Currently, corticosteroids are widely used to treat coronavirus disease 2019 (COVID-19) symptoms. However, the therapeutic role of corticosteroids remains highly controversial. To that end, we aimed to assess the efficacy of corticosteroids in treating COVID-19 patients.
MethodWe searched PubMed, Embase, and Cochrane Library to select suitable studies. Our primary study endpoint was all-cause mortality. The secondary study endpoint was the length of hospital stay.
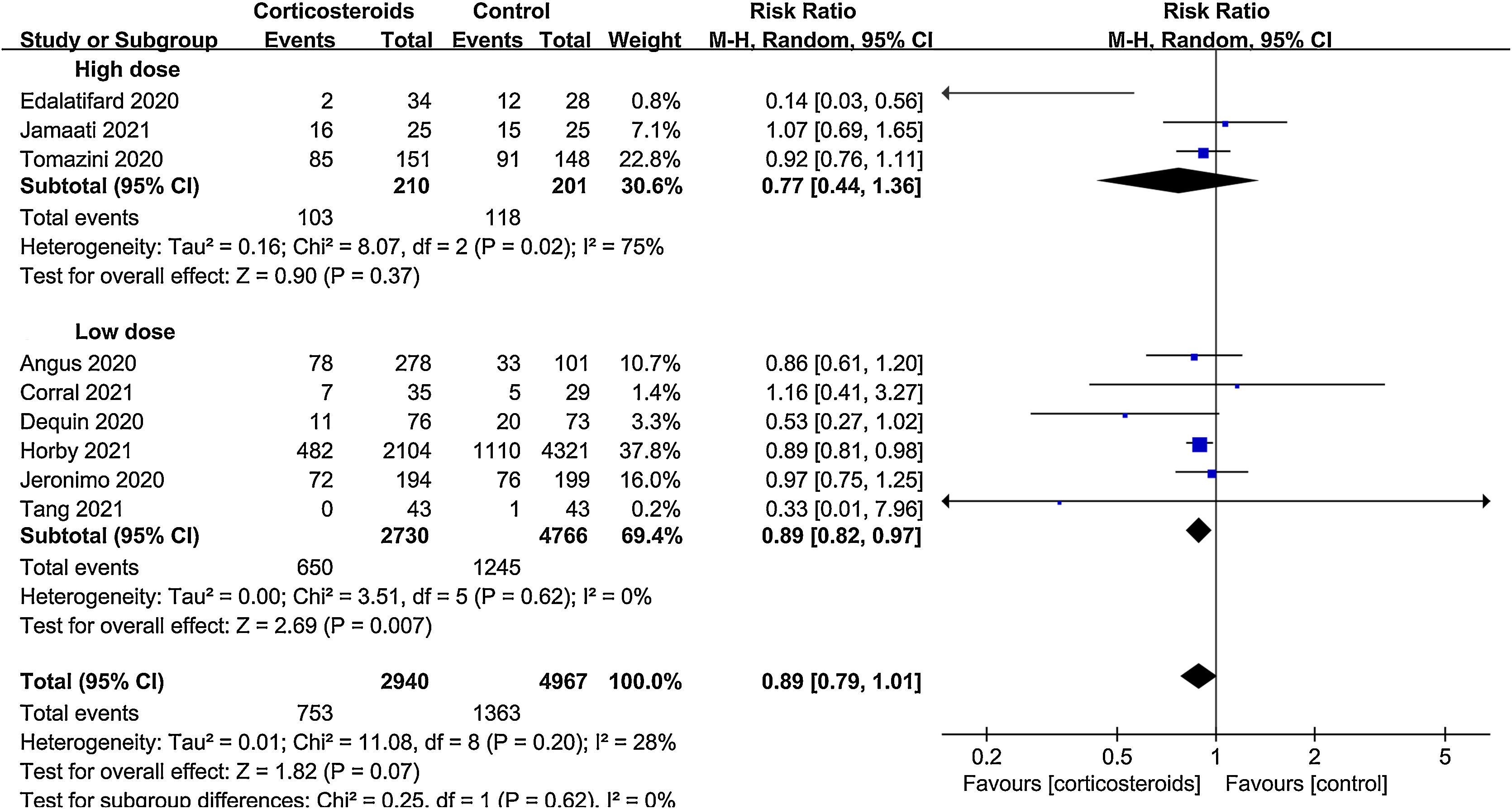
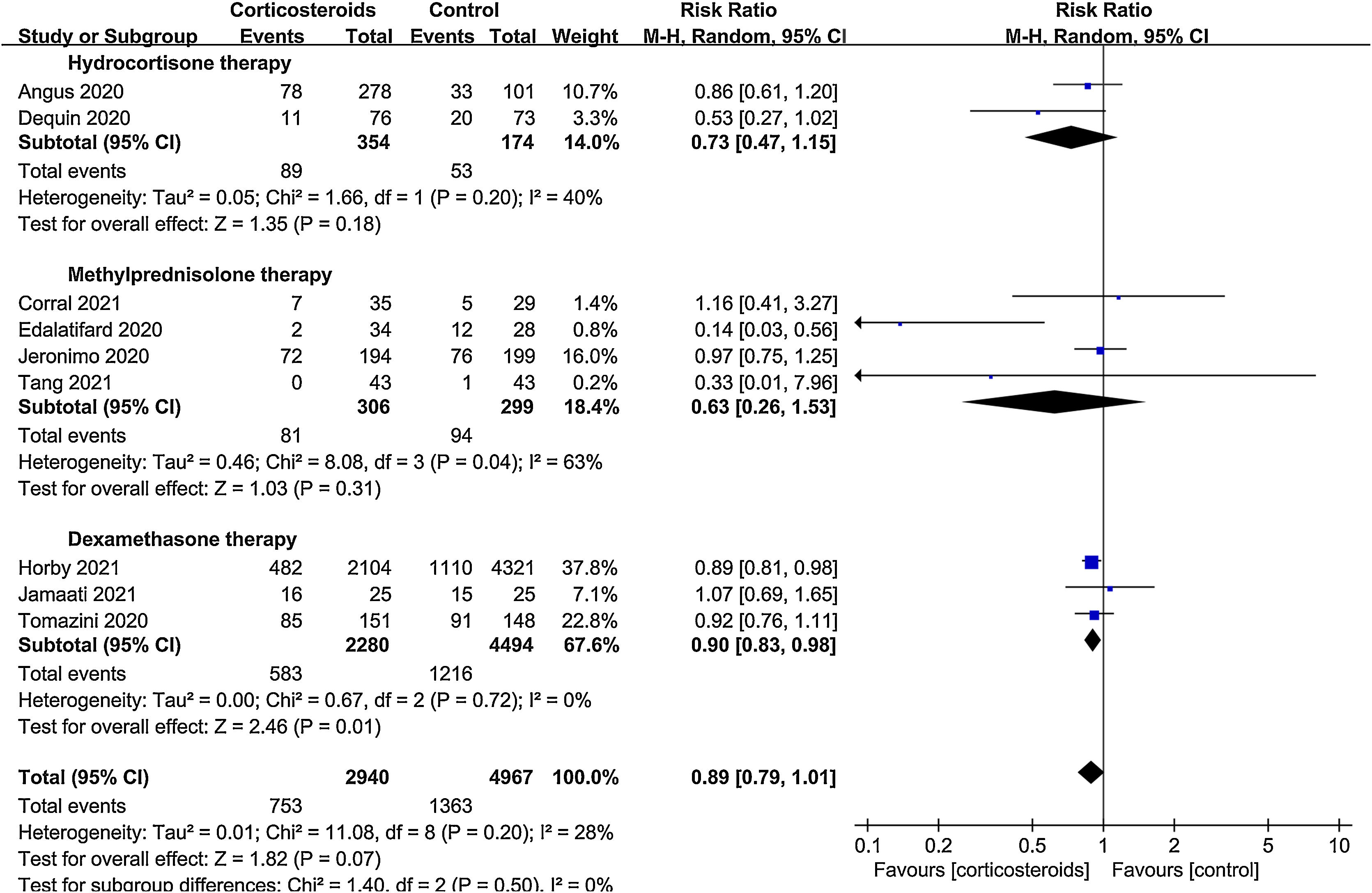
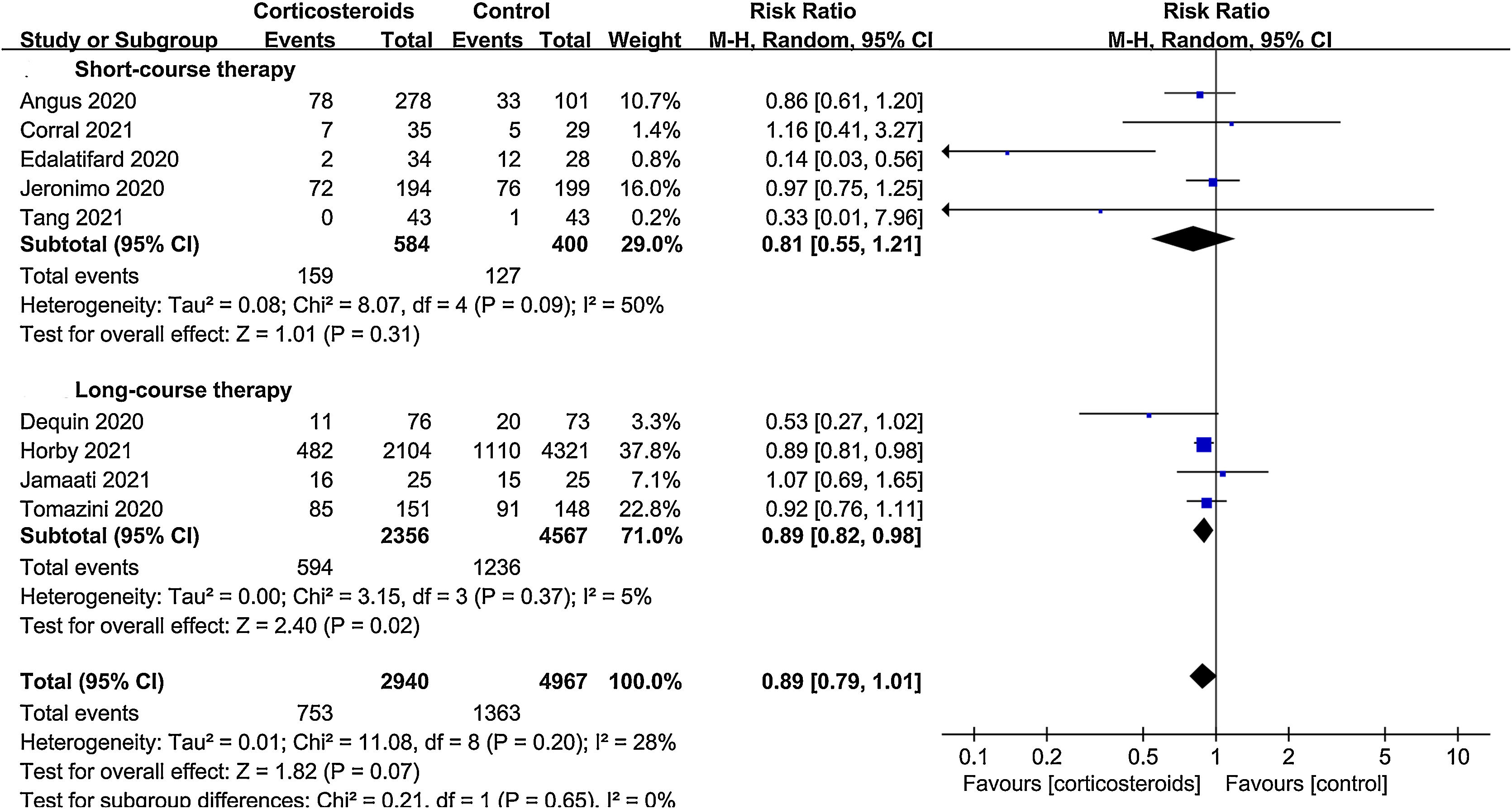
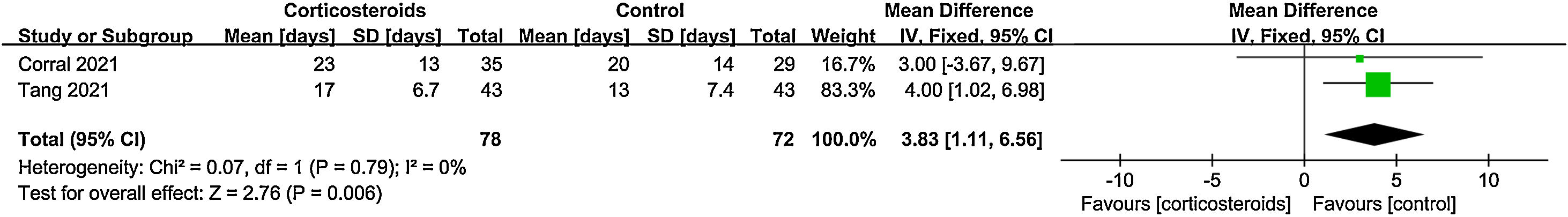
ResultsA total of 9 randomized controlled trials (RCTs) with 7907 patients were assessed. The pooled result indicated that corticosteroids treatment could significantly reduce all-cause mortality in patients with COVID-19 (RR=0.88, 95% CI [0.82, 0.95], P=0.002). When subgroup analyses were performed, we found that corticosteroids were associated