified new challenges in the clinical management of these patients. Additionally, new diagnostic criteria and/or new recommendations for indicating surgery have been put forward. They have been heterogeneously adopted by the American (AASLD)2 and European (EASL)3 associations for the study of liver diseases and the different scientific societies involved in HCC management. However, the biggest change has occurred in the systemic treatment of advanced HCC. Five new drugs (lenvatinib and the combination atezolizumab with bevacizumab in first line treatment, and regorafenib, cabozantinib and ramucirumab in second line treatment) have been shown to improve the survival of patients with HCC and have been approved by the European Medicines Agency (EMA).
Consequently, the scientific societies who participated in the 2016 HCC management guides decided that an update was timely and sought the affiliation to the panel of experts of the Spanish Association of Surgeons. Updating the guidelines employed the collaboration of working groups, each led by a coordinator (see Appendix B Supplementary Material).
These guidelines complement those published in 2016.1 The focus is on updating the previously mentioned topics and incorporating molecular aspects which are in the research phase. These aspects are not current clinical practice.
HCC epidemiology and preventionHCC is currently the sixth most common neoplasm in the world and the third leading cause of death from cancer.4 Its distribution worldwide is very heterogeneous and is closely related to the variable prevalence of the different risk factors associated with the development of this disease. According to the International Agency for Research on Cancer (IARC), in 2018 the age-specific incidence rate in East Asia was 17.7 × 100,000 inhabitants, followed by Micronesia (15.2 × 100,000 inhabitants), North Africa (14.1 × 100,000 inhabitants), Southeast Asia (13.3 × 100,000 inhabitants) and Melanesia (11.4 × 100,000 inhabitants). In this case, taking the IARC estimate into account, Southern Europe has an age-specific incidence rate of 6.8 × 100,000 inhabitants, Western Europe 5.3 × 100,000 inhabitants and North America 6.6 × 100,000 inhabitants (Appendix B Supplementary Table 1). In Spain, according to Spanish Network of Cancer Registries (REDECAN.org), the estimate of new cases of liver cancer in 2019 was 6499 (4869 in men and 1630 in women).
In all geographic areas, the risk of HCC varies according to the degree of liver fibrosis, being less than 1% annually in patients with chronic hepatitis without fibrosis and increases to 3%–7% annually when the patient develops cirrhosis.5
Established liver cirrhosis is a relevant risk factor for HCC. The intensity of the hepatic inflammation (related to viral load or genotype in the case of hepatitis) is responsible for the chronic process of necrosis/regeneration that leads to this cirrhosis, so this is the parameter that should be used to establish the acquisition of a clinically significant risk. In this regard, once liver cirrhosis is established, the risk of developing HCC is maintained despite obtaining a sustained viral response whether after interferon-based regimens or with direct-acting antivirals in the case of HCV, or after the use of nucleo(t)side analogues for inhibition of HBV replication in chronic infection. This is possibly due to structural and molecular damage already present in the liver despite clearance of the infection.6–12
Diabetes mellitus13–15 and other factors associated with metabolic syndrome such as obesity or dyslipidemia16–18 are associated with an increase in HCC-related death, as is smoking.19,20 Drinking coffee lowers the risk,21,22 but there is not enough evidence to recommend vitamin supplements, soy or alternative medicines as preventive elements of HCC.23,24 Preliminary studies show that long-term use of metformin in diabetic patients25 or propranolol in HCV infected patients26 could be associated with a decrease in the incidence of HCC. Likewise, a systematic review and meta-analysis has shown that the use of statins is associated with a reduction in the incidence of HCC.27 Finally, recent studies have suggested that the use of aspirin could be associated with a decrease in the incidence of HCC.28
Below we detail the updates in relation to each of the most frequent aetiologies:
Hepatitis BSeveral studies have shown that HBV replication increases the risk of HCC.29–31 Retrospective studies conducted predominantly in Asia indicate 30% risk reduction for HCC in cirrhotic patients with the use of entecavir and tenofovir, and 80% in patients without cirrhosis, although the evidence in Western patients is still limited.32–34 It should be noted that current antiviral agents against HBV do not completely eliminate the risk of HCC, especially in patients with cirrhosis in whom even a very low viral load (<2000 IU/mL) represents an increased risk of HCC (HR 2.20) compared to patients with undetectable HBV DNA.35 The PAGE-B score has been validated in a Caucasian population on antiviral treatment. Values ≥ 10 predict a cumulative incidence rate of HCC at 5-years of around 4% and B-PAGE ≥ 18 a cumulative incidence at 5-years of 17%.36 Likewise, a recent study suggests that the modified PAGE-B and PAGE-B scores could be used to exclude screening in patients with HBV under antiviral treatment and a very low risk of developing HCC.37
Hepatitis CThe development of a prophylactic vaccine as primary prevention is still a challenge due to the high variability of the viral genetics, although there are promising developments.38 For this reason, prevention is fundamentally based on avoiding transmission, particularly parenterally through blood transfusions or the use of contaminated needles. When the infection has already been acquired, the risk of complications and mortality associated with the development of cirrhosis, as well as the risk of developing HCC, is lower in patients who achieve a sustained virologic response (SVR), regardless of the degree of fibrosis of the patient’s liver disease.7
Despite the improvement in the SVR rate with direct-acting antiviral agents (DAAs), the projection of HCC incidents until the year 2060 is to increase39 and it is estimated that undiagnosed HCV infection represents 50% or more of all infected patients.
In patients with established cirrhosis, SVR reduces the risk of HCC compared to those patients who do not achieve SVR.40,41 However, the short follow-up of patients treated with DAAs and the lack of prospective studies aimed at evaluating HCC screening in these patients make it impossible to draw robust conclusions regarding the rate of HCC in patients treated with DAAs. Multiple risk factors for the development of ‘de novo’ HCC have been described after DAA treatment, including treatment failure, alcohol consumption, advanced age (>65 years), male, presence of cirrhosis, genotype 3, diabetes, metabolic syndrome, a high MELD index, low levels of albumin and/or platelets, and high levels of alpha-fetoprotein (AFP).12,42–44 However, at present, there are no prediction criteria/scores for the rate of HCC prior to starting DAA treatment.
A study carried out in Spain analysed the incidence of HCC in cirrhotic patients who achieved SVR and did not have nodules on the ultrasound obtained within 30 days prior to starting DAA treatment. This study showed that in this population the incidence of HCC is 3.04 (95% CI 1.45–6.37) per 100 people/year45 and that the observed median occurrence of HCC goes beyond 24 months. Another Spanish multicentre study concluded that HCC is the most frequent complication after obtaining SVR with DAA therapy.46
The expected result of HCV eradication is a decreased risk of both de novo and recurrence HCC. This expected result has been questioned in the context of patients with a history of HCC prior to starting DAA therapy.47 Clinical and experimental observations suggest that there are DAA-specific modulations of host immunity and oncogenesis.48 In addition, there are studies that suggest the presence of a time-dependent effect in the appearance of HCC after DAA, especially in patients with nodules not characterised in the screening ultrasound.42,49
Some of the suggested factors that could have an impact on the development of de novo HCC in patients treated with DAAs are known as risk factors for HCC (age, male, diabetes or elevated AFP). It is also suggested that both miRNA50 as well as serum markers other than AFP, such as angiopoietin-2,51 could condition the risk of HCC in this patient population.
Alcohol abuseThe importance of alcohol as a risk factor for HCC has not changed52 and this is the most important cause of HCC in Spain.53 There are two meta-analyses of cohort studies. One included 19 studies and showed a dose-dependent increased risk of HCC (HR 1.16)54 and the other included four studies and showed that abstinence reduced the risk of HCC by 6%–7% annually. Although there is a high level of uncertainty it is believed that, when cirrhosis is already established, it takes more than two decades to reduce the risk of HCC to the level of those who have never consumed alcohol.55 Patients with alcohol-related HCC are more often diagnosed when the disease is advanced, having reached the stage of decompensated cirrhosis, rather than in surveillance programmes, as happens with HCC of viral aetiology.52
Non-alcoholic fatty liver disease (NAFLD)NAFLD is an entity that is on the increase, and it involves the risk of progressing to cirrhosis and liver cancer.56 25% of the world population has NAFLD; 60% of biopsied NAFLD patients present non-alcoholic steatohepatitis (NASH) with an HCC rate of 5.29 per 1000 person-years.18
According to the Markov study conducted with data from China, France, Germany, Italy, Spain and the United Kingdom, it is estimated that the prevalence of HCC due to NASH will increase in all countries, predominantly in the United States, where the increase is expected to reach 130% (10,820 cases in 2016 which will increase to 24,860 in 2030).56 However, the data published to date comes from joint analyses of cirrhotic and non-cirrhotic patients.57 The data on the incidence/prevalence of HCC in non-cirrhotic patients come mainly from retrospective studies in which the primary objective of the same was not the development of HCC.57,58
Recommendations- •
Universal vaccination against HBV reduces the incidence of HCC (high evidence, strong recommendation).
- •
In patients with chronic viral hepatitis, antiviral treatment is recommended since it has demonstrated its impact on preventing the progression to cirrhosis and, therefore, preventing the development of HCC (high evidence, strong recommendation).
- •
Once liver injury associated with a clinically relevant risk is established (cirrhosis, or prior to the cirrhosis stage but the patient is HBV infected), the elimination of the aetiological agent decreases the risk of the appearance of HCC but does not eliminate it (high evidence, strong recommendation).
- •
Patients with HCV-associated cirrhosis maintain the risk of both de novo and recurrent HCC, even after having achieved a sustained viral response. It is recommended to maintain the conventional surveillance strategy in these patients (moderate evidence, strong recommendation).
- •
Coffee consumption has been shown to decrease the risk of HCC in patients with chronic liver disease (moderate evidence, strong recommendation).
Oncology screening is defined as repeatedly performing a test with the aim of reducing the mortality associated with the neoplasm.59 Bearing in mind that the only possibility of applying treatments with curative intent is by diagnosing the disease in an asymptomatic stage and given that this option is only feasible if screening is carried out in the population at risk, it is recommended to offer screening in patients with cirrhosis who would be treated if they were diagnosed with HCC. Screening should be done by ultrasound (US). Multiple cohort studies60–62 and cost-effectiveness studies63–65 reinforce the usefulness of establishing follow-up visits with US every six months. Ideally, the benefit of screening techniques should be assessed through prospective and randomised studies. There is only one prospective, randomised study published that has been conducted in China. It included approximately 20,000 patients with chronic HBV infection. They were randomised to receive screening by ultrasound (US) every six months and AFP determination versus no screening. Despite low adherence (<60%), the survival of the patients included in the screening programme was 66% at one year, 53% at three years, and 46% at five years vs. 31%, 7%, and 0%, respectively, in unscreened patients.66 The effectiveness of the programme was linked to the capacity of the US, while AFP determination was not found to be efficient.67 Conducting a new validation clinical trial in developed countries is not feasible; US studies are part of the routine evaluation of patients with chronic liver disease and the doctors’ and patients’ perception of the benefits of screening prevents recruitment.68 With all the available evidence, there is a general consensus in recommending periodic screening programmes in cirrhotic patients who would be treated if they were diagnosed with HCC. In general, cirrhotic patients in Child-Pugh class A and B should be considered for screening. Patients with poor liver function or decompensation that condition a poor vital prognosis (recurrent hepatic encephalopathy, refractory ascites, uncontrolled variceal hemorrhage, spontaneous bacterial peritonitis, malnutrition, etc.) should be evaluated for liver transplantation. The indication of a transplant will not change in these patients if HCC is detected unless the criteria for inclusion in the waiting list are exceeded and/or the HCC constitutes a contraindication to a transplant. As a transplant is considered in these patients due to liver failure with a poor short-term prognosis, the detection of HCC and its possible treatment will not have a clinically significant impact on survival. Therefore, there is no point in screening for early detection if a transplant is not feasible.
Surveillance intervalData regarding the rate of tumour growth and progression to a size detectable by imaging techniques are limited. Old case series suggest that the doubling time of tumour mass growth ranges from two and four months69,70 and these results provide the rationale for screening every six months. Similarly, this time interval was used in the only randomised clinical trial that has demonstrated the benefit of screening for HCC with ultrasound in patients with chronic liver disease.66 Some authors suggest that high-risk patients should be examined more frequently,71 but there are no data that show that a higher risk is associated with a faster rate of tumour growth. One study suggested that an annual surveillance was associated with lower survival and lower detection capacity than the semi-annual surveillance,72 and a randomised clinical trial conducted in France including 1200 cirrhotic patients concluded that there is no improvement in the HCC diagnosis or treatment when the ultrasound screening is performed every three months versus every six months.73 Finally, cost-effectiveness studies suggest that the six-month interval is more cost-effective compared to other alternatives.74 Therefore, with the current scientific evidence, it is considered that the recommended interval should be six months.
Screening toolsHCC screening techniques can be divided into radiological and serological screening. The recommended radiological test is the abdominal ultrasound. It is a non-invasive technique, accepted by the population, with a sensitivity of 60%–80% and a specificity greater than 90% for the early detection of HCC.75 In addition, a well-defined diagnostic strategy is available following the detection of a nodule suspicious for HCC. Therefore, the abdominal ultrasound, performed by expert personnel, is currently the most appropriate screening technique for the early detection of HCC. In real clinical practice, a significant number of patients are not diagnosed in the initial stages due to screening programmes being poorly applied, or lesions not being detected.53,76–79 In order to ensure that ultrasounds are performed with the necessary know-how and experience, it is important to consider establishing training programmes to certify the training and this activity. The performance of computed tomography scans (CT) as a screening technique should be discouraged due to the risk associated with irradiation80 as well as poor cost-effectiveness based on the excessive detection of false positives and little availability. This reasoning also applies to magnetic resonance imaging scans (MRI).
Regarding serological tests, a multitude of tumour markers are currently available. The most evaluated marker is the AFP, which until recently was the only tool available. However, AFP has shown poor performance since its values in many cases are normal in initial tumours81 and it is well known that patients with liver cirrhosis can present transient elevations of AFP in the absence of HCC.82,83 Different retrospective analyses evaluating the diagnostic performance using ROC curves have shown that when using different cut-off points between 10−20 ng/mL, considered optimal for screening, the sensitivity is 60% and the specificity is 80%.84–86 When looking at prospective studies where the diagnostic performance of screening tests is specifically evaluated, AFP with the same cut-off point shows a sensitivity of less than 25% and a specificity of 79%.81 A retrospective study has suggested that a progressive increase in AFP would be more useful,87 but it must be validated prospectively. Furthermore, no study is available that establishes that an increase in AFP should lead to suspect HCC if the US is negative. In this sense, studies in liver explants show that HCC may not exist even if the AFP exceeds 500 ng/mL.88 Recent studies in successfully-treated patients with HBV-related cirrhosis report a better performance of AFP, but there are still no studies that show that the use of AFP in this population is cost-effective.89–93 Finally, there is a correlation between AFP levels and tumour stage, with AFP being a marker of advanced disease. Therefore, AFP is not an effective screening tool for early detection and its use should be discouraged.94,95 Other markers have been put forward such as the lectin-bound alpha-fetoprotein fraction,85,96 des-gamma carboxyprothrombin (DGCP),85 Golgi protein 73 (GP73),97 glypican-398 or Dickkopf-1 (DKK1),99 but they have the same drawbacks as the AFP and generally cannot compete with the reliability of the US.
