Approximately 24–40% of patients with type 2 diabetes mellitus (T2DM) develop kidney damage. Our objective was to evaluate the long-term evolution of renal function using isotopic determination of GFR and urinary albumin excretion (UAE) in patients with T2DM undergoing intensive treatment for renal and cardiovascular risk factors.
Patients and methodsThis was a single-center, prospective study of 201 patients with T2DM and UAE who initiated intensive treatment. They were followed for 17.2±6.5 years. Patients were divided into three groups, according to renal function: 167(85.6%) had stable renal function, 16(8.2%) had creatinine levels that doubled and 12(6.2%) began renal replacement therapy (RRT). We performed periodic isotopic determinations of GFR using 125I-iothalamate.
ResultsThere were significant differences between the three groups with respect to age, duration of T2DM at baseline, years of follow-up in the study and systolic blood pressure, serum creatinine, isotopic GFR, and UAE at baseline. Renal function evolution slopes were −1.55mL/min/1.73m2/year in patients with stable creatinine, −2.49mL/min/1.73m2/year in those with doubled creatinine, and −8.16mL/min/1.73m2/year in those requiring RRT. We also found that differences in renal events were determined by delayed initiation of intensive treatment.
ConclusionPatients with glomerular hyperfiltration who were undergoing treatment with renin angiotensin aldosterone system blockers exhibited a better evolution in renal function, possibly because these patients initiated intensive treatment earlier. Although diabetic nephropathy is associated with classic risk factors, early initiation of intensive treatment should be a priority in order to prevent worsening renal function.
Aproximadamente el 24-40% de los pacientes con diabetes mellitus tipo 2 (DM2) desarrollan daño renal. Nuestro objetivo fue evaluar la evolución a largo plazo de la función renal mediante la determinación isotópica del filtrado glomerular (FG) y la excreción urinaria de albúmina (EUA) en pacientes con DM2 en tratamiento intensivo de los factores de riesgo renal y cardiovascular.
Pacientes y métodosEstudio prospectivo unicéntrico de 201 pacientes con DM2 y EUA que iniciaron un tratamiento intensivo. El seguimiento fue de 17,2±6,5 años. Los pacientes fueron divididos en 3 grupos según la función renal al final: 167 (85,6%) tenían función renal estable, 16 (8,2%) doblaron la creatinina y 12 (6,2%) requirieron tratamiento renal sustitutivo (TRS). Se realizaron determinaciones isotópicas periódicas del FG usando 125I-iotalamato.
ResultadosHay diferencias significativas entre los 3 grupos respecto a la edad, los años de duración de la DM2 al inicio, los años de seguimiento, la presión arterial sistólica, la creatinina sérica, el FG isotópico y la EUA basal. Las pendientes de evolución de la función renal fueron: −1,55ml/min/1,73m2/año en pacientes estables, −2,49ml/min/1,73m2/año en los que doblaron la creatinina y −8,16ml/min/1,73m2/año en los que requirieron TRS. Además, esta diferente evolución de la función renal venía determinada por el inicio tardío del tratamiento intensivo.
ConclusiónLos pacientes con hiperfiltración glomerular en tratamiento con bloqueadores del sistema renina-angiotensina-aldosterona mostraron una mejor evolución de la función renal, posiblemente debido a que estos pacientes iniciaron tratamiento intensivo antes. Aunque la nefropatía diabética se asocia a factores de riesgo clásicos, el tratamiento intensivo precoz debe ser una prioridad con el fin de prevenir el deterioro de la función renal.