with decreased all-cause mortality in severe COVID-19 patients (RR=0.77, 95% CI [0.68, 0.88], P<0.0001), however no obvious difference was observed in all-cause mortality of non-severe COVID-19 patients between the corticosteroid and control group (RR=0.96, 95% CI [0.86, 1.06], P=0.41), meanwhile, a low dose (RR=0.89, 95% CI [0.82, 0.97], P=0.007) of dexamethasone (RR=0.9, 95% CI [0.83, 0.98], P=0.01) with a long treatment course (RR=0.89, 95% CI [0.82, 0.98], P=0.02) was beneficial for all-cause mortality in COVID-19 patients. Additionally, we found that corticosteroids might be associated with a longer length of hospital stay in non-severe COVID-19 patients (MD=3.83, 95% CI [1.11, 6.56], P=0.006).
ConclusionOur results showed that corticosteroid therapy was related to a reduction in all-cause mortality in severe COVID-19 patients. However, in patients with non-severe COVID-19, the use of corticosteroids did not decrease all-cause mortality and may prolong the duration of hospital stay. In addition, we revealed that a low dose of dexamethasone with a long treatment course could reduce all-cause mortality in COVID-19 patients.
Actualmente, los glucocorticoides se utilizan ampliamente para tratar los síntomas de la enfermedad por coronavirus 2019 (COVID-19). Sin embargo, el papel terapéutico de los glucocorticoides sigue siendo muy controvertido, por ello, nos propusimos evaluar su eficacia en el tratamiento de los pacientes con COVID-19.
MétodoSe realizaron búsquedas en PubMed, Embase y Cochrane Library para seleccionar los estudios adecuados. El criterio de valoración principal del estudio fue la mortalidad por todas las causas. El criterio de valoración secundario del estudio fue la duración de la estancia en el hospital.
ResultadosSe evaluó un total de 9 ensayos controlados aleatorizados con 7.907 pacientes. En general, el tratamiento con glucocorticoides redujo la mortalidad por todas las causas en los pacientes con COVID-19 (RR=0,88, IC 95% [0,82; 0,95], p=0,002). Al realizar análisis de subgrupos, se observó que los glucocorticoides se asociaban a una disminución de la mortalidad por todas las causas en los pacientes con COVID-19 grave (RR=0,77, IC 95% [0,68; 0,88], p<0,0001), sin embargo no se observaron diferencias evidentes en la mortalidad por todas las causas de los pacientes con COVID-19 no grave entre el grupo de glucocorticoides y el de control (RR=0,96, IC 95% [0,86; 1,06], p=0,41), mientras que una dosis baja (RR=0,89, IC 95% [0,82; 0,97], p=0,007) de dexametasona (RR=0,9, IC 95% [0,83; 0,98], p=0,01) con un curso de tratamiento largo (RR=0,89, IC 95% [0,82; 0,98], p=0,02) fue beneficiosa para la mortalidad por todas las causas en los pacientes con COVID-19. Además, encontramos que los glucocorticoides podrían estar asociados con una mayor duración de la estancia hospitalaria en los pacientes con COVID-19 no grave (DM=3,83, IC 95% [1,11; 6,56], p=0,006).