HCC screening candidatesSince the main risk factor for HCC is the presence of cirrhosis, all patients with cirrhosis should be considered candidates for screening regardless of aetiology. In patients with chronic HCV-related liver disease with established cirrhosis, obtaining a persistent viral response after treatment with interferon or DAA does not eliminate the risk of developing HCC.7,49,100–103 Therefore, these patients should also be recommended for HCC screening. In patients with chronic HBV hepatitis, screening is considered cost-effective if the risk of HCC is greater than 0.2%/year. In this scenario, cost-benefit models are necessary to assess the indication for screening. The incidence of HCC in Asian or African adults with active HBV infection, with or without a family history of HCC, clearly exceeds this cut-off point,104 while the incidence of HCC ranges from 0.1% to 0.4%/year in Europe/North America.105 A high viral load is also associated with an increased risk of HCC; in Asian patients, the HBV DNA levels greater than 10,000 copies/mL are associated with a risk of HCC greater than 0.2%.104 Regarding HCV, studies from USA and Japan suggest that there is a risk of developing HCC in patients with bridging fibrosis only, in the absence of cirrhosis.106,107 Since the transition from advanced fibrosis to cirrhosis cannot be properly defined, there is agreement to offer HCC screening to this population. At the present time, we do not have information regarding the incidence of HCC in patients with advanced fibrosis due to HCV infection with SVR after DAA, which makes it impossible to make a recommendation in this population. Liver elastography appears to be a useful tool for a non-invasive determination of the presence of advanced fibrosis108 and portal hypertension,109,110 as it is able to stratify patients with different risks of developing HCC.111–116 However, most studies include patients with active HCV infection, and they have not been validated in patients who have obtained SVR. It has recently been suggested that risk stratification through genetic studies is possible117,118 and that this information could be associated with the previously discussed clinical parameters.
Patients with alcoholic liver cirrhosis do not present a risk of developing homogeneous HCC. Studies from Northern Europe report a low incidence but data from the rest of the world, including France117 and Spain, indicate the opposite.119 In the case of primary biliary cholangitis, an incidence of 3.4 cases/1000 patient-years has been established and the main predictive factors for the development of HCC are the absence of a biochemical response after medical treatment120 and the presence of stage iv of the disease.121 Finally, there is little information regarding the risk of HCC in patients with NAFLD, particularly in those who have not yet developed cirrhosis, so it is not possible to make any recommendation for screening in this population.57 Although no values exist for the percentage of patients with NAFLD who develop HCC, probably those who have already developed cirrhosis should be considered for screening, although the existence of cardiovascular comorbidity with the competing risk of mortality may prevent a positive impact on the overall survival of patients. The same concept applies to hereditary hemochromatosis and other entities that progress to cirrhosis.
Recommendations- •
Patients with liver cirrhosis of any aetiology should be considered for participation in screening programmes (moderate evidence, strong recommendation).
- •
The most appropriate screening technique is abdominal ultrasound performed by expert personnel (moderate evidence, strong recommendation).
- •
The use of AFP as a screening technique is not recommended (moderate evidence, weak recommendation).
- •
The screening abdominal ultrasound should be performed every six months. The screening interval does not need to be shortened in patients who have a higher risk of developing HCC (moderate evidence, weak recommendation).
- •
There are no data to make a recommendation in patients with fatty liver disease (NAFLD) without cirrhosis and in HCV patients without advanced fibrosis who have achieved SVR (low evidence, weak recommendation).
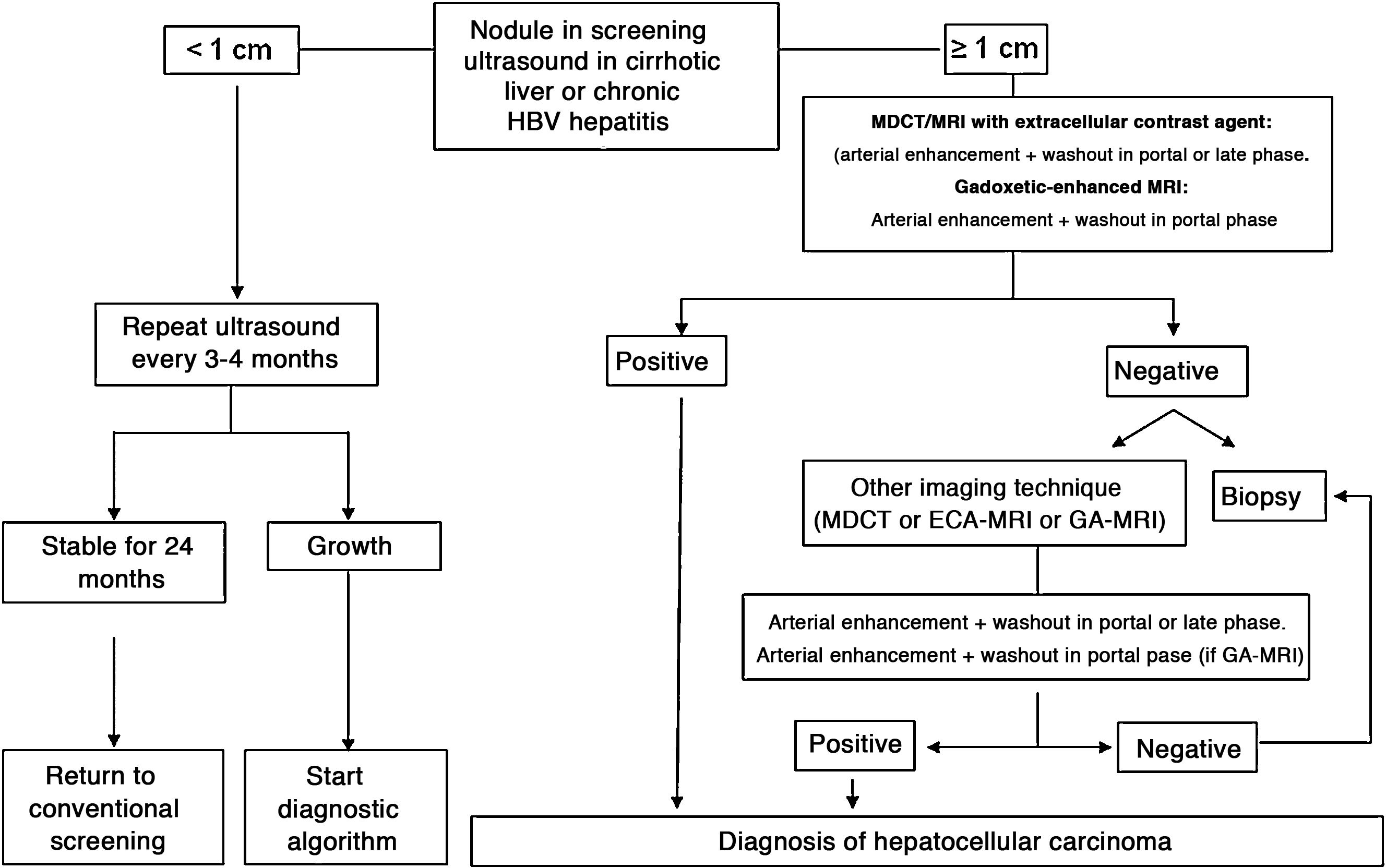
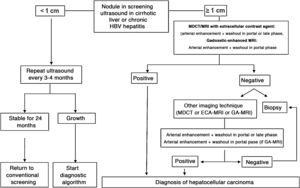
In patients with liver cirrhosis, the probability that a new nodule detected by ultrasound is an HCC is very high, especially if its diameter exceeds 10 mm.81 Therefore, if the detected nodule reaches or exceeds this limit, it is advisable to continue the studies so as to reach a definitive diagnosis. HCCs present predominant arterial vascularity (neovascularisation), as well as a gradual decrease in the number of portal tracts as the hepatocarcinogenesis process progresses,122 in contrast to the liver parenchyma where the vascularisation is mixed: arterial and portal. This determines the typical vascular pattern of HCC, characterised by an intense contrast enhancement in the late arterial phase, followed by a contrast wash-out of the lesion in the venous phases. The imaging techniques show a lesion with high signal intensity/density in the late arterial dynamic phase of the study (wash-in), and a hypodensity/hypointensity of the lesion as compared to the surrounding liver parenchyma in the portal and/or delayed phase (wash-out). This characteristic pattern, favoured due to the high pretest probability of HCC in patients with chronic liver disease, has shown a specificity close to 100% for the diagnosis of HCC when it has been correlated with the pathological analysis of explants, surgical resection pieces or percutaneous biopsies.81,123–129 However, this vascular pattern is penalised by 60%–70% sensitivity in small-sized lesions, and it has been reported that about 15% of small HCCs are hypovascular as they have not yet developed their neovascularisation, though this does not mean that these lesions behave less aggressively.130 Since the first proposal for non-invasive diagnosis of HCC at the Barcelona-2000 EASL consensus conference,131 the diagnostic criteria have been refined regarding the size and image characteristics of the lesion, with the intention of increasing its sensitivity while maintaining its high specificity. In accordance with these criteria, as proposed in the latest version of the AEEH clinical guidelines,1 it is possible to establish the diagnosis of HCC without the need for pathological confirmation when 1 cm in size or larger nodule is detected in a chronic liver disease patient, and the dynamic imaging study (CT or MRI) shows intense contrast enhancement in the arterial phase followed by wash-out in the portal phase (and/or venous phase if it is a CT scan or MRI with extracellular contrast).
Numerous studies and several meta-analyses have been published in recent years to determine the diagnostic efficacy of CTs and MRIs for the diagnosis of small HCC in patients at risk, with very disparate results.132–136 In conclusion, a trend has been observed that indicates a greater diagnostic efficacy of MRI over CT, although without significant differences that would allow a formal recommendation of one technique over the other.
Gadoxetic acid is an MRI combined contrast agent, with an extracellular component that allows dynamic phase studies to be obtained, and a hepatobiliary component that causes normal functioning hepatocytes to capture the contrast in delayed phase. This particular contrast is captured by cells very early, so the images obtained beyond the portal dynamic phase should not be interpreted as late venous phases, since they are actually transitional phases with mixed extracellular and hepatobiliary components.137,138 There is a lot of information published that points to greater sensitivity of gadoxetic-enhanced MRIs compared to CTs and extracellular contrast MRIs, without providing specific data on their specificity.139–144 The retrospective design with its associated selection biases, and the use of non-validated imaging criteria for the final confirmation of the diagnosis of HCC, constitute the major limitations of these studies. In addition, most of these studies have been conducted in Asia, where HCC appears frequently in patients with chronic HBV infection without well-established liver cirrhosis. This fact could mark a difference with respect to the performance of this imaging test when applied in a population composed of patients with established liver cirrhosis. The prospective studies published to date evaluating the diagnostic efficacy of extracellular contrast MRIs and gadoxetic-enhanced MRIs showed that the MRI with the organ-specific contrast agent did not offer greater sensitivity or diagnostic precision compared to the extracellular contrast MRI.145–147 No data are yet available from prospective studies in patients with established liver cirrhosis due to either alcohol abuse or HCV infection included in screening programmes for HCC ≤ 2 cm that would allow the performance of the gadoxetic-enhanced MRIs in this selected group of patients to be evaluated.
The use of gadoxetic acid in MRI studies is accepted in the recent update of the EASL guidelines3 and the AASLD148 guidelines for HCC management. Both scientific societies consider HCC non-invasive diagnostic criterion to be a vascular pattern defined as arterial enhancement and washout in the portal phase. The lesion characteristics in the transitional and hepatobiliary phases are not taken into account as they are mixed or exclusively hepatobiliary phases in which the hyposignal of the lesion is not a consequence of the decrease or absence of portal vessels in the HCC, but of the decrease or absence of the OATP8 expression, which is a transporter responsible for the cellular enhancement of the contrast and is generally absent in HCC. However, it can be present in some HCC, especially in well differentiated ones. Inclusion of the hypointensity of the lesion in delayed phases as a sign of washout and therefore applicable to non-invasive imaging criteria, would lead to an increase in the sensitivity for the detection of HCC, but it would be at the cost of reducing its specificity.149 On the other hand, transient respiratory artifacts have been described during the arterial phase of gadoxetic acid in a percentage of studies that range between 2.4% and 18% These are attributed to the difficulty for patients to maintain apnoea during the first seconds after the contrast injection.150–152 Finally, the sequence in the hepatobiliary phase is usually poor in patients with significant liver failure. Therefore, gadoxetic-enhanced MRI with the reading of the venous washout limited to the portal dynamic phase to avoid possible over-diagnoses is an accepted technique that can be used in the non-invasive diagnosis of HCC. However, the lack of prospective studies comparing its diagnostic precision with respect to MRI obtained with extracellular contrast means that at the present time there is not enough scientific evidence to support the recommendation of its use as the first diagnostic technique, ahead of extracellular contrast MRI.