Diabetic nephropathy is the most frequent cause of chronic kidney disease in patients requiring renal replacement therapy (RRT) worldwide.1 According to the World Health Organization, the prevalence of diabetes is expected to double within the next 20 years.2 Type 2 diabetes mellitus (T2DM) is the most common form of diabetes, with approximately 24–40% of these patients developing kidney damage.3
Physiopathologically, diabetic nephropathy is characterized by an initial period of hyperfiltration, and this event plays a central role in the pathogenesis and progression of renal disease in experimental diabetes.4 However, small cohort studies suggest that type 1 and type 2 diabetic subjects with glomerular hyperfiltration may be at increased risk of accelerated renal function loss or progression to albuminuria.5 Further, this initial period of hyperfiltartion is followed by a subsequent period of deterioration in glomerular filtration and albuminuria.6 Therefore, the importance of starting treatment at this hyperfiltration stage may affect the progression of renal injury in T2DM. In fact, albuminuria is used as an early marker of glomerular and vascular injury.3
On the other hand, long-term studies evaluating the evolution of renal function using isotopic determination of the glomerular filtration rate (GFR), and the associated long-term consequences of diabetic nephropathy, are scarce.7 Because formulas assessing renal function are not ideal in patients with GFR greater than 60mL/min, the use of isotopic determination of GFR is indicated in these patients.8 The objective of the study was to evaluate the long-term evolution of glomerular filtration using annual isotopic determination of GFR and urinary albumin excretion (UAE) in patients with T2DM undergoing intensive treatment for renal and cardiovascular risk factors. We also evaluated possible independent risk factors affecting the progression of renal function to identify patients at greater risk for diabetic nephropathy.
Materials and methodsPatientsWe carried out a single-center prospective study9 of 201 patients with T2DM with no prior history of cardiovascular disease, who were consecutively referred to our hospital's outpatient clinic to test for UAE. Patients attended the outpatient clinic between the years 1985–1995 and were followed until 2011 or until the appearance of a renal event or death. Renal events occurring during the study were considered significant if the patient required RRT, renal transplantation, and/or when baseline creatinine doubled. Diabetic nephropathy was defined as impaired renal function and/or UAE in T2DM patients with diabetic retinopathy. Individuals with other causes of primary nephropathy and cardiovascular events were excluded from the study. Cardiovascular events were defined as ischemic heart disease in the form of angina or myocardial infarction, stroke, and severe peripheral vascular disease requiring surgery or sympathectomy.
This study was presented at the Ethics Committee and approved. Furthermore, all patients provided their informed consent to participate in the study.
At the start of the study, patients were undergoing intensive treatment for glycemic control, blood pressure, UAE, and dyslipidemia, according to the current guidelines for each time period. (All patients were treated with renin angiotensin aldosterone system [RAAS] blockers. Initially, patients were given captopril 100–150mg/day; later, lisinopril 20mg/day was used. When angiotensin II receptor antagonists (ARA II) began to be used, some patients switched to irbesartan 150–300mg/day.)
Intensive treatment (control of blood pressure, metabolism, and lipids) was defined according to the ADA guidelines approved in 1985 and 199010,11; we subsequently followed current ADA guidelines for each time period over the course of the follow-up.
The following demographic data were collected: age, gender, body mass index (BMI), evolution (in years) from diagnosis of T2DM until the beginning of the study, and systolic and diastolic pressure. In addition, we performed isotopic determinations of GFR every 12 months during the follow-up period.
Patients were classified by the duration of T2DM at baseline, prior to the start the study, using tertiles.
MethodologyBlood samples were collected at the same time that GFR was measured. Annual biochemical parameters (serum creatinine, glucose, albumin, cholesterol, HDL cholesterol, LDL cholesterol, triglycerides) were measured using an autoanalyzer (Technicon Autoanalyzer, Tarrytown, New York, USA). In the earlier years of the study, HbA1c was measured using chromatography (Biosystem, Barcelona, Spain; normal range: 5.0–6.7%). After 1995, an HbA1c analyzer with ion-exchange high-performance liquid chromatography (Hitachi L-9100; normal range: 3.3–6.0%) was used. UAE was measured using nephelometry (CV: inter-study 5%, intra-study 3%, sensitivity 1.9mg/l). UAE was categorized as less than 30mg/24h (normal) and 30–300mg/24h (positive) in three consecutive determinations analyzed in a period of six months in the absence of urinary infection.12 All measurements of serum and urinary creatinine were performed in the same laboratory and determined using Jaffe's alkaline picrate method (normal range of SCr: 0.6–1.2mg/dL), and calibrated using a SET point Calibrator (Bayer Corporation). Annual Isotopic determinations of GFR were carried out using a single-shot clearance technique using an intravenous injection of 30–50mCi 125I-iothalamate; blood was drawn at specific time intervals and values were corrected for body surface area of 1.73m2. Glomerular hyperfiltration was considered when the isotopic GFR was greater than 120mL/min/1.73m2.7 Both the UAE as GFR were categorized based on clinical criteria cut-offs recognized in the literature.