ConclusiónNuestros resultados mostraron que el tratamiento con glucocorticoides estaba relacionado con una reducción de la mortalidad por todas las causas en los pacientes con COVID-19 grave. Sin embargo, en los pacientes con COVID-19 no grave, el uso de glucocorticoides no disminuyó la mortalidad por todas las causas y puede prolongar la duración de la estancia hospitalaria. Además, descubrimos que una dosis baja de dexametasona con un curso de tratamiento largo podría reducir la mortalidad por todas las causas en los pacientes con COVID-19.
The COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) appeared at the end of December 2019 and has since brought about an unprecedented challenge to public health worldwide.1 Reportedly, 20% of COVID-19 patients will progress to severe respiratory failure requiring intensive care.2,3 Severe cytokine and chemokine storms are believed to be involved in respiratory and multi-organ failure. Thus, immunosuppressive drugs such as corticosteroids have been widely used in the treatment of COVID-19 patients. Nevertheless, the role of corticosteroids in the management of COVID-19 remains a subject of controversy.
Recently, the RECOVERY trial reported a notable survival benefit of a daily low dosage of dexamethasone for up to 10 days in subjects with COVID-19 who were receiving oxygen therapy or mechanical ventilation.4 However, other clinical studies showed different results. The Metcovid trial found no benefit in the 28-day mortality of a low methylprednisolone dosage for 5 days in COVID-19 patients.5 Similarly, in the CAPE COD trial,6 compared with the placebo group, using low-dose hydrocortisone for 10 days or 8 days could not reduce mortality or the requirement for respiratory support. Additionally, a study by Jamaati et al.7 suggested that a 10-day high dosage of dexamethasone did not decrease mortality in patients with non-severe COVID-19 compared to the control group.
Previous meta-analyses of several RCTs mainly focused on the effect of corticosteroids treatment on severe COVID-19 patients. In this meta-analysis, we included more valuable RCTs to evaluate the efficacy of corticosteroids among not only severe but also non-severe COVID-19 patients.
Materials and methodsSearch strategy and data sourcesThe present study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Eligible RCTs were identified through a systematic search of PubMed, Embase and Cochrane Library from December 31, 2019 to March 31, 2021. The following search terms were used: (adrenal cortex hormone or corticosteroid or glucocorticoid or corticoid or steroid) and (COVID-19 or 2019 nCoV or coronavirus disease-19 or 2019 novel coronavirus disease or 2019-nCoV disease or coronavirus disease 2019 or SARS CoV-2 or nCov 2019). In addition, the references were manually searched to make the results more comprehensive. The work was done independently by two authors. A third investigator resolved all encountered disagreements.
Inclusion and exclusion criteriaStudies that met all of the following criteria were selected: (1) patients in each study were adults with laboratory-confirmed or clinically suspected COVID-19. (2) The participants were assigned to a corticosteroid group using corticosteroids plus standard care, and the control group received standard care without corticosteroids. We excluded conference abstracts, case reports, articles not in English and studies without full text or missing important data.
Our primary study endpoint was the all-cause mortality rate at the longest follow-up available. The secondary study endpoint was the length of stay in the hospital.
Data extractionTwo reviewers independently extracted data from the included studies. If there was any dispute, it was discussed or resolved by the third author. The following data were collected: first author, year of publication, study region, study design, inclusion criteria, type, dose, and duration of corticosteroid use, control intervention, outcome in each study, as well as the longest follow-up.
Quality assessmentThe quality of each study was independently evaluated by two authors using the Cochrane Collaboration risk of bias, consisting of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases.
Statistical analysisThe meta-analyses were performed using RevMan 5.4 software (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). Dichotomous data were analyzed as the pooled relative risk (RR) with its 95% confidence interval (CI), while for continuous data, we calculated the mean difference (MD) and the 95% CI. A P-value of <0.05 was considered statistically significant. Statistical heterogeneity between studies was estimated using statistic I2. A random-effects model was used when either P<0.1 or I2>50% defined significant heterogeneity; otherwise, the fixed-effects model was used.
For the primary study endpoint, subgroup analyses were carried out according to the severity of disease, corticosteroids dosage, type and treatment time. Severe COVID-19 patients were defined as those patients admitted to the Intensive Care Unit (ICU), and the remaining were non-severe. A low or high dosage was defined based on the cutoff values: dexamethasone 15mg/day, hydrocortisone 400mg/day, or equivalent methylprednisolone 80mg/day.8 Besides, treatment duration was classified as short (≤7 days) or long course (>7 days).