When the vascular pattern of a nodule is atypical on the CT or MRI, and there are no imaging findings that suggest it could be a malignant process, the use of contrast-enhanced ultrasound (CEUS) has been suggested as a second line test in the latest EASL guidelines update.3 The quality of evidence is moderate, and the strength of recommendation is weak. The justification for this incorporation is the recent refinement of the ultrasound criteria that favours the differential diagnosis between HCC and intrahepatic cholangiocarcinoma (ICC). Venous washout is observed in CEUS in both HCC and ICC,153,154 so the specificity of the MRI and the CT is superior to that of CEUS.155,156 However, the majority of ICCs (50%–88%) have an early washout that takes place within 60 s of the injection of contrast, but this type of washout appears only in 16% of HCC lesions because the washout of the lesion in most HCCs occurs 60 s after the injection of contrast agent.157–159 The vascular pattern of HCC in the CEUS is the homogeneous arterial enhancement of the lesion and a slow and moderate washout, which occurs more than 60 s after the injection of the contrast agent, in contrast to the ICC that shows predominantly peripheral arterial enhancement and fast and intense washout. These new CEUS criteria for the diagnosis of HCC have been adopted by the American College of Radiology (ACR), who have recently developed the CEUS LI-RADS criteria version 2017.160 This is pending prospective validation that will allow us to know its diagnostic precision. A retrospective study applying the CEUS LI-RADS criteria showed a 99% positive predictive value for the LR-5 categorisation for the diagnosis of HCC.161 However, in another retrospective study, Shin et al. reported sensitivity and specificity values of CEUS of 91.1% and 83.3% respectively to establish the differential diagnosis between HCC and ICC.162 A multicentre study published by Aubé et al.163 compares the diagnostic performance of CT, MRI and CEUS independently and in combination, for the diagnosis of HCC between 1 and 3 size. This is a retrospective analysis of a group of patients collected prospectively from the screening programmes of the included sites, and analyses 544 nodules studied in 381 patients. The sensitivity and specificity of the vascular pattern of arterial enhancement and venous washout of HCC between 1 and 2 cm in size (n = 342 lesions) for CT was 67.9% and 76.8% respectively, for MRI was 70.6% and 83.2% respectively, and for CEUS was 39.6% and 92.9% respectively. The sensitivity and specificity for the diagnosis of HCC of 1−2 cm of the 25 patients who had diagnostic confirmation after surgical treatment for CT was 55.6% and 71.4% respectively, for MRI was 61.1% and 85.7% and respectively, and for CEUS was 22.2% and 85.7% respectively. The low specificity of CT and MRI in this study is surprising, as it ranges between 96% and 100% in other series.81,126–128 There is currently a lack of prospective studies that validate the results of CEUS for the diagnosis of HCC in nodular lesions between 10 and 20 mm detected in the screening US, as the subjectivity in the evaluation of the intensity of the washout could be a limitation. Although CEUS can be helpful in specific cases, there is currently not enough scientific evidence to justify its routine use after a non-diagnostic CT or MRI. Therefore, and in an attempt not to delay the diagnosis, when the vascular pattern of the lesion is not typical, the confirmation of the diagnosis of HCC should be based on the biopsy. Finally, in the case of a nodule smaller than 1 cm, given the low probability that it is malignant in nature81 and the difficulty of its correct characterisation, close surveillance using ultrasound every 3–4 months is recommended in order to detect possible growth, and then apply the diagnostic criteria already stated, as shown in Fig. 1. These non-invasive criteria based on the detection of the HCC specific vascular pattern have been externally validated in Europe,81,126,127 North America128 and Asia129 and they are only applicable in patients with established liver cirrhosis or in patients with long-standing HBV infection acquired in the perinatal/childhood period. In the rest of the patients, a pathological study is necessary to obtain a definitive diagnosis of the lesion. The detection of other imaging parameters such as the presence of intralesional fat, isolated signal hypodensity/hypointensity of the lesion in venous phases, or the presence of a pseudocapsule, does not significantly increase the diagnostic performance.164 In 2011, the ACR created LI-RADS (Liver Imaging - Reporting and Data System) for CT and MRI image reading. This would provide a standardised interpretation of liver CT and MRI reports in patients with chronic liver disease and then present a clinical recommendation according to the degree of suspicion that the detected lesion corresponds to HCC. The ACR has updated LI-RADS on several occasions and its latest version has been published recently.165,166 LI-RADS classifies the observations into six broad categories: LR-1 (100% benign), LR-2 (probably benign), LR-3 (intermediate probability for HCC), LR-4 (probably HCC), LR-5 (100% definite HCC) and LR-M (other malignancies: lesions with a high probability of being malignant neoplasms other than HCC). The retrospective evaluation of a prospectively collected cohort of cirrhotic patients included in a HCC screening programme in whom US had detected a single new-onset nodule measuring between 1 and 2 cm in size showed that 69% of the lesions categorised as LR-3 were HCC and that the LR-4 criterion according to the 2014 LI-RADS version was as effective as the LR-5 for the diagnosis of HCC, which meant that uniting both categories would increase the sensitivity without affecting the specificity.167
Algorithm for the diagnosis of hepatocellular carcinoma.
MDCT: Multidetector CT, ECA-MRI: Magnetic resonance imaging with extracellular contrast agents, GA-MRI: Gadoxetic acid-enhanced magnetic resonance imaging.
*Given that the probability of obtaining a false negative result can reach up to 30% in nodules smaller than 2 cm, if the biopsy is negative, consider repeating it, or close surveillance by imaging.
According to these results, the latest version of LI-RADS recommends that LR-5 category should include any nodule not less than 10 mm in size, which in a CT or MRI study shows non-rim arterial enhancement with non peripheral venous washout. This agrees with the classic criteria already validated. The LR-5 category also includes hypervascular lesions that, although they do not show venous washout, present more than 50% tumour growth in under six months. But prospective studies are still required regarding the value of the increased tumour size as an unequivocal criterion for the diagnosis of HCC.166,168,169 According to these criteria, the probability of HCC progressively increases in categories LR 2–4. According to previous publications167,170 and applying the LI-RADS versions of the years 2014 and 2017, the percentage of HCC among the lesions categorised as LR2 ranges between 13% and 23%. In the case of LR3 lesions the percentage ranges between 38% and 69%, and in LR4 lesions it ranges between 74% and 91%. Therefore, pending a prospective validation of the latest version of LI-RADS 2018 criteria, pathological confirmation of lesions categorised as LR 2–4 should be recommended to avoid delays in patient diagnosis and treatment. Perhaps the risk scores of HCC in LR 2–4 lesions could be of value when a second biopsy is indicated after a negative first biopsy,171 although there is no data available to recommend this proposal. The systematic reading of the imaging studies according to the LI-RADS criteria could homogenise the readings of various findings and observations, but the clinical impact of correlating the different categorisations with decision making is not justified. The LR 2–4 categorisations all have a non-negligible risk of HCC, and therefore, to continue with progress surveillance of the lesion instead of indicating a biopsy could lead to a delay in the diagnosis and with it, a worse prognosis of the patient. Also, these criteria have not been validated in prospective studies that would allow us to know their diagnostic precision.
Diffusion-weighted sequences have shown potential for the diagnosis of HCC, but to date there are no prospective studies that demonstrate a clear increase in diagnostic performance.172,173 Positron emission tomography (PET) with 18F-FDG performs poorly for the diagnosis of HCC, especially in the case of small, well-differentiated lesions, which are usually PET-negative. Other radiotracers such as 11C-Choline, have shown promising initial results, but not comparable with those of CT or MRI.174–176
Despite the improvement of imaging techniques, in many cases needle puncture of the liver nodule is still necessary so as to reach the diagnosis of HCC. However, the diagnostic performance of a biopsy in these small nodules is not optimal. There is a false negative rate close to 30%.81 This can be due to a sampling error and the difficulty of making a differential diagnosis between dysplastic nodules and very early HCC when presented with the minute sample obtained from a percutaneous biopsy.122 Therefore, even with a negative biopsy the diagnosis of HCC cannot be ruled out, and the need to obtain a new biopsy must be assessed.3,81,177 Regarding the technique to obtain material for anatomical-pathological analysis, there is no study that has adequately compared the performance of fine needle aspiration-puncture with cutting needle puncture, so no generalised recommendation can be made. Cytology provides a high diagnostic yield, but when the architectural pattern has to be analysed, then the cell block examination or “mini-biopsy” can provide valuable information. The usefulness of performing a non-tumour liver biopsy is controversial.
In recent years, diagnosis based on different gene signatures have been proposed178 as well as immunostaining to reflect this different protein expression.179–181 It is worth highlighting the panel composed of glypican 3 (GPC3), heat-shock protein 70 (HSP70) and glutamine synthetase (GS); these were initially evaluated in tumours obtained after surgical resection or liver transplantation179 and subsequently validated in samples obtained by percutaneous biopsy.180,182 When the staining is positive for two of these proteins, the diagnosis can be assured, but the immunohistochemical panel is not a substitute for expert evaluation and should be reserved to confirm the diagnostic suspicion of HCC, particularly in those samples with little material or for pathologists with little experience in evaluating liver tumours.182 Finally, some authors have warned about the risk of local seeding after the puncture of these nodules. However, the incidence of this complication is very low, less than 0.1%,183,184 and the risk associated with puncture should always be assessed with the risk of applying treatment in patients without confirmed validated criteria of HCC.88 There is controversy over the recommendation to perform biopsies for diagnostic confirmation in order to obtain histological material that allows different molecular markers to be evaluated. The need to obtain samples is indisputable in order to further our understanding of the molecular pathways associated with HCC. But given that radiological techniques can confirm the diagnosis of HCC in a significant number of patients, the systematic obtaining of biopsies should be carried out in the context of a research project with the informed consent of the patient and acceptance by the ethics committees of each site.3,185
Finally, the performance of AFP as a diagnostic is poor.81,129,186 Different neoplasms such as cholangiocarcinoma or metastases of gastrointestinal origin can present high levels of AFP.84,129,187,188 Therefore, despite finding elevated levels of AFP of any magnitude, if the liver mass does not present a specific vascular pattern on imaging, a confirmatory biopsy should be performed.
Recommendations- •
Nodules larger than or equal to 1 cm detected by ultrasound in cirrhotic patients can be diagnosed as HCC without the need for histological confirmation if they present contrast enhancement in the arterial phase followed by washout in venous phases in an imaging technique (MDCT or extracellular contrast MRI) (high evidence, strong recommendation). When using gadoxetic acid-enhanced MRI the washout should only be evaluated in the portal venous phase (moderate evidence, weak recommendation). Studies are required to validate the utility of contrast ultrasound.
- •
Nodules smaller than 1 cm detected by screening ultrasound should be followed-up by ultrasound every 3–4 months. If after two years no growth is detected, the surveillance can return to routine screening every six months (low evidence, weak recommendation).
- •
If the vascular pattern of a focal lesion is not specific for HCC, a biopsy should be recommended. If the pathology test is negative, the diagnosis of HCC cannot be ruled out. A new diagnostic biopsy should be considered, or the lesion should be closely monitored (moderate evidence, strong recommendation).
- •
In the case of patients without chronic liver disease, the application of these imaging criteria is not valid, and a biopsy is necessary to obtain the diagnosis (moderate evidence, strong recommendation).
- •
The determination of AFP is not useful for the diagnosis of HCC (moderate evidence, strong recommendation).
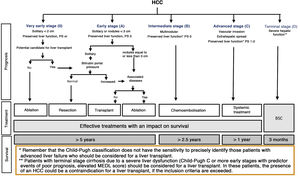
Once the diagnosis is obtained, it is necessary to carry out a study of the extension of the disease and a prognostic evaluation. This makes it possible to inform the patient and family members about life expectancy, to choose the most appropriate treatment and to evaluate the patient’s response. The prognosis of solid tumours depends fundamentally on the tumour stage. However, given that HCC mostly appears in association with liver cirrhosis, and that the degree of alteration in liver function determines the therapeutic options and survival regardless of the presence of HCC, it is essential to jointly consider the degree of liver dysfunction and the tumour extension. There are different scores/classifications that assess the degree of liver dysfunction, such as the Child-Pugh classification,189 the MELD system190 or the ALBI classification.191 The ALBI classification was designed to predict the evolution of patients with HCC and includes albumin and bilirubin in its nomogram.191 It has been evaluated in patients with different stages of the tumour and treatments, but currently its use is not part of the recommendations of the majority of clinical guidelines in terms of making treatment decisions.2,3 Finally, the use of validated scales such as the ECOG performance status192 or the Karnofsky index193 to adequately assess the presence of cancer-related symptoms, have shown great prognostic value and, in the same way as the hepatic functional reserve, they determine the applicability of the different available treatments. Therefore, those prognostic systems that take into account a single dimension of the disease (tumour extension, liver function or the presence of symptoms associated with cancer) are inaccurate and are only useful for detecting terminal disease. Multiple staging systems have appeared during the past decade that take into account factors associated with tumour extension and liver function.194 Unfortunately, most of them either do not consider the presence of symptoms, or they evaluate the tumour extension inaccurately. The only one that links staging with treatment and that has also been externally validated is the Barcelona Clinic Liver Cancer system (BCLC).194 Since its original publication in 1999,195 the system has been refined until its latest version in 2018.194 It is the staging system recommended by the most relevant scientific societies, although each one has suggested modifications to the BCLC.2,3,196,197