Blood pressure was taken in the right arm with the patient in the supine position using a standard mercury sphygmomanometer. Subjects rested for 15min before blood pressure readings; two readings were taken and the mean of the two readings was calculated.
Statistical analysisDescriptive statistics are frequencies (%), mean±standard deviation or median (percentile 25–percentile 75, P25–P75), as appropriate, or otherwise specified. The Chi-square test was used for categorical variables, the t-test or the one-way ANOVA for Gaussian distributed variables (for 2 or more than two groups, respectively) and for non-Gaussian continuous variables we used non-parametric methods (Mann–Whitney test for two groups or Kruskal–Wallis for more than two groups). Mixed models for repeated measurements (MMRM) were used for the repeated measurements based on the likelihood of estimating the slope of decline for the isotopic determinations of GFR. The survival function was calculated using the Kaplan–Meier method. Variables with a p-value<0.2 on the univariate Cox analysis were considered for the multivariate model using a backward elimination approach. We used the following statistical analysis packages: SAS version 9.2 software (SAS Institute Cary, NC, USA) and SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA), and the level of significance was established at the two-sided 0.05 level, except for the multivariate Cox model (0.10 two-sided level).
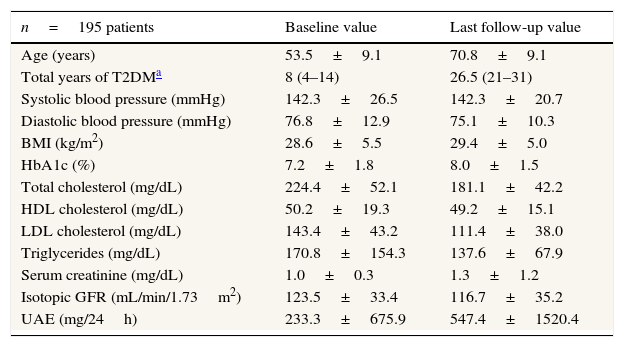
ResultsThe study sample included 201 patients with T2DM and UAE with no history of cardiovascular events who received intensive treatment at our hospital for renal and cardiovascular risk factors. The sample included 120 women (59.7%) and 81 men (40.3%), with a mean baseline age of 53.5±9.1 years (range 25–70 years). Six patients (3%) were lost in follow-up, mainly because of changing residency, bringing the total number of patients that completed the study to 195. Follow-up was 19 (13–22) years. Diabetes was diagnosed at a mean of 8 (4–14) years prior to the beginning of the study, rest of characteristics at baseline and at the last control visit shown in Table 1.
Characteristics of the type 2 diabetic study patients at baseline since 1985 and at the last follow-up visit in 2011.
| n=195 patients | Baseline value | Last follow-up value |
|---|---|---|
| Age (years) | 53.5±9.1 | 70.8±9.1 |
| Total years of T2DMa | 8 (4–14) | 26.5 (21–31) |
| Systolic blood pressure (mmHg) | 142.3±26.5 | 142.3±20.7 |
| Diastolic blood pressure (mmHg) | 76.8±12.9 | 75.1±10.3 |
| BMI (kg/m2) | 28.6±5.5 | 29.4±5.0 |
| HbA1c (%) | 7.2±1.8 | 8.0±1.5 |
| Total cholesterol (mg/dL) | 224.4±52.1 | 181.1±42.2 |
| HDL cholesterol (mg/dL) | 50.2±19.3 | 49.2±15.1 |
| LDL cholesterol (mg/dL) | 143.4±43.2 | 111.4±38.0 |
| Triglycerides (mg/dL) | 170.8±154.3 | 137.6±67.9 |
| Serum creatinine (mg/dL) | 1.0±0.3 | 1.3±1.2 |
| Isotopic GFR (mL/min/1.73m2) | 123.5±33.4 | 116.7±35.2 |
| UAE (mg/24h) | 233.3±675.9 | 547.4±1520.4 |
Data using mean±SD.
T2DM: type 2 diabetes mellitus; BMI: Body Mass Index; HbA1c: glycated hemoglobin; HDL: high-density lipoprotein; LDL: low-density lipoprotein; isotopic GFR: isotopic glomerular filtration rate; UAE: urinary albumin excretion; SD: standard deviation.