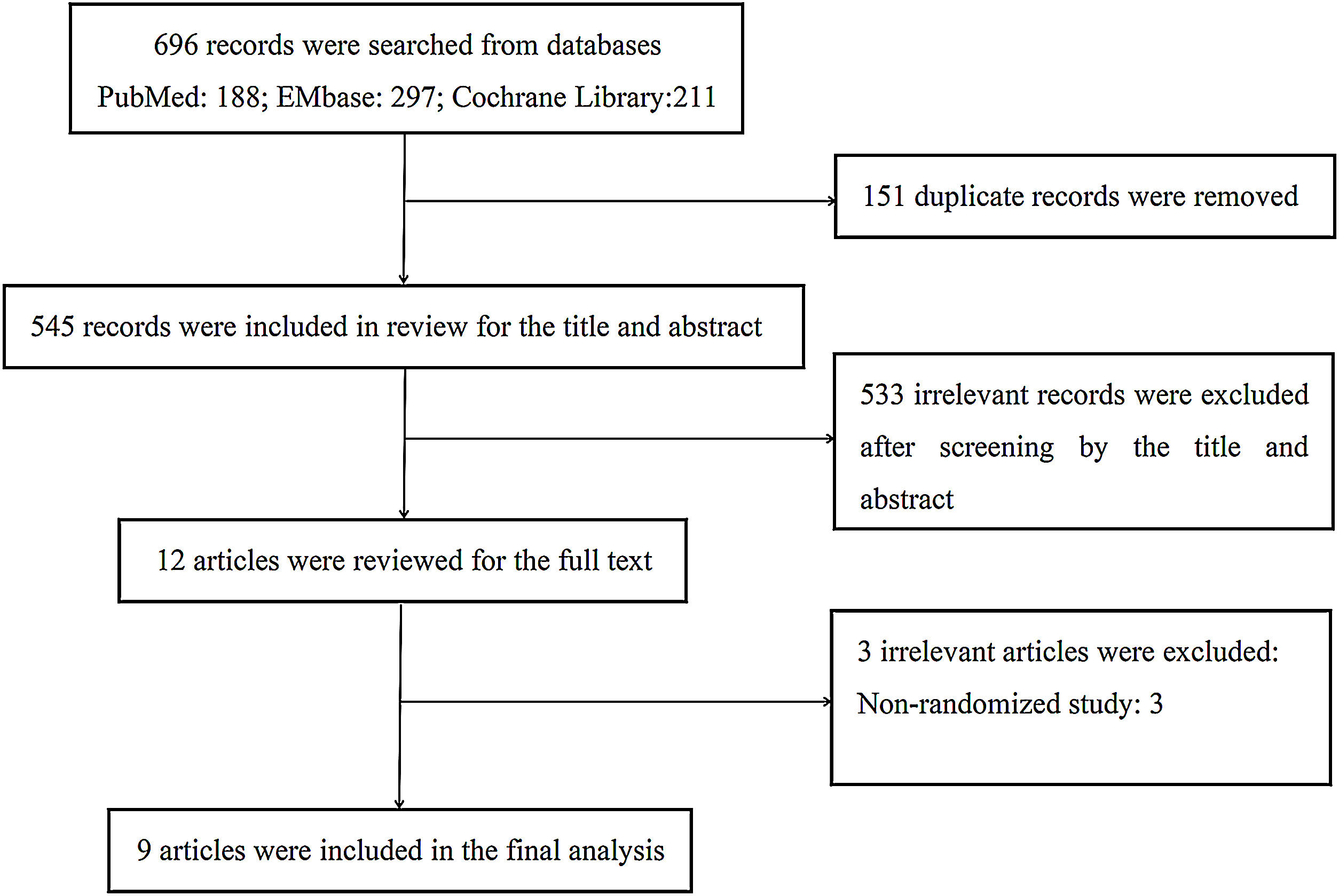
ResultsStudy selectionA total of 696 articles were obtained from our database search, 188 in PubMed, 297 in Embase, 211 in the Cochrane Library. After removing 151 duplicates and another 533 records by screening the title and abstract, there were 12 remaining full-text articles, among which 3 studies were removed due to non-randomized controlled trials. Ultimately, 9 trials4–7,9–13 were included. The literature screening workflow is shown in Fig. 1.