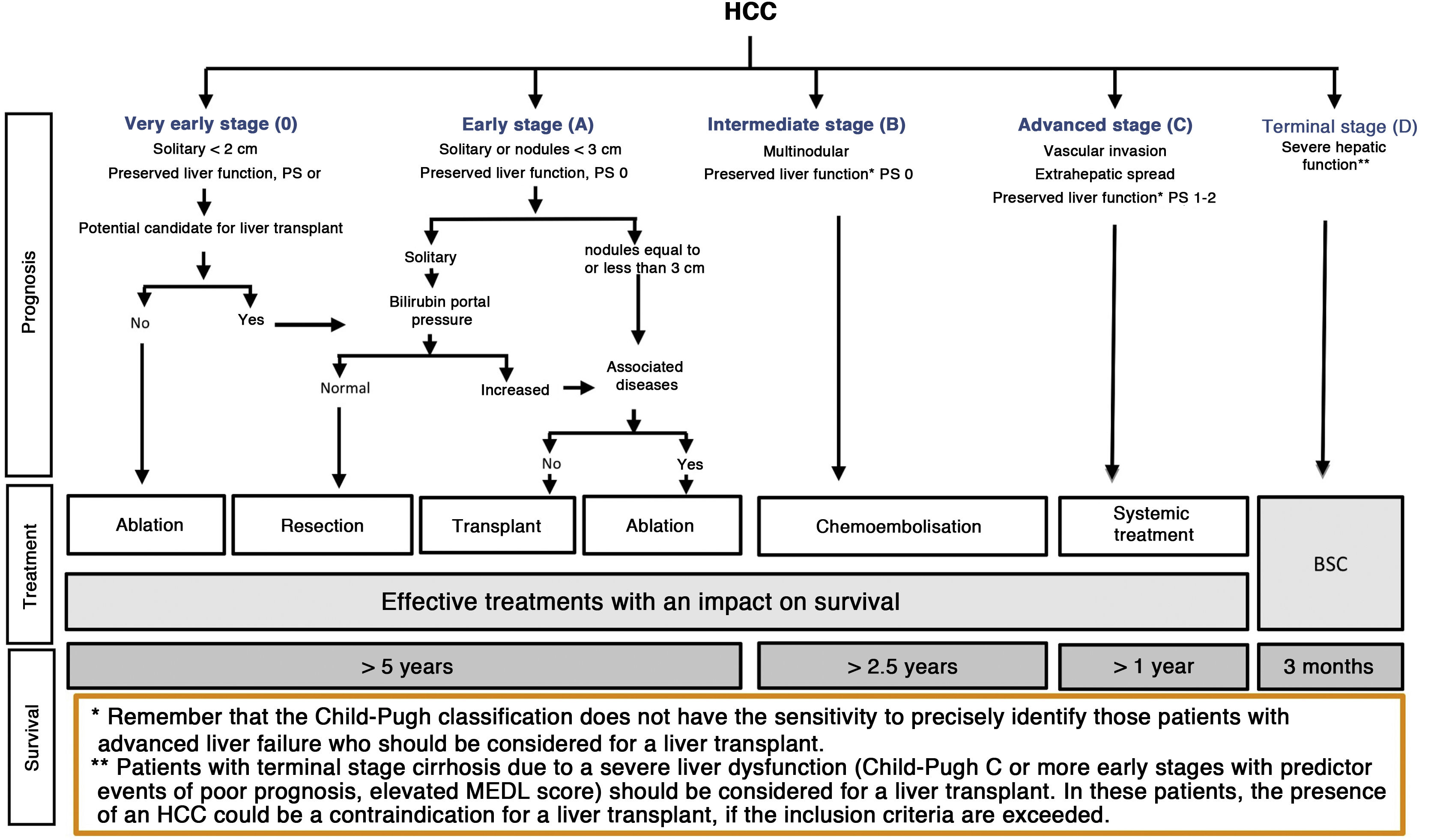
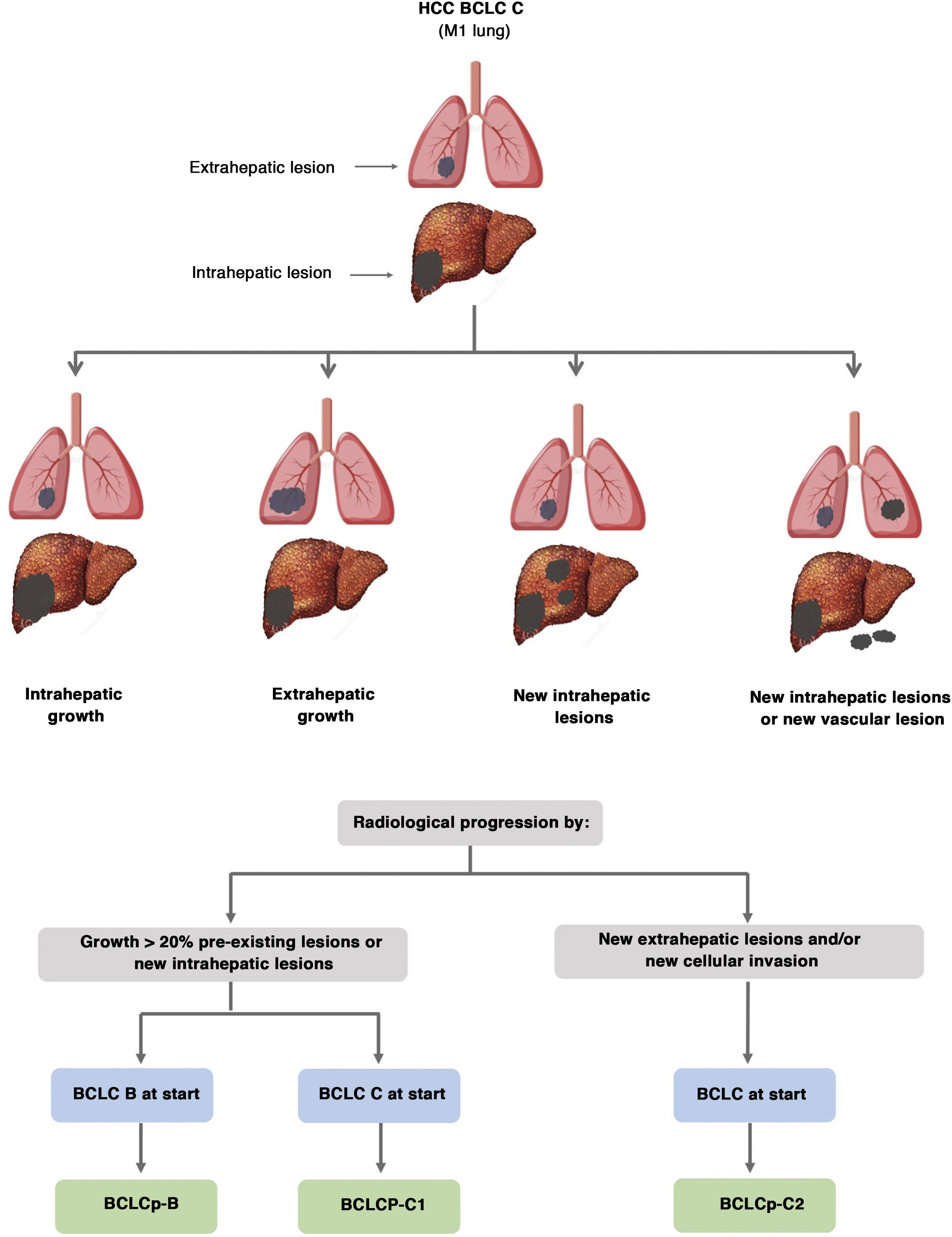
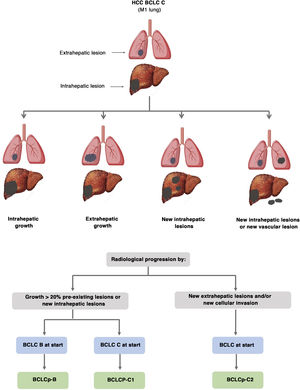
The BCLC system includes variables associated with tumour stage, liver function, and presence of symptoms and establishes the prognosis according to five stages that are linked to the possible indication for treatment (Fig. 2). The very early stage (stage 0) constitutes a group with an especially good prognosis that includes patients with compensated liver cirrhosis (Child-Pugh A), totally asymptomatic, who present single tumours less than or equal to 2 cm without vascular invasion and not spreading. This very early stage would correspond to the concept of carcinoma in situ.122 In these cases, percutaneous ablation offers a high probability of cure, with survival rates similar to those obtained with surgical resection, but with lower cost and morbidity. It is considered the first therapeutic option, particularly in those patients without future options for liver transplantation. The early stage (stage A) includes asymptomatic patients with preserved liver function (Child-Pugh A and B not meeting liver transplantation criteria due to liver function) with a solitary HCC or a maximum of three nodules up to 3 cm in diameter. These patients can receive curative-intent treatment using surgical resection, percutaneous ablation, and liver transplantation, with a 50%–75% expected 5-year survival rate. The intermediate stage (stage B) consists of patients with multinodular tumours that exceed the criteria described above, absence of vascular or extrahepatic invasion, preserved liver function and general health condition. Expected treatment-free survival in this group of patients is 49.6% (95% CI 32%–75%) at one year198 and the only treatment that has shown efficacy in terms of survival is liver chemoembolisation (transarterial chemoembolisation, TACE).194 In 2012, Bolondi et al. proposed a subclassification of stage B into four subgroups according to tumour burden, presence of symptoms, and degree of liver dysfunction.199 However, this subclassification includes patients with severe liver dysfunction who should be evaluated for liver transplantation and in whom the presence of HCC is only a contraindication if the tumour extension exceeds the criteria to be able to offer this option. Additionally, it suggests considering PS 1 patients as BCLC-B despite the Bolondi et al. study showing that the presentation of PS 1 in TACE-treated patients implied significantly lower survival.200 Finally, it should be noted that according to the BCLC model, single tumours without PS involvement should be considered BCLC A, but many analyses and proposals to reform the BCLC model mistakenly include them as BCLC B. Patients with preserved liver function, but who present an HCC with vascular invasion and/or extrahepatic metastasis or with slightly deteriorated general health condition are classified as advanced stage (stage C). The median treatment-free survival in this group of patients is 4–8 months and they are candidates for systemic treatment. In the context of patients with advanced HCC treated with sorafenib, a complementary classification to the BCLC classification was developed, and it is called BCLC at the time of progression.201 This classification is applicable to patients with HCC undergoing systemic treatment who develop radiological progression but maintain their general health condition and preserved liver function. Patients are classified according to the baseline BCLC stage and the radiological progression pattern (Fig. 3).202 Patients who begin systemic treatment at BCLC-B stage and develop new lesions within the liver are called BCLCp-B; patients who begin systemic treatment at BCLC-C stage and develop new lesions within the liver or pre-existing lesions grow regardless of location are called BCLCp-C1; and finally, patients who develop new lesions outside the liver, regardless of the baseline BCLC stage are called BCLC-C2. These groups of patients have a median post-progression survival that differs from each other and varies between 24, 15 and 7 months respectively.201
BCLC staging system (Barcelona-Clinic-Liver-Cancer).
Performance status; BSC: Best supportive care.
Adapted from Forner et al.194
Prognostic value of the progression patterns. The progression pattern in first-line treatment with sorafenib is a prognostic factor in patients with hepatocellular carcinoma (HCC). Patients who develop new extrahepatic lesions have a lower survival rate than those with other patterns. According to the baseline stage and the progression pattern, different prognostic groups are established, known as the BCLC (Barcelona-Clinic-Liver-Cancer) classification after progression (BCLCp). BCLCp-B defines radiological progression due to the growth of existing liver nodules or new intrahepatic foci, but the patient is still in intermediate stage (BCLC B) due to the absence of vascular invasion or extrahepatic spread. Patients with radiological progression and progressing to advanced stage (BCLC C) or progressing within BCLC C are divided into BCLCp-C1 (growth of pre-existing nodules or new intrahepatic sites) and BCLCp-C2 (progression by a new extrahepatic lesion and/or or vascular invasion).
Adapted from Bruix et al.201
Finally, patients with severely deteriorated general health condition and/or compromised liver function (Child-Pugh C or Child-Pugh B cirrhosis with decompensations associated with a poor prognosis such as refractory ascites, chronic/recurrent hepatic encephalopathy or spontaneous bacterial peritonitis) and who are not candidates for liver transplantation correspond to stage D or terminal stage. The median survival for these patients is less than three months198 and only symptomatic treatment should be indicated.
Recommendation- •
To assess the prognosis of HCC, not only must the tumour stage be considered but also the liver function and the presence of symptoms related to the tumour. The BCLC system takes these parameters into account and is the only system that combines the prognostic prediction with the recommended therapeutic option (high evidence; strong recommendation).
The treatment of HCC is particularly complex due to the development of cancer in the context of chronic liver disease. At the same time, the liver disease determines the need for an expert radiological evaluation of the image findings, both for non-invasive diagnosis and for the assessment of the application/extension and evaluation of locoregional treatment. The consideration of surgical treatment should include liver resection (open surgery or laparoscopic surgery) and liver transplantation. Systemic therapy involves the detection and management of toxicity, especially liver toxicity. This means providing a healthcare environment with various specialists, especially when combined treatments are applied. Finally, when the underlying liver disease decompensates and a specific cancer treatment is not possible, a healthcare arrangement must be in place to meet the needs for symptomatic treatment of liver decompensation, in advance of end-of-life palliative care.
These concerns plus the fact that HCC is not a common tumour led to the recommendation of caring for patients with this neoplasm in reference centres, where the needed specialities and fields of expertise are available. This ensures the application of scientific evidence-based medicine and progress towards precision medicine. In this sense, the Liver Cancer departments should incorporate liver oncology advanced practice nurses to provide the relevant health education and promote empowerment of patients and their families.
Liver resectionSurgical resection is the treatment of choice for tumours that develop over a non-cirrhotic liver, where more extensive resections can be performed with a low risk of morbidity and mortality and acceptable survival.203–205 HCC can emerge without underlying cirrhosis in patients with NAFLD or HBV. In these patients, the hepatic functional reserve may be adequate, but the existence of comorbidities associated with these entities must be taken into account. The same happens when the patient is along in years; age is not a contraindication in itself, but the presence of comorbidities should be carefully assessed.
In our setting, most HCCs appear in the context of chronic liver disease, usually in the cirrhotic phase. This creates a high risk of morbidity and mortality due to postoperative liver failure when performing large liver resections. So before indicating surgery, liver function and remaining liver volume should be carefully evaluated. Decompensated cirrhosis is a formal contraindication, and the treatment of choice would be a liver transplant once the contraindications for the same are excluded.3
The best candidates for surgical resection are patients with solitary tumours without vascular invasion or extrahepatic metastasis, with normal bilirubin levels and without clinically significant portal hypertension (CSPH). CSPH is diagnosed based on the absence of gastroesophageal varices and hepatic venous pressure gradient less than or equal to 10 mmHg.206,207 The presence of CSPH is associated with an increased risk of postoperative complications and a worse medium term prognosis. Thus, in multifocal tumours or in the presence of portal hypertension, resection could be technically feasible and immediate morbidity and mortality apparently acceptable; however, five-year survival may be lower than 50%, so these patients should be considered for liver transplantation if they meet the selection criteria.
The existence of CSPH is confirmed with the observation of oesophageal varices or ascites. Detection of splenomegaly and a platelet count lower than 100,000/mm3 does not accurately identify the presence of CSPH.208 Liver elastography can detect CSPH, with a value greater than 21 kPa being highly suggestive of CSPH and a value less than 13.6 kPa ruling it out.110 Unfortunately, an unequivocal cut-off point does not exist and elastography only allows the correct evaluation of CSPH in half of the cases.110,209
There is no size limit to consider surgical resection in solitary tumours. It is known that the risk of satellite nodules and/or microscopic vascular invasion increases in line with tumour size but, once these characteristics are ruled out by adequate staging, tumour size is not seen to be connected with a greater risk of recurrence. If the pathological examination detects microscopic vascular invasion and/or satellites, the risk of recurrence is higher.210
Liver resection should be guided by intraoperative ultrasound and the goal should be anatomic resection with a disease-free margin in order to eliminate microscopic satellitosis not visible by imaging. In <20 mm tumours, microvascular invasion with satellitosis is rare and the advantages of the margin are not evident. There may be benefits in larger tumours, but if macroscopic vascular invasion or satellites are already registered preoperatively, the benefits regarding recurrence risk or survival disappears.
In patients selected according to the previously mentioned criteria and operated on by experienced teams, postoperative mortality should be less than 3% and serious postoperative complications less than 30% with a transfusion rate of less than 10%.3 However, the latest version of the EASL clinical guidelines for the management of HCC proposes to broaden these criteria and the prospective evaluation of the proposed criteria, as well as to define the preoperative risk of said expansion.3
The risk of recurrence is related to various pathological findings such as the tumour differentiation grade, the presence of satellite nodules, multifocality or the presence of microscopic vascular invasion.210 Sometimes the risk of recurrence is high, or there is a risk that the tumour extent will exceed the transplant indication criteria. A strategy has been proposed to enlist patients for liver transplantation who present pathological findings of poor prognosis (ab initio indication) before tumour recurrence but after a six-month observation period. This allows more aggressive HCCs with a high risk of recurrence as well as posttransplant tumour recurrence to be discriminated.211
Advances in laparoscopic and robotic surgery provide less invasive surgical treatments with comparable results in terms of recurrence and survival, but with lower perioperative morbidity and shorter hospital stays.212,213 Certain locations (posterior superior segments) represent greater difficulty and open surgery continues to be used. The minimally invasive approach is playing an important role in terms of the possibility of expanding surgical criteria beyond the theoretical “ideal candidate” previously described. This possibility should be evaluated prospectively in specialised centres.
Adjuvant or neoadjuvant treatmentTumour recurrence complicates 70% of cases at five years post surgical resection, reflecting intrahepatic metastasis (recurrence known as “true” dissemination) or due to the development of de novo HCC. Neither entity has a clear definition based on clinical or temporal data. There is no therapeutic strategy that has been shown to be effective in reducing the recurrence risk. It is hoped that the hepatitis C virus treatment may decrease the risk of de novo HCC, but confirmation with a five-year follow up is not possible, as the published works have median follow-up of approximately 3–4 years.9,10
A clinical trial in Asia demonstrated the efficacy of adoptive immunotherapy in reducing recurrence and increasing early survival (to two years) after curative treatment,214 suggesting that check-point inhibitors such as CTLA4, PD-1 or PDL1 could play a role in this indication. A second study published by the same group described the five-year follow-up of these patients and the authors concluded that both the risk of recurrence and of death were lower in the immunotherapy group compared to the control group (survival HR 0.33; 95% CI 0.15−0.76; P = .006).215 This adds weight to the potential of immunotherapy to lower the recurrence risk, and clinical trials are currently underway to establish its efficacy.