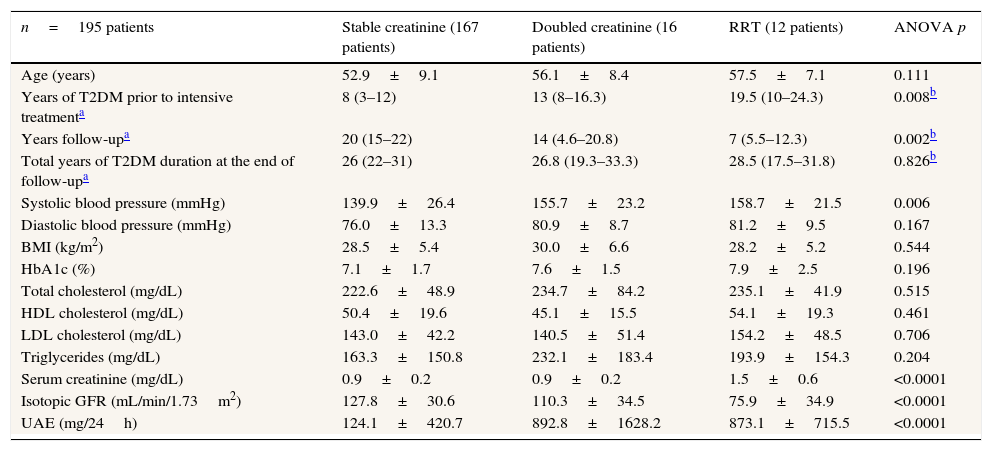
The patients were divided into groups according to the evolution of renal function: 167 patients (85.6%) had stable creatinine, 16 patients (8.2%) had doubled creatinine levels, and 12 patients (6.2%) initiated RRT. We found significant differences between the three groups with respect to the duration of T2DM prior to the initiation of the study, years of follow-up in the study, baseline systolic blood pressure, serum creatinine, isotopic GFR, and UAE (Table 2).
Baseline clinical characteristics of the type 2 diabetic study patients divided into three groups according to renal evolution at the end of the study.
| n=195 patients | Stable creatinine (167 patients) | Doubled creatinine (16 patients) | RRT (12 patients) | ANOVA p |
|---|---|---|---|---|
| Age (years) | 52.9±9.1 | 56.1±8.4 | 57.5±7.1 | 0.111 |
| Years of T2DM prior to intensive treatmenta | 8 (3–12) | 13 (8–16.3) | 19.5 (10–24.3) | 0.008b |
| Years follow-upa | 20 (15–22) | 14 (4.6–20.8) | 7 (5.5–12.3) | 0.002b |
| Total years of T2DM duration at the end of follow-upa | 26 (22–31) | 26.8 (19.3–33.3) | 28.5 (17.5–31.8) | 0.826b |
| Systolic blood pressure (mmHg) | 139.9±26.4 | 155.7±23.2 | 158.7±21.5 | 0.006 |
| Diastolic blood pressure (mmHg) | 76.0±13.3 | 80.9±8.7 | 81.2±9.5 | 0.167 |
| BMI (kg/m2) | 28.5±5.4 | 30.0±6.6 | 28.2±5.2 | 0.544 |
| HbA1c (%) | 7.1±1.7 | 7.6±1.5 | 7.9±2.5 | 0.196 |
| Total cholesterol (mg/dL) | 222.6±48.9 | 234.7±84.2 | 235.1±41.9 | 0.515 |
| HDL cholesterol (mg/dL) | 50.4±19.6 | 45.1±15.5 | 54.1±19.3 | 0.461 |
| LDL cholesterol (mg/dL) | 143.0±42.2 | 140.5±51.4 | 154.2±48.5 | 0.706 |
| Triglycerides (mg/dL) | 163.3±150.8 | 232.1±183.4 | 193.9±154.3 | 0.204 |
| Serum creatinine (mg/dL) | 0.9±0.2 | 0.9±0.2 | 1.5±0.6 | <0.0001 |
| Isotopic GFR (mL/min/1.73m2) | 127.8±30.6 | 110.3±34.5 | 75.9±34.9 | <0.0001 |
| UAE (mg/24h) | 124.1±420.7 | 892.8±1628.2 | 873.1±715.5 | <0.0001 |
Data using mean±SD.