Study characteristicsThe characteristics of included studies are summarized in Table 1. In total, 7907 patients from 9 studies were analyzed. Of the 9 studies, 2940 patients were treated with corticosteroids and 4967 were treated without corticosteroids. There were 54–6,9,10 articles describing the use of corticosteroids in severe patients and another 54,7,11–13 in non-severe patients. The corticosteroids utilized in the studies included hydrocortisone,6,9 dexamethasone,4,7,10 and methylprednisolone.5,11–13
Characteristics of trials.
| Study | Study region | Study design | Inclusion criteria | Dosage and duration of corticosteroids (n) | Control intervention (n) | Primary outcome | Longest follow-up |
|---|---|---|---|---|---|---|---|
| Angus 2020REMAP-CAP | Australia, Canada, France, Ireland, the Netherlands, New Zealand, the UK, the USA | Multicenter Open-labelRCT | Aged at least 18 years confirmed or suspected COVID-19 admitted to ICU receiving respiratory or cardiovascular support | A fixed 7-day course of intravenous hydrocortisone (50 or 100mg every 6h) (n=137) OR A shock-dependent course (50mg every 6h up to 28 d for shock patients) (n=141) | Standard care (n=101) | Respiratory and cardiovascular organ support-free days up to day 21 | 21 days |
| Jeronimo 2020Metcovid | Brazil | Single centerRCT | Aged at least 18 years confirmed or suspected COVID-19 in use of oxygen therapy or under invasive mechanical ventilation | Methylprednisolone 1mg/kg/d for 5d (n=194) | Standard care (n=194) | 28-day mortality | 28 days |
| Dequin 2020CAPE COD | France | MulticenterDouble-blindRCT | Aged at least 18 years confirmed or suspected COVID-19 admitted to ICU with acute respiratory failure | Hydrocortisone 200mg/d for 7d, then 100mg/d for 4d and 50mg/d for 3 d; if symptoms improved by day 4, then followed with hydrocortisone 100mg/d for 2 d and 50mg/d for 2 d (n=76) | Standard care (n=73) | Death or persistent respiratory support on 21 d | 21 days |