Recommendations- •
Surgical resection is recommended in patients with solitary HCC arising in non-cirrhotic liver or in a cirrhotic liver with preserved liver function, normal bilirubin and hepatic venous pressure gradient less than or equal to 10 mmHg. Above these limits, survival is significantly lower (high evidence, strong recommendation).
- •
In referral centres, liver resection should be considered using minimally invasive approaches, following the guidelines for open liver resection (moderate evidence; weak recommendation).
- •
At present, no adjuvant treatment has demonstrated efficacy in preventing recurrences after surgery in HCC (high evidence, strong recommendation). Evaluation of new treatments in clinical trials is recommended.
Although liver transplant is theoretically the oncological treatment of choice in HCC patients, its use is limited. This is due to the risk of post-transplant recurrence (usually disseminated, with few therapeutic alternatives and high mortality)216 and because of its potential impact on waiting lists. An excessive increase on these lists of candidates with HCC (who often have preserved liver function and, therefore, have other treatment options) could limit access to transplantation for patients with hepatocellular failure or those without a therapeutic alternative.
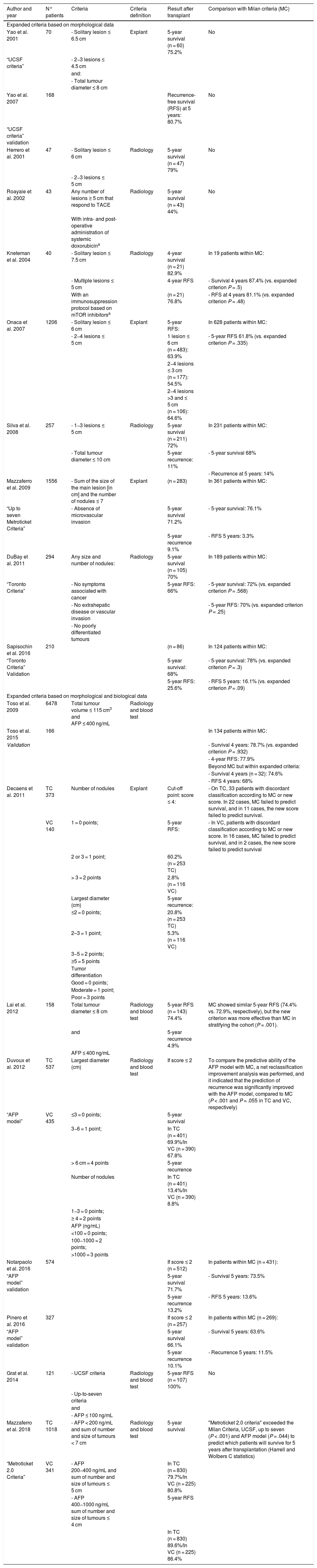
The Milan criteria (a nodule less than or equal to 5 cm or up to 3 nodules less than or equal to 3 cm, in the absence of macrovascular invasion or extrahepatic disease) are widely validated to select candidates for liver transplantation.217 However, some authors consider that these criteria are too restrictive since they exclude patients who could benefit from the transplant. In addition, the Milan criteria only consider morphological factors which, in turn, depend on the reliability of the radiological examinations,218 and do not consider other variables related to the biological behaviour of the tumour. Several studies have shown that with somewhat less restrictive “expanded criteria” it is possible to obtain post-transplant survival results similar to those of the Milan criteria, although they are associated with an increase in the recurrence rate.
These “expanded criteria” exclude cases with macrovascular invasion and/or extrahepatic spread and, in addition to broadening the morphological limits (larger size and/or number of lesions), many programmes recommend the use of AFP levels and/or response neoadjuvant therapy (downstaging) as favourable biological behaviour markers to be included in the selection criteria.219,220Table 1 summarises the most accepted expansion proposals.
Expanded criteria for liver transplantation.
| Author and year | N° patients | Criteria | Criteria definition | Result after transplant | Comparison with Milan criteria (MC) |
|---|---|---|---|---|---|
| Expanded criteria based on morphological data | |||||
| Yao et al. 2001 | 70 | - Solitary lesion ≤ 6.5 cm | Explant | 5-year survival (n = 60) 75.2% | No |
| “UCSF criteria” | - 2−3 lesions ≤ 4.5 cm | ||||
| and: | |||||
| - Total tumour diameter ≤ 8 cm | |||||
| Yao et al. 2007 | 168 | Recurrence-free survival (RFS) at 5 years: 80.7% | No | ||
| “UCSF criteria” validation | |||||
| Herrero et al. 2001 | 47 | - Solitary lesion ≤ 6 cm | Radiology | 5-year survival (n = 47) 79% | No |
| - 2−3 lesions ≤ 5 cm | |||||
| Roayaie et al. 2002 | 43 | Any number of lesions ≥ 5 cm that respond to TACE | Radiology | 5-year survival (n = 43) 44% | No |
| With intra- and post-operative administration of systemic doxorubicina | |||||
| Kneteman et al. 2004 | 40 | - Solitary lesion ≤ 7.5 cm | Radiology | 4-year survival (n = 21) 82.9% | In 19 patients within MC: |
| - Multiple lesions ≤ 5 cm | 4-year RFS | - Survival 4 years 87.4% (vs. expanded criterion P = .5) | |||
| With an immunosuppression protocol based on mTOR inhibitorsa | (n = 21) 76.8% | - RFS at 4 years 81.1% (vs. expanded criterion P = .48) | |||
| Onaca et al. 2007 | 1206 | - Solitary lesion ≤ 6 cm | Explant | 5-year RFS: | In 628 patients within MC: |
| - 2−4 lesions ≤ 5 cm | 1 lesion ≤ 6 cm (n = 483): 63.9% | - 5-year RFS 61.8% (vs. expanded criterion P = .335) | |||
| 2−4 lesions ≤ 3 cm (n = 177): 54.5% | |||||
| 2−4 lesions >3 and ≤ 5 cm (n = 106): 64.6% | |||||
| Silva et al. 2008 | 257 | - 1−3 lesions ≤ 5 cm | Radiology | 5-year survival (n = 211) 72% | In 231 patients within MC: |
| - Total tumour diameter ≤ 10 cm | 5-year recurrence: 11% | - 5-year survival 68% | |||
| - Recurrence at 5 years: 14% | |||||
| Mazzaferro et al. 2009 | 1556 | - Sum of the size of the main lesion [in cm] and the number of nodules ≤ 7 | Explant | (n = 283) | In 361 patients within MC: |
| “Up to seven Metroticket Criteria” | - Absence of microvascular invasion | 5-year survival 71.2% | - 5-year survival: 76.1% | ||
| 5-year recurrence 9.1% | - RFS 5 years: 3.3% | ||||
| DuBay et al. 2011 | 294 | Any size and number of nodules: | Radiology | 5-year survival (n = 105) 70% | In 189 patients within MC: |
| “Toronto Criteria” | - No symptoms associated with cancer | 5-year RFS: 66% | - 5-year survival: 72% (vs. expanded criterion P = .568) | ||
| - No extrahepatic disease or vascular invasion | - 5-year RFS: 70% (vs. expanded criterion P = .25) | ||||
| - No poorly differentiated tumours | |||||
| Sapisochin et al. 2016 | 210 | (n = 86) | In 124 patients within MC: | ||
| “Toronto Criteria” Validation | 5-year survival: 68% | - 5-year survival: 78% (vs. expanded criterion P = .3) | |||
| 5-year RFS: 25.6% | - RFS 5 years: 16.1% (vs. expanded criterion P = .09) | ||||
| Expanded criteria based on morphological and biological data | |||||
| Toso et al. 2009 | 6478 | Total tumour volume ≤ 115 cm3 and AFP ≤ 400 ng/mL | Radiology and blood test | ||
| Toso et al. 2015 | 166 | In 134 patients within MC: | |||
| Validation | - Survival 4 years: 78.7% (vs. expanded criterion P = .932) | ||||
| - 4-year RFS: 77.9% | |||||
| Beyond MC but within expanded criteria: | |||||
| - Survival 4 years (n = 32): 74.6% | |||||
| - RFS 4 years: 68% | |||||
| Decaens et al. 2011 | TC 373 | Number of nodules | Explant | Cut-off point: score ≤ 4: | - On TC, 33 patients with discordant classification according to MC or new score. In 22 cases, MC failed to predict survival, and in 11 cases, the new score failed to predict survival. |
| VC 140 | 1 = 0 points; | 5-year RFS: | - In VC, patients with discordant classification according to MC or new score. In 16 cases, MC failed to predict survival, and in 2 cases, the new score failed to predict survival | ||
| 2 or 3 = 1 point; | 60.2% (n = 253 TC) | ||||
| > 3 = 2 points | 2.8% (n = 116 VC) | ||||
| Largest diameter (cm) | 5-year recurrence: | ||||
| ≤2 = 0 points; | 20.8% (n = 253 TC) | ||||
| 2–3 = 1 point; | 5.3% (n = 116 VC) | ||||
| 3−5 = 2 points; | |||||
| ≥5 = 5 points | |||||
| Tumor differentiation | |||||
| Good = 0 points; | |||||
| Moderate = 1 point; | |||||
| Poor = 3 points | |||||
| Lai et al. 2012 | 158 | Total tumour diameter ≤ 8 cm | Radiology and blood test | 5-year RFS (n = 143) 74.4% | MC showed similar 5-year RFS (74.4% vs. 72.9%, respectively), but the new criterion was more effective than MC in stratifying the cohort (P = .001). |
| and | 5-year recurrence 4.9% | ||||
| AFP ≤ 400 ng/mL | |||||
| Duvoux et al. 2012 | TC 537 | Largest diameter (cm) | Radiology and blood test | If score ≤ 2 | To compare the predictive ability of the AFP model with MC, a net reclassification improvement analysis was performed, and it indicated that the prediction of recurrence was significantly improved with the AFP model, compared to MC (P < .001 and P = .055 in TC and VC, respectively) |
| “AFP model” | VC 435 | ≤3 = 0 points; | 5-year survival | ||
| 3−6 = 1 point; | In TC (n = 401) 69.9%/In VC (n = 390) 67.8% | ||||
| > 6 cm = 4 points | 5-year recurrence | ||||
| Number of nodules | In TC (n = 401) 13.4%/In VC (n = 390) 8.8% | ||||
| 1−3 = 0 points; | |||||
| ≥ 4 = 2 points | |||||
| AFP (ng/mL) | |||||
| <100 = 0 points; | |||||
| 100−1000 = 2 points; | |||||
| >1000 = 3 points | |||||
| Notarpaolo et al. 2016 | 574 | If score ≤ 2 (n = 512) | In patients within MC (n = 431): | ||
| “AFP model” validation | 5-year survival 71.7% | - Survival 5 years: 73.5% | |||
| 5-year recurrence 13.2% | - RFS 5 years: 13.6% | ||||
| Pinero et al. 2016 | 327 | If score ≤ 2 (n = 257) | In patients within MC (n = 269): | ||
| “AFP model” validation | 5-year survival 66.1% | - Survival 5 years: 63.6% | |||
| 5-year recurrence 10.1% | - Recurrence 5 years: 11.5% | ||||
| Grat et al. 2014 | 121 | - UCSF criteria | Radiology and blood test | 5-year RFS (n = 107) 100% | No |
| - Up-to-seven criteria | |||||
| and | |||||
| - AFP ≤ 100 ng/mL | |||||
| Mazzaferro et al. 2018 | TC 1018 | - AFP < 200 ng/mL and sum of number and size of tumours < 7 cm | Radiology and blood test | 5-year survival | "Metroticket 2.0 criteria" exceeded the Milan Criteria, UCSF, up to seven (P < .001) and AFP model (P = .044) to predict which patients will survive for 5 years after transplantation (Harrell and Wolbers C statistics) |
| “Metroticket 2.0 Criteria” | VC 341 | - AFP 200−400 ng/mL and sum of number and size of tumours ≤ 5 cm | In TC (n = 830) 79.7%/In VC (n = 225) 80.8% | ||
| - AFP 400−1000 ng/mL sum of number and size of tumours ≤ 4 cm | 5-year RFS | ||||
| In TC (n = 830) 89.6%/In VC (n = 225) 86.4% | |||||
AFP, alpha-fetoprotein; MC, Milan Criteria; RFS, recurrence-free survival; TACE, transarterial chemoembolisation; TC, training cohort; VC, validation cohort; UCSF, University of California, San Francisco criteria.
Elevated AFP (or its progressive increase) is a predictor of microvascular invasion and poor tumour differentiation. Although there is no defined cut-off value, the risk clearly increases after 100 ng/mL. Many sites establish levels of 400−500 ng/mL as a contraindication (at least relative), and it is commonly accepted that values > 1000 ng/mL is associated with a prohibitive risk of post-transplant recurrence.221–223
The response to neoadjuvant therapy (downstaging) is considered another surrogate marker of the favourable tumour biological profile.219,220 Stable regression (usually six months) to the Milan limits after locoregional treatment by patients who previously exceeded those limits, seems to identify a subgroup of candidates with survival and recurrence results comparable to conventional criteria.
There are different composite criteria that include size/number, volume, AFP and/or locoregional treatment response222 or even histological data obtained by biopsy.224 However, studies advocating the expansion of criteria and/or the application of downstaging frequently suffer from various methodological flaws: retrospective vs. prospective design; inadequate sample size; “expanded” cohorts that are poorly representative; variable waiting-list times; use of radiological vs. histological expanded criteria; lack of definition of the tumour staging criteria; or the use of non-validated reports, insufficient observation periods and short follow-up periods. Therefore, at this moment a recommendation in this regard is not feasible.
When considering the abovementioned limitations, a moderate expansion of the Milan criteria could be appropriate as long as fair access to transplantation is assured for patients with indications other than HCC. In the current situation characterised by a progressive decrease in the number of patients on the transplant waiting list in our country, the SETH (Spanish Liver Transplant Society) has drawn up a consensus document to expand transplantation indications, which includes HCC.225 In said document the recommendation was given to adopt the “up to seven” expanded criterion, as this had solid scientific support (the sum of the number of nodules and the size of the largest nodule in cm must be less than or equal to 7),226,227 as long as there is a favourable tumour biology context (estimated by the serum AFP level and by the response to locoregional ablation therapy).
When putting this recommendation into practice, it is important to consider the epidemiological heterogeneity between different Spanish autonomous regions, as well as the variations in donation rates and the composition of the waiting lists. Therefore, the application of SETH recommendations in a certain region will require a specific analysis and a strategic planning initiative. Moreover, it will be essential to closely monitor the impact of expanding the criteria on the waiting lists, and implement the necessary corrective actions that arise, so as to uphold the ethical principles of utility, equity and justice in liver transplants. With respect to downstaging, the SETH has not issued recommendations. Given the heterogeneity of the scientific evidence in this field, it seems a suboptimal source of expanding criteria, and caution should prevail. In any case, the ideal strategy continues to be an increase in organ availability by means of non-heart beating organ donations and/or living donors.