RRT: renal replacement therapy; T2DM: type 2 diabetes mellitus; BMI: Body Mass Index; HbA1c: glycated hemoglobin; HDL: high-density lipoprotein; LDL: low-density lipoprotein; isotopic GFR: isotopic glomerular filtration rate; UAE: urinary albumin excretion; SD: standard deviation; NS: non-significant.
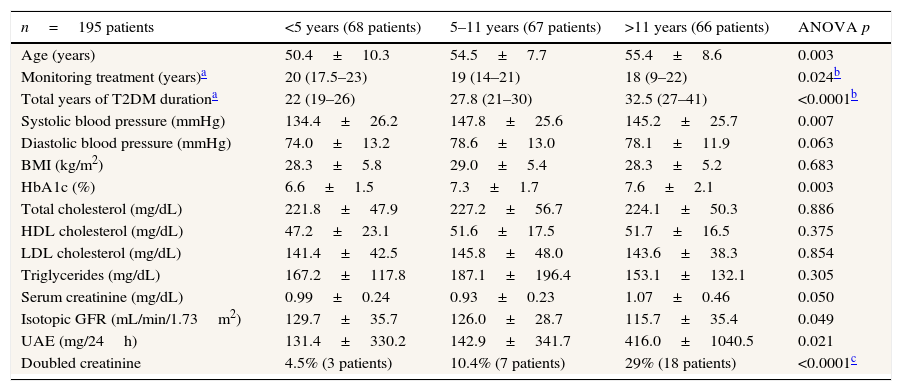
When patients were grouped into one of three groups according to the time (in years) from diagnosis of T2DM until the initiation of intensive treatment (0–5, 5–11, more than 11 years) using tertiles, significant differences were observed in age at baseline, total years of follow-up for T2DM, systolic blood pressure, HbA1c, isotopic GFR, and UAE (Table 3). However, when data was analyzed at the end of the follow-up period, differences were only detected in isotopic GFR and UAE. And, when patients were grouped according to total years of evolution of T2DM, no significant differences were found.
Clinical parameters of the type 2 diabetic study patients classified according to the total years of T2DM known evolution at baseline using tertiles.
| n=195 patients | <5 years (68 patients) | 5–11 years (67 patients) | >11 years (66 patients) | ANOVA p |
|---|---|---|---|---|
| Age (years) | 50.4±10.3 | 54.5±7.7 | 55.4±8.6 | 0.003 |
| Monitoring treatment (years)a | 20 (17.5–23) | 19 (14–21) | 18 (9–22) | 0.024b |
| Total years of T2DM durationa | 22 (19–26) | 27.8 (21–30) | 32.5 (27–41) | <0.0001b |
| Systolic blood pressure (mmHg) | 134.4±26.2 | 147.8±25.6 | 145.2±25.7 | 0.007 |
| Diastolic blood pressure (mmHg) | 74.0±13.2 | 78.6±13.0 | 78.1±11.9 | 0.063 |
| BMI (kg/m2) | 28.3±5.8 | 29.0±5.4 | 28.3±5.2 | 0.683 |
| HbA1c (%) | 6.6±1.5 | 7.3±1.7 | 7.6±2.1 | 0.003 |
| Total cholesterol (mg/dL) | 221.8±47.9 | 227.2±56.7 | 224.1±50.3 | 0.886 |
| HDL cholesterol (mg/dL) | 47.2±23.1 | 51.6±17.5 | 51.7±16.5 | 0.375 |
| LDL cholesterol (mg/dL) | 141.4±42.5 | 145.8±48.0 | 143.6±38.3 | 0.854 |
| Triglycerides (mg/dL) | 167.2±117.8 | 187.1±196.4 | 153.1±132.1 | 0.305 |
| Serum creatinine (mg/dL) | 0.99±0.24 | 0.93±0.23 | 1.07±0.46 | 0.050 |
| Isotopic GFR (mL/min/1.73m2) | 129.7±35.7 | 126.0±28.7 | 115.7±35.4 | 0.049 |
| UAE (mg/24h) | 131.4±330.2 | 142.9±341.7 | 416.0±1040.5 | 0.021 |
| Doubled creatinine | 4.5% (3 patients) | 10.4% (7 patients) | 29% (18 patients) | <0.0001c |
Data using mean±SD.