Recommendations- •
Liver transplantation is recommended as the first choice for patients with HCC within the Milan criteria not susceptible to liver resection (high evidence, strong recommendation). Milan criteria are the reference framework for liver transplantation for HCC and the basis for comparison for any other alternative (high evidence, moderate recommendation).
- •
Patients who discretely exceed conventional criteria could be considered for liver transplantation if such expansion does not significantly limit access to transplantation for the rest of the patients and indications. Criteria such as “up to seven” are considered as a reference to expand the transplantation criteria in HCC, especially in patients with low serum AFP and a favourable response to locoregional therapies. This strategy should be developed in the context of well-defined prospective protocols (moderate evidence, weak recommendation).
- •
“Composite criteria” that, in addition to the size/number of nodules, provide information on tumour biology (AFP is the most evaluated marker to date) and include the tumour progression pattern and the response to previous treatment, could eventually replace the current criteria. These criteria should be determined beforehand and validated afterwards (low evidence, strong recommendation).
- •
The presence of macroscopic vascular invasion and/or extrahepatic metastases is an absolute contraindication for liver transplantation (high evidence, strong recommendation).
In very early-stage patients, percutaneous ablation could offer similar survival results as surgical resection. In selected cases where percutaneous ablation is not feasible due to the location of the tumour, laparoscopic-assisted ablation can be used.
At the present time, radiofrequency (RF) ablation constitutes the benchmark technique, while percutaneous ethanol injection (PEI) is reserved for limited indications due to location or to complete the ablation when there is minimal residual activity.228 RF ablation has shown greater ablative capacity,229–233 and survival advantage over PEI,233–236 especially in tumours larger than 2 cm.236 However, its drawbacks are greater frequency and severity of adverse effects,237–239 higher costs, and less applicability. In subcapsular tumours, adjacent to the gallbladder, hepatic hilum or heart, its use is limited due to the risk of complications. Furthermore, its efficacy is limited in tumours close to large blood vessels due to a phenomenon of thermal energy dissipation that makes it difficult to completely ablate the lesion.
Microwave ablation (MW) has shown great ablative capacity, and unlike RF ablation, its efficacy is not affected by the presence of blood vessels adjacent to the tumour.240 However, to date, there is no prospective randomised study that has demonstrated the superiority of this technique over RF ablation.241 Other ablation techniques such as laser,242 cryoablation,243 HIFU (high-intensity focused ultrasound),244 or irreversible electroporation245,246 have not shown superiority over RF ablation and they are more costly with greater technical complexity.
CT-guided electromagnetic navigation systems and image fusion are readily available allowing further refinement of percutaneous ablation. They provide more precise and safer punctures, reduce the operator-dependent factor, and allow access to hard-to-reach locations. Moreover, they identify the problem nodule regardless of its visibility on ultrasound.
Like surgical resection, the main drawback of percutaneous ablation is high recurrence (80% at 5 years) despite obtaining an initial complete response.238,247,248 Clinical trials conducted to prevent relapse were negative and clinical trials with immunotherapy are currently underway.
Percutaneous ablation in solitary tumours ≤ 2 cm offers complete necrosis rates greater than 95% and its efficacy has been compared to that obtained with surgical resection,247,249–255 with conflicting results. Unfortunately, these studies present methodological problems, particularly in the allocation of treatment and selection biases, which invalidate their results. Cost-effectiveness studies exploring which of these two options is more cost-effective conclude that RF ablation can offer survival rates similar to resection but at a lower cost.256,257 Therefore, percutaneous ablation may be the first-line treatment in patients with very early stage HCC.
Recommendations- •
Tumour ablation is an effective treatment in patients with early-stage HCC who are not candidates for surgical resection, or as treatment during the waiting time for liver transplantation (high evidence, strong recommendation).
- •
Ethanol injection and radiofrequency have similar efficacy in tumours smaller than 2 cm. Radiofrequency is the gold standard ablative treatment (high evidence, strong recommendation).
- •
In tumours smaller than 2 cm, radiofrequency presents a therapeutic efficacy similar to surgical resection (moderate evidence, strong recommendation).
TACE is the first-line treatment for patients with intermediate stage HCC (stage B of the BCLC classification). It consists of selective hepatic artery branch embolisation, and superselective catheterisation of the tumour-feeding arteries. It combines the injection of chemotherapy plus arterial flow occlusion by means of an embolising substance.258 TACE is indicated in patients with compensated liver disease with multifocal tumours without vascular invasion or extrahepatic spread. The major contraindications include decompensated cirrhosis, and/or multicentric involvement of both liver lobes that prevent selective intervention, severely reduced portal vein blood flow (thrombosis or hepatofugal blood flow), untreatable arteriovenous fistula, bilioenteric anastomosis or biliary stents and creatinine clearance < 30. In these cases there is a high risk of liver disease decompensation and although an objective tumour response can be achieved, the survival benefit is marginal.259 The most common adverse event of TACE is postembolisation syndrome, while liver failure, abscesses, ischemic cholecystitis, or even death affect less than 1% of patients. Fever is a marker of tumour necrosis and prophylactic antibiotic therapy does not prevent the risk of infection.260
TACE has robustly demonstrated survival benefit.261–263 The technique has improved in recent years but there are still many aspects to investigate.264,265 The treatment modality that has shown benefits in survival rates consists of the administration of chemotherapy mixed with lipiodol as a transport vehicle, followed by vascular occlusion with Spongostan gelatine sponge pieces. This treatment modality is known as conventional TACE. In recent years, safety and efficacy studies have evaluated the use of adriamycin-loaded synthetic microspheres (known as DEB [drug-eluting bead]-TACE). The charged beads achieve vascular occlusion simultaneously with a slow release of the chemotherapeutic agent within the tumour. This reduces the passage of the chemotherapeutic agent into the systemic circulation, thereby reducing the potential toxicity of chemotherapy once the beads are injected.266
In addition, it is possible to choose the calibre of the microspheres depending on the vessel to be embolised and increase the homogeneity of the resulting embolisation. The response to treatment evaluated by the magnitude of tumour necrosis obtained and the survival of patients is not increased with the use of loaded spheres, but systemic toxicity is decreased and tolerance to the procedure is improved.200,267
TACE can be repeated at regular intervals or according to tumour response, but it should be stopped in the event of refractory progression.201,202 This situation is defined as: progression associated with a profile that determines a contraindication for the procedure, such as liver disease decompensation, vascular invasion, or extrahepatic spread. In recent years, multiple point score indexes have been published to guide the decision for retreatment.268–270 However, the applicability of these scores is controversial and very possibly they identify patients who should not have been treated with TACE from the early stage (mainly because they include patients with limited functional reserve). Moreover, performing repeated TACE sessions, especially in extensive treatments, can have a significant impact on the hepatic functional reserve, depriving patients the opportunity to access the different therapeutic steps within the current systemic treatment. Therefore, the decision to repeat TACE should be made following the concept of refractory progression.259,264,271
The association of TACE with molecular agents such as sorafenib or brivanib has not conclusively demonstrated an improvement in response rate, time to tumour progression or survival,272,273 so its use is not recommended. Currently there are ongoing studies evaluating the combination with immunotherapy, with no results reported to date. For several years, the combination with ablation has been suggested as a strategy to optimise results in terms of local necrosis and survival, but no reliable studies have been published that demonstrate its benefit in survival.274
Transarterial radioembolisation (TARE), also known as selective internal radiation therapy (SIRT) using Yttrium-90 microspheres has been evaluated in multiple prospective cohort studies, including patients with different stages of the disease. Positive results have been demonstrated in terms of radiological response, good clinical tolerance to treatment together with apparently comparable survival to that obtained in patients treated with TARE or sorafenib.275–280 These data have provided the rationale for phase III randomised clinical trials comparing TARE associated or not with sorafenib versus sorafenib. The results of three phase III clinical trials are available at present. Two of these trials (SARAH281 and SIRveNIB282) randomised patients with advanced HCC to receive TARE vs. sorafenib, and the primary end point was to demonstrate the survival benefit of TARE. Both studies were limited by an intention-to-treat applicability of TARE of 72%–77%, and although TARE offered a higher objective response rate and an apparent better safety profile, this did not translate into an improvement in survival, and therefore both studies were negative.281,282 The SORAMIC study evaluated whether the combination of TARE with sorafenib offered better survival than sorafenib in patients with advanced HCC.283 Although the association of TARE with sorafenib was not associated with greater toxicity, the study was negative. The benefit of improved irradiation dosing should be explored and subgroups of patients in which the therapeutic effect translates into survival benefit should be investigated.
Therefore, at present there is no scientific evidence to recommend this therapeutic option in patients with advanced HCC and the completion of a final trial evaluating the combination of TARE with sorafenib compared with sorafenib is pending (STOP-HCC trial, NCT01556490). The efficacy of TARE in other settings such as: intermediate stage HCC compared to TACE284; a bridging treatment during the transplant waiting list285; or as a tool to rescue patients for surgical resection,286 is based on cohort studies284 or clinical trials287–289 including a limited number of cases, which prevents any recommendation from being made. Finally, there is increasing evidence that the efficacy of TARE depends directly on the dose of radiation delivered to the tumour as measured by dosimetry.290 Therefore, future studies to evaluate the usefulness of TARE should be carried out in sites with experience, with an appropriate selection of patients, and with an adequate technique.
New radiation therapy techniques such as three-dimensional conformal radiation therapy (3D-CRT), intensity-modulated radiation therapy (IMRT), image-guided stereotactic body radiation therapy (SBRT), or proton pump radiation therapy allow high doses of radiation to be delivered to the tumour without damaging the surrounding tissue. These treatments are currently used for the treatment of metastases. The results reported in HCC are promising, but their efficacy needs to be confirmed in larger series and in randomised studies.291
Recommendations- •
TACE is the treatment of choice in asymptomatic patients with preserved liver function who present with multinodular HCC without vascular or extrahepatic invasion (high evidence, strong recommendation).
- •
TARE is effective in terms of radiological response and adequate safety profile, but it has not been able to demonstrate a significant increase in terms of survival in advanced HCC (high evidence, strong recommendation). Its evaluation in the context of clinical trials is recommended (strong recommendation).
- •
There is insufficient evidence to recommend external radiation therapy (low evidence, weak recommendation). Its potential utility should be evaluated in the context of clinical studies.
Since the publication of the HCC management guidelines in 2016,1 the survival benefit of new systemic treatments has been demonstrated, both in first-line (lenvatinib and the combination of atezolizumab and bevacizumab),292,293 and in second-line treatments (regorafenib, cabozantinib and ramucirumab).294–296 These drugs have been approved by the European Medicines Agency (EMA) and the Food and Drug Administration (FDA). Likewise, immunotherapy was evaluated both in the first line (nivolumab297 and the combination atezolizumab and bevacizumab vs. sorafenib293) and second line treatment (nivolumab in phase II298 and pembrolizumab vs. placebo299). In this regard, nivolumab and pembrolizumab received FDA’s conditional approval for second-line treatment based on the results of the phase I/II study Check-Mate 040298 and the phase II trial KEYNOTE-224,300 respectively. However, the CheckMate 459 study297 which compared nivolumab with sorafenib for first-line treatment was negative and the study that compared pembrolizumab with placebo for second-line treatment did not show any benefits in terms of survival either, according to the premises established by the clinical trials.299
The only clinical trial conducted to date in patients with HCC and mild liver dysfunction (Child-Pugh B7-8), evaluated the safety profile of nivolumab for second-line treatment.301 In this population, the safety profile was similar to that reported in HCC patients, who were second-line candidates but with preserved liver function (Child-Pugh-A). However, as previously mentioned, the impact of nivolumab in terms of second-line treatment survival is unknown to date.
Tables 2 and 3 detail the characteristics of the first- and second-line clinical trials that have shown benefit in terms of survival published up to April 2020, including time to progression, disease-free progression, survival and frequency of discontinuation due to treatment-related adverse events. The trials with negative results have not been included in these tables.297,299,302–312 The second-line treatment trials are phase III, randomised and double blind studies, designed to superiority.294–296 The patients included in the three studies had received sorafenib as first-line treatment and the control arm for all the studies was placebo.
Description of clinical trials that demonstrated a benefit in the survival of patients who were candidates for first-line treatment.
| SHARP311 | Asia-Pacific312 | REFLECT292 | IMbrave150 trial293 | |||||
|---|---|---|---|---|---|---|---|---|
| Sorafenib | Placebo | Sorafenib | Placebo | Lenvatinib | Sorafenib | Atezolizumab + bevacizumab | Sorafenib | |
| Exclusion criteria | n/a | n/a | Main portal invasion | NA | ||||
| Different from SHARP study | Infiltration of > 50% liver | |||||||
| > 2 anti-hypertension drugs | ||||||||
| Baseline characteristics and progress during the treatment received in the Clinical Trial. | ||||||||
| Child-Pugh A (%) | 95 | 98 | 97.3 | 97.4 | 99 | 99 | 99 | 100 |
| PS 0 (%) | 54 | 54 | 25.3 | 27.6 | 64 | 63 | NAa | |
| Vascular invasion (%) | 36 | 41 | 36 | 34.2 | 23 | 19 | 38 | 43 |
| Metastasis (%) | 53 | 50 | 68.3 | 68.4 | 61 | 62 | 63 | 56 |
| BCLC-C (%) | 82 | 83 | 95.3 | 96.1 | NR | 82 | 81 | |
| Hepatitis B (%) | 19 | 18 | 70.7 | 77.6 | 53 | 48 | 49 | 46 |
| Hepatitis C (%) | 29 | 27 | 10.7 | 3.9 | 19 | 26 | 21 | 22 |
| Alcohol (%) | 26 | 26 | NR | 8 | 4 | NR | ||
| NAFLD (%) | NR | NR | ||||||
| Median time in treatment (months) | 5.3 | 4.3 | NR | 5.7 | 3.7 | A 7.4; Bev 6.9 | 2.8 | |
| Discontinuation due to treatment-related AE (%) | 11 | 5 | 19.5 | 13.3 | 9 | 7 | 7 | 10 |
| Median time to progression (RECIST) | 5.5 | 2.8 | 2.8 | 1.4 | 7.4 | 3.7 | NA | |
| HR (95% CI) | 0.58; 0.45−0.74; P < .001 | 0.57; 0.42–0.79; P = .0005 | 0.61; 0 51−0 72;< 0 0001 | |||||
| Median time to progression (mRECIST) | n/a | n/a | 7.4 | 3.7 | ||||
| HR (95% CI) | 0.60; 0 51−0 71; P < .0001 | |||||||
| Median progression-free survival (RECIST) | NR | NR | 7.3 | 3.6 | 6.8 | 4.3 | ||
| HR (95% CI) | 0.65; 0 56−0 77; P < .0001 | 0.59; 0.47. 0.76; P < .0001 | ||||||
| Median progression-free survival (mRECIST) | n/a | NR | 7.3 | 3.6 | ND | |||
| HR (95% CI) | 0.64; 0 55−0 75; P < .0001 | |||||||
| Median overall survival (months) | 10.7 | 7.9 | 6.5 | 4.2 | 13.6 | 12.3 | NE | 13.2 |
| HR (95% CI) | 0.69; 0.55−0.87; P < .001 | HR 0.92. 0.79–1.06 | 0.58; 0.42. 0.79; P = .0006 | |||||
n/a, not applicable; NA, not available; NR, not reported; HTA, arterial hypertension; PS, performance status; BCLC, Barcelona Clinic Liver Cancer; NAFLD, fatty liver disease; A, atezolizumab; Bev, bevacizumab; RECIST, Response Evaluation Criteria in Solid Tumours; mRECIST, Modified Response Evaluation Criteria in Solid Tumours.
Description of clinical trials that demonstrated a benefit in the survival of patients who were candidates for second-line treatment.
| RESORCE294 | CELESTIAL295 | REACH-2296 | ||||
|---|---|---|---|---|---|---|
| Regorafenib | Placebo | Cabozantiib | Placebo | Ramucirumab | Placebo | |
| Candidates | Sorafenib tolerant | Second- or third-line | Alpha-fetoprotein ≥ 400 mg/dL | |||
| Description of progress during treatment with sorafenib before entering the Clinical Trial | ||||||
| Median time on sorafenib (months) | 7.8 | 7.8 | 5.3 | 4.8 | 4.1 | 4.1 |
| Median time between last sorafenib dose and start of clinical trial | 0.9 | 0.9 | 1.4 | 1.2 | 1.2 | 1.1 |
| Reason why sorafenib was discontinued | ||||||
| Radiological progression | 100 | NR | 84 | 80 | ||
| Sorafenib intolerance* | NA | 16 | 20 | |||
| Baseline characteristics and progress during the treatment received during the Clinical Trial. | ||||||
| Child-Pugh A (%) | 98 | 97 | 98 | 99 | 62 | 57 |
| PS 0 (%) | 65 | 67 | 52 | 55 | 57 | 58 |
| Vascular invasion (%) | 29 | 28 | 27 | 34 | 36 | 35 |
| Metastasis (%) | 70 | 76 | 79 | 77 | 72 | 74 |
| BCLC-C (%) | 86 | 89 | 91 | 90 | 83 | 89 |
| Hepatitis B (%) | 38 | 38 | 38 | 38 | 36 | 38 |
| Hepatitis C (%) | 21 | 21 | 24 | 23 | 24 | 29 |
| Alcohol (%) | 24 | 28 | 24 | 16 | 24 | 22 |
| NAFLD (%) | 7 | 7 | 9 | 10 | 10 | 4 |
| Median of treatment (months) | 3.6 | 1.9 | 3.8 | 2 | 3 | 2 |
| Discontinuation due to treatment-related AEs (%) | 10 | 4 | 16 | 3 | 11 | 3 |
| Median time to progression (RECIST) | 3.9 | 1.5 | NR | 3 | 1.6 | |
| HR (95% CI) | 0.41. 95% CI 0.34–0.51; P < .0001 | 0.427 [95% CI 0.313–0.582]; P < .0001 | ||||
| Median time to progression (mRECIST) | 3.2 | 1.5 | NR | |||
| HR (95% CI) | 0.44 (95% CI 0.36–0.55); | |||||
| Median progression-free survival (RECIST) | 3.4 | 1.5 | 5.2 | 1.9 | 2.8 | 1.6 |
| HR (95% CI) | 0.43 95% CI 0.35–0.52; P < .0001 | 0.44 (95% CI. 0.36−0.52; P < .001 | 0.452 [95% CI 0.339–0.603]; P < .0001 | |||
| Median progression-free survival (mRECIST) | 3.1 | 1.5 | NR | NR | ||
| HR (95% CI) | 0.46 (95% CI 0.37–0.56); P < .0001 | |||||
| Median Overall survival (months) | 10.6 | 7.8 | 10.2 | 8 | 8.5 | 7.3 |
| HR (95% CI) | 0.63 (0.50–0.79); P < .0001 | 0.76 (95% CI. 0.63−0.92); P = .005 | 0.710 [95% CI 0.531–0.949]; P = .0199 | |||
NA, not applicable; NR, not reported; HTA, arterial hypertension; PS, performance status; BCLC, Barcelona Clinic Liver Cancer; NAFLD, fatty liver disease; RECIST, Response Evaluation Criteria in Solid Tumours; mRECIST, Modified Response Evaluation Criteria in Solid Tumours.
Since the only treatment available in the last 10 years has been sorafenib, post-marketing publications focus mainly on this drug.
Mechanism of action of the drugs indicated for the systemic treatment of HCCSorafenib, lenvatinib, and regorafenib are part of the tyrosine kinase inhibitors family, but the target of each drug differs, and it has its own safety profile. Cabozantinib is a tyrosine kinase inhibitor that acts on multiple receptors including VEGF 1, 2 and 3, MET and AXL. Ramucirumab is a monoclonal antibody that binds specifically and with high affinity to the extracellular domain of VEGFR-2, thus preventing the binding of VEGF to its receptor. Atezolizumab is a PD-L1 inhibitor and bevacizumab is a monoclonal antibody against VEGF.313
Efficacy in first-line treatmentThe REFLECT study292 is a randomised, double-blind, non-inferiority study, which compares lenvatinib vs. sorafenib in HCC patients with no main portal vein or bile duct invasion, and with a hepatic tumour burden not exceeding more than 50% of liver occupation. This study demonstrated that patients treated with lenvatinib had a longer time on treatment, longer progression-free survival time, and longer time-to-progression than those treated with sorafenib, but survival was similar for the patients treated in both arms. This study did not consider safety within its endpoints, so the safety profile between the two drugs was not compared. Therefore, there are no data available to define whether the safety profile of sorafenib or lenvatinib is more or less favourable in this population. Both lenvatinib and sorafenib are approved in Europe and Spain as first-line marketed systemic treatments for HCC.
Phase III of CheckMate 459 study297 evaluated the efficacy of nivolumab versus sorafenib in first line treatment, but it failed to demonstrate a significant improvement in survival. This study included 743 patients, who were randomised 1:1 to either the nivolumab arm (n = 371) or the sorafenib arm (n = 372). The study was negative in terms of survival according to the pre-established definitions (HR 0.84; P = .0419). The study did not describe a safety profile that differed from that previously seen with nivolumab and sorafenib. The quality of life analysis showed that nivolumab had better indicators of quality of life for patients compared to sorafenib.
The IMbrave150 study293 is a phase III clinical trial which compared the combination of atezolizumab and bevacizumab (A + B) versus sorafenib (S) in patients with HCC who had not previously received systemic treatment. 501 patients were included and randomised 2:1 into two groups: A + B (n = 336) and S (n = 165). The majority of the patients belonged to the BCLC-C stage (80%), with the predominant cause of liver disease being the hepatitis B virus (50%), 40% of the patients had a baseline AFP value ≥ 400 mg/dL and approximately 40% of patients had previously received TACE. All patients in the sorafenib arm were Child-Pugh A and <1% in the Child-Pugh B 7 points combination. In August 2019, the mean follow-up was 8.6 months (8.9 months in A + B and 8.1 in S) and at that time 28.6% of the patients who received the A + B combination and 39.4% of those who received S had died, the HR for mortality being 0.58 (95% CI 0.42−0.79); P < .001. The median survival in the combination arm was not calculable and the overall survival was 84.8% and 67.2% at 6 and 12 months, respectively. Median survival in the sorafenib arm was 13.2 months (95% CI 10.4) and observed survival was 72.2% and 54.6% at 6 and 12 months, respectively. Progression-free survival was significantly longer in the combination arm, with a median of 6.8 months (95% CI 5.7–8.3) in patients who received the combination and 4.3 months (95% CI% 4.0–5.6) in those who received sorafenib (HR 0.59; 95% CI 0.47−0.76; P < .001).
The median duration of treatment was 7.4 months for the combination arm and 2.8 months for the sorafenib arm. The overall frequency of adverse events (AE) was similar in both arms and severe AE were more frequent in the combination arm with respect to the sorafenib arm (38% and 30.8%, respectively). The percentage of patients who discontinued the clinical trial due to treatment-related AE was 15.5% in the combination arm and 10.3% in the sorafenib arm. The EA grade III-iv that occurred most frequently in both arms was arterial hypertension (HT) being 15.2% in the combination arm and 12.2% in sorafenib. However, the most frequent AE regardless of the degree of severity was hypertension for the combination arm (29.8%) and hand-foot reaction for the sorafenib arm (48.1%).
The greatest difficulty in extrapolating the results of the IMbrave150 clinical trial293 in our population is due to the fact that more than 40% of the patients in the clinical trial were Asian and around 50% of the cases had a chronic HBV infection, potentially without established cirrhosis. This population profile is reflected in the low percentage of patients (26% in both arms) who presented oesophageal varices at the beginning of the clinical trial. In the combination arm, eight patients (2.4%) developed gastrointestinal bleeding due to oesophageal varices of any grade and six (1.8%) developed grade 3–4. In the sorafenib arm, one patient (0.6%) had gastrointestinal bleeding due to oesophageal varices and it was grade 3–4.293 Although none of these haemorrhages were considered by the investigators to be related to treatment, the gastrointestinal bleeding due to oesophageal varices was one of the reasons for discontinuation of the clinical trial. In relation to cancer treatment after the trial, 20.5% in the combination arm and 44.2% in sorafenib received treatment.
Efficacy in second line treatmentThe phase III RESORCE study compared regorafenib with placebo in patients with sorafenib-tolerant HCC (defined as those patients who tolerated at least 400 mg/day for a minimum period of 20 days, during the last 28 days of treatment), who presented radiological progression on sorafenib, good liver function and a preserved general health condition.294 The CELESTIAL study compared cabozantinib with placebo for second- and third-line treatment, regardless of the reason for discontinuing sorafenib.295 Finally, the REACH-2 study compared ramucirumab vs. placebo and included patient intolerance or with radiological progression to sorafenib with a baseline AFP value ≥ 400 ng/dL.296 The KEYNOTE 240study was a phase III study which randomised 278 patients to receive pembrolizumab and 135 to receive placebo in HCC patients with second-line treatment. The primary endpoint was survival.299 The patients treated with pembrolizumab had better survival rates (HR 0.78; one-sided P = .0238) and disease-free progression (HR 0.78; one-sided P = .0209) than placebo-treated patients, but the difference between pembrolizumab and placebo was not statistically significant as per prespecified statistical criteria.
In summary, currently there are drugs that increase survival both in first- and second-line treatments, and the decision to indicate one treatment or another may be conditioned by the patient’s characteristics, the profile of adverse events reported in each study, and the availability of the different treatments in each medium. The progress spectrum of the patients depends not only on the tumour extension or liver function but also on the patient’s comorbidities. For this reason, the sub-analyse results of each study and/or any post-marketing cohort studies would be very useful to define the applicability of the different drugs. In this sense, the only data available for the application of systemic treatments in special populations, such as patients with HIV, liver transplant recipients, elderly patients or those on dialysis come from cohort studies.314–316
Profile of adverse events of first- and second-line treatmentA comparison of the safety profiles in the different clinical trials is not possible as their design does not suit this purpose. Therefore, the decision to indicate one drug or another based on a direct comparison, between clinical trials, of the percentage of patients who discontinued treatment due to adverse events (AE) may lead to an under/over assessment of the safety impact.
The safety analysis in the context of systemic treatments is complex and requires a critical analysis. Sorafenib-treated patients who develop AE ≥ grade II have shown better survival rates than patients who do not develop them.317 However, as AE are progressive events that develop over time, their development could be conditioned by time in treatment and induce an interpretation bias. Currently, the only AE that has been validated as a predictor of survival is the development of early dermatological AE (eDtAE - within 60 days of starting sorafenib and that this AE conditions a dose modification) with sorafenib318–320 or regorafenib.321 Patients who develop eDtAE present a significantly longer median survival rate than those who do not. However, the median survival rate of patients who do not develop these adverse effects is similar to that described in clinical trials that demonstrated the benefit of sorafenib in patients with HCC.
Efficacy and safety of drugs based on the stratification parameters of clinical trials or data from post-marketing studiesDespite the fact that the clinical trials stratify patients according to different factors, the safety results according to this stratification could be biased by the limited number of patients in each sub-analysis. That is why the sub-analyses results allow for new hypotheses, but do not provide solid evidence to make decisions. Likewise, post-marketing studies provide data for applicability in patients outside of clinical trials, but do not modify the quality of evidence generated in the clinical trials.
Applicability of sequential treatmentDue to the availability of first- and second-line treatments, the decision to continue with first-line drugs until symptomatic progression, as occurred in the SHARP322 and Asia-Pacific323 studies more than 10 years ago, is currently controversial. Likewise, we do not have randomised studies that consider sorafenib or lenvatinib as a control arm in second-line treatment trials, so the decision to continue or not with first-line drugs post-progression is based on the empirical interpretation of the information available to date. The patient’s general health condition,192 the pattern of progression,201,202 and the liver function189–191 are parameters that are considered when defining whether to modify first-line treatment or not.
Post-progression radiological treatment data with sorafenib come from the SHARP,322 Pacific Asia323 and cohort studies.324 In 2013 a study was published which included the impact of radiological patterns of progression in HCC patients treated with sorafenib who were candidates for second-line treatments.202 This study demonstrated that patients who present radiological progression due to the development of new extrahepatic lesions or the appearance of new vascular invasion have a significantly lower median post-progression survival (PPS) than those who develop other types of progressions. These data were validated in the RESORCE,294 METIV,311 and REACH/REACH-2325 clinical trials and cohort studies of patients treated with sorafenib,201 but to date the impact of the pattern of progression in patients treated with lenvatinib, atezolizumab plus bevacizumab, or cabozantinib is not available.
The survival of patients who start the second-line treatment due to progression of the first-line drug is conditioned by the type of progression during sorafenib treatment. For this reason, the BCLC classification at the time of progression (Fig. 3) is better for characterising the prognosis for second-line treatment candidates. This classification, as mentioned previously, is complementary to the BCLC classification and has been validated by cohort studies in patients treated with sorafenib and second-line treatment clinical trials evaluating regorafenib and tivantinib.201
However, the progress of HCC patients undergoing systemic treatment requires not only the radiological progression to be analysed, but also the other progression events that develop during patient follow-up (including the development of early AEs or the impact of the time that elapses between the suspension of the first-line treatment and the start of the second-line treatment). In this regard, although the impact of the development of eDtAE on survival has been demonstrated,320 patient progress information that considers both factors together is scarce and these data are not available in patients treated with lenvatinib, cabozantinib or ramucirumab.
Clinical and radiological follow-up in patients with systemic treatmentThere is no consensus regarding the clinical or radiological follow-up scheme of patients undergoing systemic treatment. Close clinical follow-up is recommended during this period of time, which can vary between the first 15–30 days after the start of treatment, which is when most early AEs develop, and then follow-up every 30–60 days. Patients receiving intravenous treatments require a pre-infusion analysis, and in this scenario, follow-up is conditioned by the administration schedule of each drug.