T2DM: type 2 diabetes mellitus; BMI: Body Mass Index; HbA1c: glycated hemoglobin; HDL: high-density lipoprotein; LDL: low-density lipoprotein; isotopic GFR: isotopic glomerular filtration rate; UAE: urinary albumin excretion; SD: standard deviation; NS: non-significant.
During follow-up, the group who had been diagnosed with T2DM less than 5 years prior to intensive treatment had only 3 patients (4.5%) with doubled creatinine, while the group who had T2DM for more than 11 years prior to intensive treatment had 18 patients (29%) with doubled creatinine (p<0.0001).
The slopes for renal function evolution were calculated using repeated isotopic determinations of GFR according to renal function evolution. Patients with stable creatinine had a slope of −1.55mL/min/1.73m2/year; those in whom creatinine had doubled had −2.49mL/min/1.73m2/year; and those requiring RRT had −8.16mL/min/1.73m2/year, with statistically significant differences between the first and third groups (p=0.044). Although it did not reach statistical significance, the difference in slopes between the second and third group was 5mL/min/1.73m2/year (p=0.129).
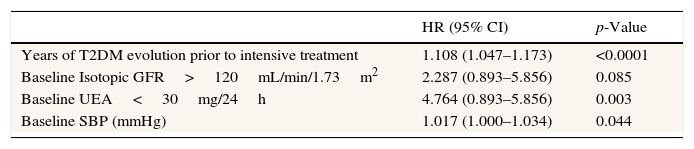
The hazard ratio of doubling creatinine was calculated using doubled creatinine plus RRT as the dependent variable in the Cox regression analysis. Independent risk factors included the duration of T2DM prior to entry in this study, UAE below 30mg/24h, systolic blood pressure, and isotopic GFR above 120mL/min/1.73m2 (Table 4).
Relative risk of doubling creatinine and RRT in the type 2 diabetic study patients.a
| HR (95% CI) | p-Value | |
|---|---|---|
| Years of T2DM evolution prior to intensive treatment | 1.108 (1.047–1.173) | <0.0001 |
| Baseline Isotopic GFR>120mL/min/1.73m2 | 2.287 (0.893–5.856) | 0.085 |
| Baseline UEA<30mg/24h | 4.764 (0.893–5.856) | 0.003 |
| Baseline SBP (mmHg) | 1.017 (1.000–1.034) | 0.044 |
Relative risk was calculated using the following variables: age, years of T2DM at baseline, systolic blood pressure, diastolic blood pressure, HbA1c, serum triglycerides, isotopic GFR superior or inferior to 120mL/min/1.73m2 and UAE superior or inferior to 30mg/24h (the last two are considered categorical variables, with the reference category defined as isotopic GFR>120mL/min/1.73m2 and UAE<30mg/24h).
Independent factors included the years of T2DM prior to study entry, baseline UAE below 30mg/24h, baseline systolic blood pressure, and baseline isotopic GFR above 120mL/min/1.73m2.
RR: hazard ratio; CI: confidence interval; T2DM: type 2 diabetes mellitus; isotopic GFR: isotopic glomerular filtration rate; UAE: urinary albumin excretion; SBP: systolic blood pressure.