There is also no consensus regarding which is the most appropriate follow-up scheme to perform radiological surveillance, but it is usually performed every 2−3 months in the context of clinical practice. A dynamic imaging test is recommended to evaluate both the abdomen and the thorax (CT or MRI). Since second-line treatments are currently available, the need for radiological control should be a priority.
Recommendations- •
Sorafenib and lenvatinib improve survival in HCC patients compared to first-line placebo. The combination atezolizumab with bevacizumab demonstrated improved survival and disease-free progression compared to sorafenib as first-line treatment (high evidence, strong recommendation).
- •
Regarding second-line treatment, regorafenib improves survival in patients who progress and are tolerant to sorafenib, cabozantinib in patients who are candidates for second- and third-line treatments, and ramucirumab in patients who are candidates for second-line treatment who present an AFP value ≥ 400 ng/dL (high evidence, strong recommendation).
- •
Making the decision to use one treatment option rather than another should be based on the clinical trials’ inclusion/exclusion criteria and the patient profile. Choosing a first-/second-line treatment should take into consideration not only the patient’s liver function, general health condition and tumour burden, but also the comorbidities that could link to a higher risk of developing adverse events. For this reason, it is recommended to consider the profile of adverse events and impact of each treatment according to the pattern of progression when choosing second-/third-line treatment (moderate evidence, strong recommendation).
The development of sufficiently robust serological markers for the detection of HCC remains a necessity in clinical practice. Current research in the field of liquid biopsy has achieved promising results in terms of identifying new screening tools for this disease. The so-called liquid biopsy is a minimally invasive procedure that is based on the identification of tumour components released into biological fluids, primarily blood. These tumour components are circulating tumour cells (CTCs), circulating tumour nucleic acids, DNA (ctDNA) and RNA (miRNA and long noncoding RNA), and extracellular vesicles.326
The number of CTCs is very low (it is estimated at 0–10 CTC for every 7 × 106 nucleated cells) and, therefore, their detection, based on physical properties or on the presence of surface antigens, is still a challenge in practice. A recent meta-analysis that includes 20 studies showed high diagnostic precision, although the authors do not recommend the CTC analysis as a diagnostic tool.327 Other papers have shown improved performance with the combination of CTC detection and isolation in conjunction with qPCR analysis of the stem cell marker panel. This approach has shown accuracy for the diagnosis of HCC with normal AFP in the early stage.328 The potential usefulness in HCC of single-cell mRNA sequencing of CTC has recently been published.329 In summary, it is a promising field, but technological improvements and procedure standardisation are still needed before the detection and isolation of CTC is of clinical utility.330
There is also great interest in exploring the utility of ctDNA analysis. The ctDNA contains relevant information on specific genetic and epigenetic tumour alterations, including specific mutations, copy number variations, chromosomal rearrangements and DNA methylation that have been shown to be related to those evident in the corresponding tumour tissues.331 Until now, most studies have profiled mutations in ctDNA using highly sensitive detection methods in targeted (digital PCR) and non-targeted approaches (next generation sequencing, NGS).330 The cfDNA levels in patients with HCV-related HCC have long been linked with extrahepatic recurrence and worse survival after resection.332,333 A recent pilot study showed that it is feasible to detect ctDNA mutations by ultra-deep targeted sequencing in patients with HCC in early stages. The results showed a high correlation with the mutations present in their corresponding tumoral origin.334 Changes in methylation can also be analysed in ctDNA.335 The first studies have already demonstrated that the methylation of the RASSF1A gene could be detected in 90% of HCC patients, which rules out healthy individuals.336 In this regard, a study that used plasma samples from a large number of patients with HCC described a methylation marker panel with high sensitivity (83.3%) and specificity (90.5%) in a validation cohort for early diagnosis of HCC and with prognostic value.337 Even so, technical issues such as the selection of HCC-specific methylation sites and non-tumour associated methylation, need to be refined. The combination of ctDNA analysis with established tumour protein markers can also improve liquid biopsy performance for early tumour detection.338 Circulating miRNA panels with good diagnostic sensitivity for HCC have been described, although most of these biomarkers are in their early stages of development.335 In summary, technical refinements and systematic studies that include liver cirrhosis samples are needed before clinically implementing the use of liquid biopsy for the early diagnosis of HCC.
Prognosis and follow-up of patients with HCCFor the present, no analysis based on liquid biopsy has reached clinical practice to be able to evaluate the follow-up of HCC patients. But there is experimental evidence that supports the potential of this technology for stage evaluation, tumour burden, potential recurrence after treatment, and, ultimately, disease prognosis.
The first reported studies described an association between the presence of EpCAM-positive CTCs and tumour recurrence in HCC patients treated with surgical resection, as well as its prognostic value in terms of the presence of vascular invasion and overall survival.330 Recently, an improvement in the characterisation of CTCs with stem cell phenotypes has demonstrated clinical value in predicting tumour recurrence after surgical resection.328
The analysis of ctDNA is also promising in terms of the follow-up of HCC patients. It makes it easy to obtain serial samples that can provide a snapshot of the genetic profile of the tumour and its clonal evolution over time, while avoiding the problems derived from tumour heterogeneity in single biopsy samples.336 Recently, a diagnostic panel with ten ctDNA methylation markers was highly correlated with tumour burden, treatment response, stage, and a prognostic prediction model of survival.337 Similarly, circulating levels of specific miRNAs and long noncoding RNAs have been associated with tumour size, early recurrence, and prognosis.335 However, despite the interesting results, these studies need additional validation in multicenter trials with a larger number of patients and standardised technological platforms.
Prognostic evaluation and prediction of therapeutic response with biomarkers in HCCThe National Cancer Institute defines a biomarker as a biological molecule (in tissue, blood or other fluids) that is a sign of a normal or abnormal process, of a condition or disease, or that determines how well the body responds to a treatment.339 Apart from the clinical variables (ECOG-PS, vascular invasion, extrahepatic spread, BCLC) described above, some biomarkers have prognostic or predictive capacity for response in HCC. The level of evidence to accept a biomarker in a clinical guideline depends on whether it has been obtained in the context of a controlled clinical trial specifically designed to address the biomarker (category A), if it has been obtained by studying samples from patients included in a phase III study not designed to address the biomarker (category B), based on cohort studies (category C) or retrospective studies (category D).340 Category A or B studies with external validation are determined to be Level of Evidence I; Category B studies without validation or C with external validation are determined to be Level of Evidence II. Level of Evidence I (and some studies with LOE II) can be sufficient to change clinical practice, while the rest of the LOEs have no clinical impact.340
Prognostic biomarkersAFT plasma levels have an independent predictive capacity for mortality, both in early and advanced stages of HCC. Although AFP has prognostic value as a quantitative variable, most studies establish two prognostic levels, AFP > 200 ng/mL or >400 ng/mL, and both have been accepted in European clinical guidelines as prognostic tools and useful in the stratification of patients in clinical trials.3 In the early stages of the disease, these AFP levels have been associated with a worse prognosis after resection, liver transplantation, and locoregional treatments (radiofrequency ablation and chemoembolisation).3 In the advanced stage (BCLC C), patients with AFP > 200−400 ng/mL in the context of phase III studies have presented a worse prognosis,294,296,341,342 with median survival rates of 5–7 months.296 Also, elevated AFP levels in multivariate studies have proved to be independent predictors of mortality (level I-A).341
Apart from AFP, multiple studies have evaluated the prognostic ability of survival of molecular subclasses of HCC and of gene-expression signatures or other molecular profiles. The gene-expression signature of 182 genes from non-tumour tissue343 and that of five genes from tumour tissue344 showed independent predictive capacity for mortality, together with other clinical variables (e.g., platelets, BCLC or vascular invasion). These studies, however, have a II-C LOE, and the biomarkers have not been transferred into clinical practice. The biomarker and molecular profile prospective cohort study of sorafenib compared with placebo for the prevention of post-resection recurrence, identified the pERK activation as a predictor of increased risk (II-B LOE). None of the signatures previously proposed in retrospective studies had independent prognostic capacity.345
Biomarkers predictive of therapeutic responseIn solid tumours, biomarkers which are predictive of therapeutic response have been approved in more than 30 indications, for example, vemurafenib in melanoma with BRAF V600E mutations or crizotinib in lung cancer with ALK gene aberrations (I-A LOE).346 Generally, these markers define a subpopulation of tumours with a dominant oncogene that is effectively blocked by molecular therapy. In HCC, the most prevalent suppressor oncogenes/tumours (TERT, CTNNB1, TP53) do not have effective drugs. The less prevalent ones that do, (i.e., FGF19, IGF2, VEGFA, TSC1/TSC2) have not yet demonstrated efficacy in the context of phase III trials.347,348
Currently, there are six effective drugs in advanced HCC that have shown better survival rates in the context of phase III studies: sorafenib,322,323 lenvatinib292 and the atezolizumab-bevacizumab combination293 as first-line treatment, and regorafenib,294 cabozantinib295 and ramucirumab296 as second-line treatment. Studies with biomarkers to predict response to sorafenib have been negative.341 In contrast, recent studies have identified plasma proteins and miRNAs associated with better survival with regorafenib, with II-B LOE.349 Likewise, plasma AFP > 400 ng/mL has also been shown to predict the benefit of ramucirumab in HCC patients who had progressed to sorafenib.296 This is the first phase III study, positive in HCC in which the target population has been selected by a biomarker (I-A LOE), which will determine the use of this drug solely in this subpopulation, which represents around 40% of patients with advanced HCC in the second-line of treatment.
Immunotherapy treatments (nivolumab and pembrolizumab) have been approved by the FDA and other regulatory agencies for advanced second-line treatment of HCC based on encouraging results obtained in phase II studies (14%–18% objective response). Although patients whose tumours exhibit PD-L1 in the tumour cells, or PD-1 in the lymphocytes, have tumour responses with greater frequency and better survival, the differences are not discriminatory enough to be of use in patient selection. The predictors of response and/or survival of these drugs in HCC are not currently known.298,350
Scholarships and acknowledgments- •
Maria Reig: Research grant from the Carlos Health Institute III (PI15/00145 and PI18/00358).
- •
Alejandro Forner: Research grant from the Carlos Health Institute III (PI18/00542).
- •
Matías A. Ávila: «Hepacare Project» of the «La Caixa» Foundation.
- •
Beatriz Mínguez: Research grant from the Carlos Health Institute III (PI18/00961).
- •
Bruno Sangro: Research grant from the Carlos III Health Institute (PI16/01845, PI19/00742 and AC16/00065).
- •
Jordi Bruix: Research grant from the Carlos III Health Institute (PI18/00768), AECC (PI044031), Secretary of Universities and Research of the Economy and Knowledge Department (2014 SGR 605) and WCR (AICR)16-0026.
- •
CIBEREHD is funded by the Carlos III Health Institute.
Maria Reig: Consultant for Bayer HealthCare Pharmaceuticals, BMS, Roche, Ipsen, AstraZeneca and Lilly. Lectures for Bayer, BTG, BMS, Gilead and Lilly. Bayer and Ipsen research projects. Trips: Bayer, Gilead, Lilly.
Alejandro Forner: Consultant for Bayer HealthCare Pharmaceuticals, AstraZeneca and Guerbert. Lectures for Bayer HealthCare Pharmaceuticals, Gilead and MSD.
Carmen Ayuso: Lectures for Bayer HealthCare Pharmaceuticals.
Beatriz Mínguez: Consultant for Bayer HealthCare Pharmaceuticals. Lectures for Bayer HealthCare Pharmaceuticals, Gilead and Eisai-Merck.
Maria Varela: Consultant for Bayer HealthCare Pharmaceuticals, BMS, Sirtex, Ipsen, Roche, Astra-Zéneca. Lectures for Boston Scientific, Gilead, MSD and Bayer HealthCare Pharmaceuticals.
José Ignacio Bilbao: Proctor for Sirtex Medical Europe.
Marta Burrel: Lectures for Terumo, BTG and Guerbet.
Joana Ferrer: Lectures for Bayer HealthCare Pharmaceuticals.
Miguel Ángel Gómez: Declares not to have any conflict of interest.
Josep María Llovet: Research projects for Bayer HealthCare Pharmaceuticals, Eisai Inc., Bristol-Myers-Squibb, Boehringer-Ingelheim and Ipsen. Consultant for Bayer HealthCare Pharmaceuticals, Merck, Eisai Inc., Bristol-Myers Squibb, Celsion Corporation, Eli Lilly, Roche, Genentech, Glycotest, Nucleix, Can-Fite Biopharma, AstraZeneca and Exelixis.
Ana Matilla: Consultant for Bayer HealthCare Pharmaceuticals. Lectures for consultant of Bayer HealthCare Pharmaceuticals, BTG and Bristol-Myers Squibb.
Josep Tabernero: Consultant for Array Biopharma, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Chugai, Genentech Inc., Genmab A/S, Halozyme, Imugene Limited, Inflection Biosciences Limited, Ipsen, Kura Oncology, Lilly, MSD, Menarini, Merck Serono, Merrimack, Merus, Molecular Partners, Novartis, Peptomyc, Pfizer, Pharmacyclics, ProteoDesign SL, Rafael Pharmaceuticals, F. Hoffmann-La Roche Ltd, Sanofi, SeaGen, Seattle Genetics, Servier, Symphogen, Taiho, VCN Biosciences, Biocartis, Foundation Medicine, HalioDX SAS and Roche Diagnostics.
Ruth Vera: Consultant for Roche, Amgen, Sanofi, MERCK, Bristol-Myers Squibb, MSD, SERVIER, Bayer HealthCare Pharmaceuticals, Astra-Zeneca.
Bruno Sangro: Consultant for Adaptimmune, Astra-Zeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers-Squibb, BTG, Eisai, Eli Lilly, Ipsen, Onxeo, Roche, Sirtex. Lectures for Astra-Zeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers-Squibb, Sirtex. Research projects with Bristol-Myers-Squibb, Onxeo, Sirtex.
Jordi Bruix: Consultant for Arqule, Bayer HealthCare Pharmaceuticals, Novartis, Bristol-Myers-Squibb, BTG-Biocompatibles, Eisai, Kowa, Terumo, Gilead, Bio-Alliance/Onxeo, Roche, AbbVie, Merck, Sirtex, Ipsen, Astra-Medimmune, Incyte, Quirem, Adaptimmune, Lilly. Research projects with Bayer HealthCare Pharmaceuticals and BTG. Collaboration for educational events Bayer HealthCare Pharmaceuticals and BTG. Lectures for Bayer HealthCare Pharmaceuticals, BTG- Biocompatibles, Eisai, Terumo, Sirtex and Ipsen.
Matías A Ávila, Itxarone Bilbao, Javier Bustamante, Manuel de la Mata, Fernando Pardo, Miguel A. Pastrana, Manuel Rodríguez-Perálvarez and José Urbano: Declare they have no conflict of interests.
Please cite this article as: Reig M, Forner A, Ávila MA, Ayuso C, Mínguez B, Varela M, et al. Diagnóstico y tratamiento del carcinoma hepatocelular. Actualización del documento de consenso de la AEEH, AEC, SEOM, SERAM, SERVEI y SETH. Med Clin (Barc). 2021;156:463.