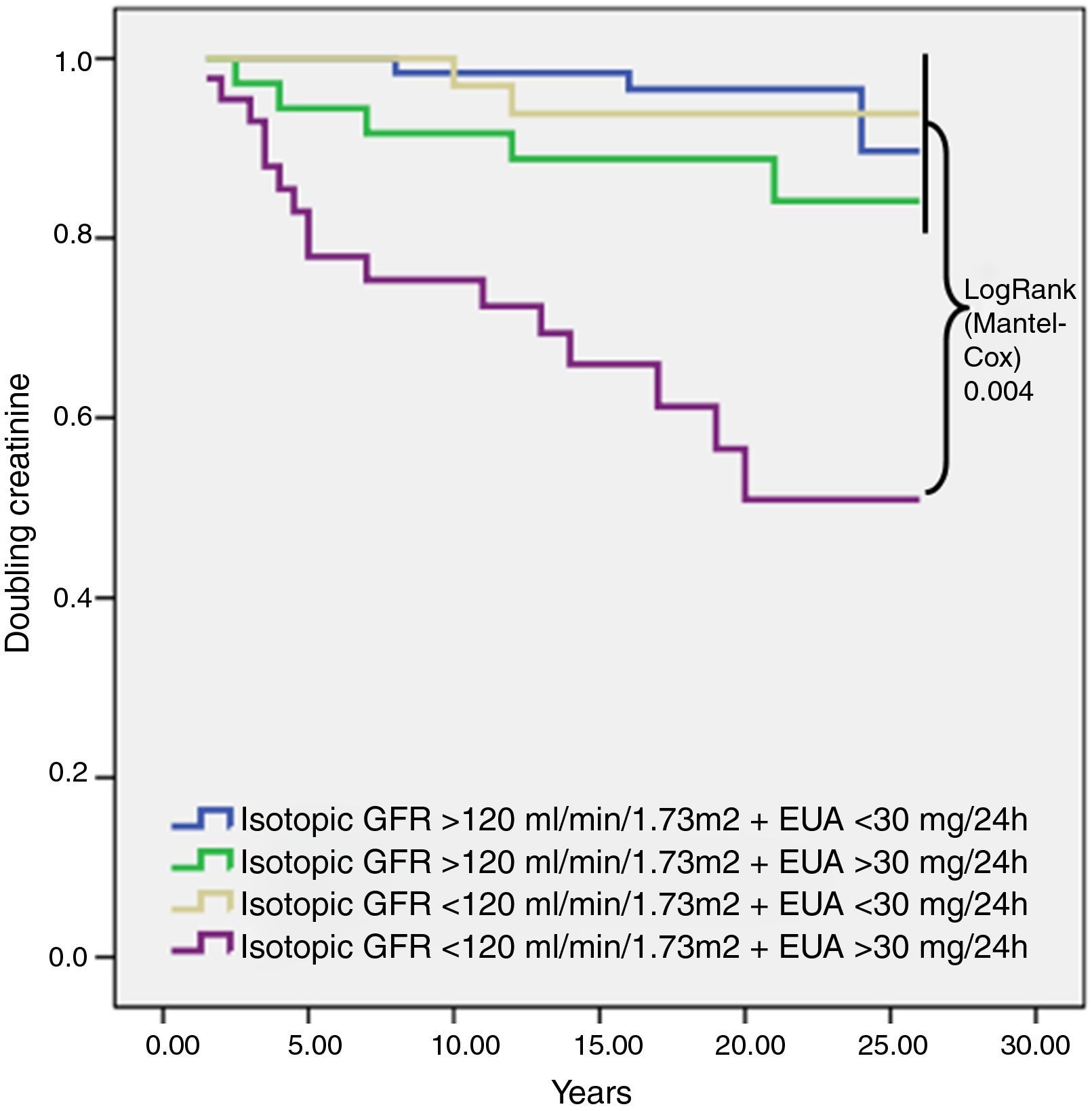
For the survival analysis, the Kaplan–Meier method was used to analyze the risk of renal event, using glomerular hyperfiltration and UAE greater than 30mg/24h as categorical variables. We observed that patients with glomerular hyperfiltration (isotopic GFR>120mL/min/1.73m2) UAE at any level had a lower rate of doubling creatinine. Patients with an isotopic GFR<120mL/min/1.73m2 and UAE>30mg/24h had the worst evolution in renal function (Fig. 1).
Kaplan–Meier survival analysis of the risk of doubling creatinine according to baseline isotopic GFR and UAE over a mean of 17 years of follow-up. The only difference was observed between baseline isotopic GFR<120mL/min/1.73m2 and baseline UAE>30mg/24h and the other three groups (LogRank 0.004). Isotopic GFR, isotopic glomerular filtration rate; UAE, urinary albumin excretion.
Isotopic determination of GFR revealed that our patients with T2DM and UAE undergoing intensive therapy for renal and cardiovascular risk factors had a small percentage of renal events (14.4%) over long-term follow-up – a lower percentage than that found in other studies.3 Other authors observed that independent risk factors for the prognosis of diabetic nephropathy included GFR, UAE, and evolution from diagnosis of T2DM.13 Our results suggest that, although isotopic GFR and UAE should be taken into account when defining and classifying chronic kidney disease,14 the evolution of T2DM should also be considered with respect to when to start treatment with RAAS blockers and other treatments for renal and cardiovascular risk factors.
RAAS blockers have been shown to reduce UAE and preserve renal function in both clinical and experimental studies. These drugs result in early normalization of podocyte morphology, and therefore have an anti-proteinuric effect.15 Blood pressure control improves renal evolution, because RAAS blockers produce intraglomerular hemodynamic changes and a subsequent decrease in UAE.16 Indeed, a subgroup of patients with uncontrolled blood pressure from the UK Prospective Diabetes Study (UKPDS) showed that only 29% of patients undergoing intensive metabolic control achieved a reduction in UAE to less than 50mg/L, while those with albuminuria over 300mg/L or with renal failure did not achieve such reductions.17 Thus, in our study, all patients were treated with RAAS blockers and the target blood pressure was 140/90mmHg.10,11 Systolic blood pressure was detected as an independent factor in the evolution of renal function. As such, intensified and especially early blood pressure control with RAAS blockers should be a goal in patients with emerging diabetic nephropathy to slow the decline in renal function.
Some studies have shown a positive association between baseline HbA1c and the progression of diabetic nephropathy,18,19 but in our group there were no significant differences in metabolic control measured by HbA1c. However, it should be noted that 43.3% of our patients were treated with insulin.
We measured renal function using isotopic determinations of GFR with 125I-iothalamate, because it is known that equations used to predict renal function underestimate GFR in T2DM patients with normal renal function.8,20 We found that renal function status at the onset of intensive therapy is a determining factor in predicting its evolution over time, as demonstrated in Table 2. A large percentage of our patients had glomerular hyperfiltration at the start of intensive therapy, and it is known that this increases the risk of albuminuria and the progression of diabetic nephropathy in patients with type 1 diabetes.21 Schmieder and colleagues found an association between hyperfiltration and early renal damage in individuals with essential hypertension.22,23 In contrast, in our study glomerular hyperfiltration patients undergoing intensive treatment maintained stable renal function for at least 18 years. As shown by Okada et al., early treatment of hyperglycemia and high blood pressure in patients with hyperfiltration prevented the progression to chronic kidney disease.24 In our study, patients with glomerular hyperfiltration had a lower incidence of renal events, independently of UAE level. However, patients who did not have glomerular hyperfiltration with a UAE>30mg/24h had a greater incidence of renal events (Fig. 1). This was related to a longer delay in intensive treatment after diagnosis of T2DM, as patients with no glomerular hyperfiltration and UAE greater than 30mg/24h had passed the stage of glomerular hyperfiltration before beginning intensive treatment.
On the other hand, UAE is an early sign of diabetic nephropathy and a main predictor of the progression and speed of renal damage.17,25,26 As such, an elevated UAE predicts greater renal damage, and, as a consequence, a faster decline in renal function. Thus, in the post hoc analysis of the RENAAL trial, proteinuria was observed to be an independent risk factor for doubling creatinine or developing advanced chronic kidney disease.27
Studies have also shown that the slope of decline for GFR is associated with multiple factors, such as albuminuria, arterial blood pressure, HbA1c≥7%, duration of diabetes, obesity, and intensive treatment.27–29 In our study, there were no significant differences between the GFR slopes of patients with doubled creatine and those requiring RRT, the difference being 5mL/min/1.73m2 slower. However, the time interval until kidney failure has been described as approximately 10 years when the decline is ≥4mL/min/1.73m2.30 We also observed that the patients with stable renal function had an isotopic GFR progression 50% slower than those who had a renal event.
Study limitationsOur study had some limitations. It did not contain a control group and was a single-center study, and “intensive treatment” was defined according to the American Diabetes Association guidelines approved at each time period during follow-up.
ConclusionIn conclusion, according to our study, patients with T2DM with glomerular hyperfiltration at baseline intensively treated for renal and cardiovascular risk factors exhibited a better progression in renal function than patients without hyperfiltration over long follow-up. This could be due to the early initiation of intensive treatment. Although diabetic nephropathy is associated with classic risk factors such as hypertension and UAE, early initiation of intensive treatment should be a priority to prevent renal function decline.
Conflict of interestThe authors declare no conflict of interest.
This work was supported by grant from Instituto de Salud Carlos III (ISCIII-RETICS REDinREN RD 06/0016) Spain.