| Edalatifard 2020 | Iran | MulticenterSingle-blindRCT | Aged at least 18 years confirmed COVID-19 receiving oxygen therapy but not intubation or ventilation | Methylprednisolone 250mg/d for 3d (n=34) | Standard care (n=28) | Time to clinical improvement and hospital discharge or death | Until clinical improvement and hospital discharge or death |
| Tomazini 2020CoDEX | Brazil | Multicenter Open-labelRCT | Aged at least 18 years confirmed or suspected COVID-19 receiving mechanical ventilation for ARDS | Dexamethasone 20mg/d for 5d, then 10mg/d for 5d or until ICU discharge (n=151) | Standard care (n=148) | Ventilator-free days at 28d | 28 days |
| Corral 2021GLUCOCOVID | Spain | Multicenter Open-labelRCT | Aged at least 18 years confirmed COVID-19, not intubated or ventilated | Methylprednisolone 80mg/d for 3d, then 40mg/d for 3d (n=35) | Standard care (n=29) | A composite of death, ICU admission, or requirement of noninvasive ventilation | Until composite endpoint happened |
| Horby 2021RECOVERY | UK | Multicenter Open-labelRCT | Confirmed or suspected COVID-19 | Oral or intravenous dexamethasone 6mg/d for up to 10d (or until hospital discharge if sooner) (n=2104) | Standard care (n=4321) | All-cause mortality within 28d after randomization | 28 days |
| Tang 2021 | China | MulticenterSingle-blindRCT | Aged at least 18 years confirmed COVID-19 admitted to general wardsless than 72h | Methylprednisolone 1mg/kg/d for 7 d (n=43) | Standard care (n=43) | Incidence of clinical deterioration 14 days after randomization. | 14 days |
| Jamaati 2021 | Iran | Single centerRCT | Aged at least 18 years confirmed COVID-19 (PaO2/FiO2) between 100 and 300mmHg | Dexamethasone 20mg/d for 5d, then 10mg/d for 5d (n=25) | Standard care (n=25) | Need for invasive mechanical ventilation and death rate | 28 days |
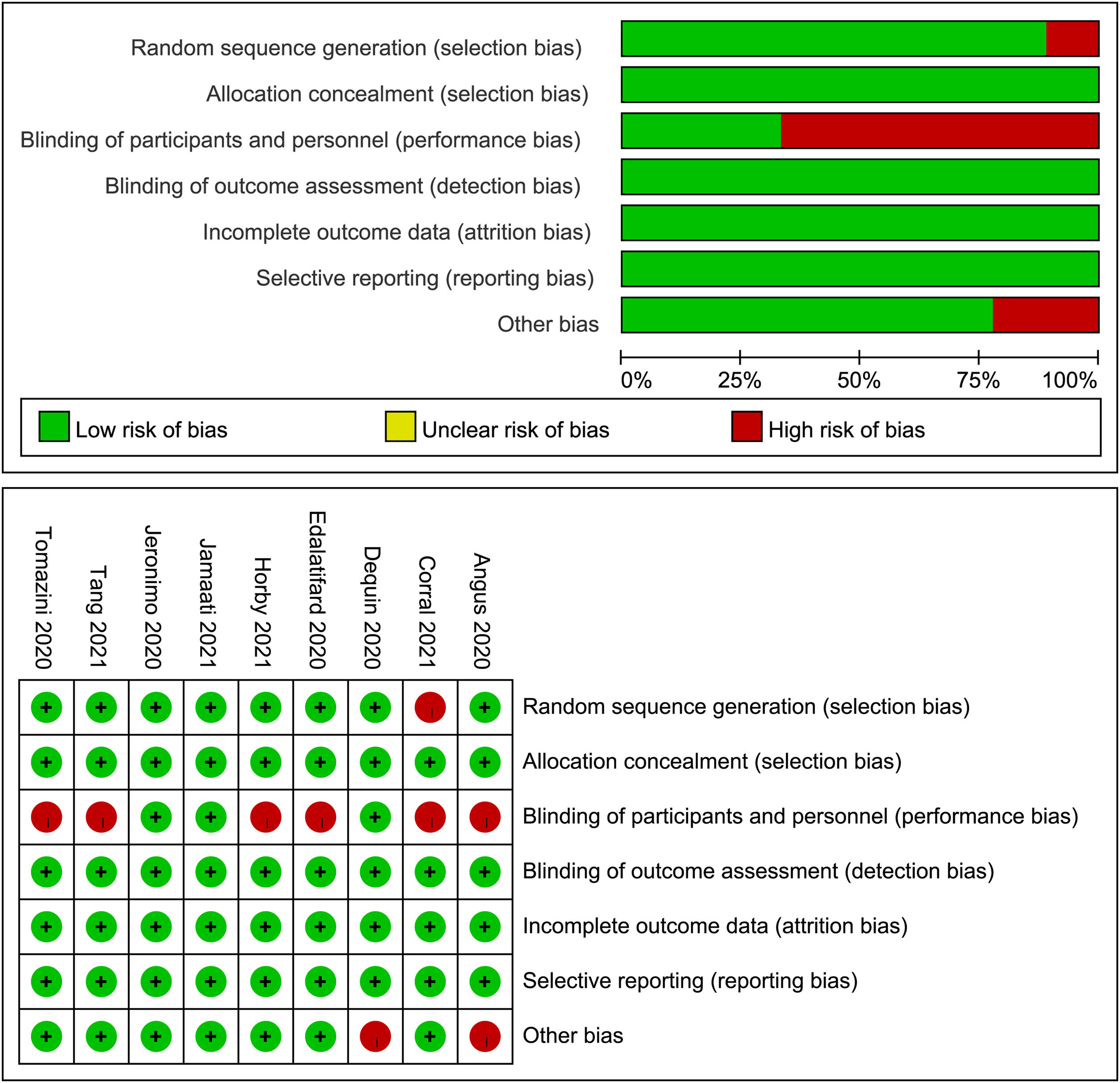
Results of the methodological quality assessment of included studies are presented in Fig. 2. As indicated, two trials5,7 have a low risk of bias, while the other seven trials4,6,9–13 were judged to have a high risk of bias.
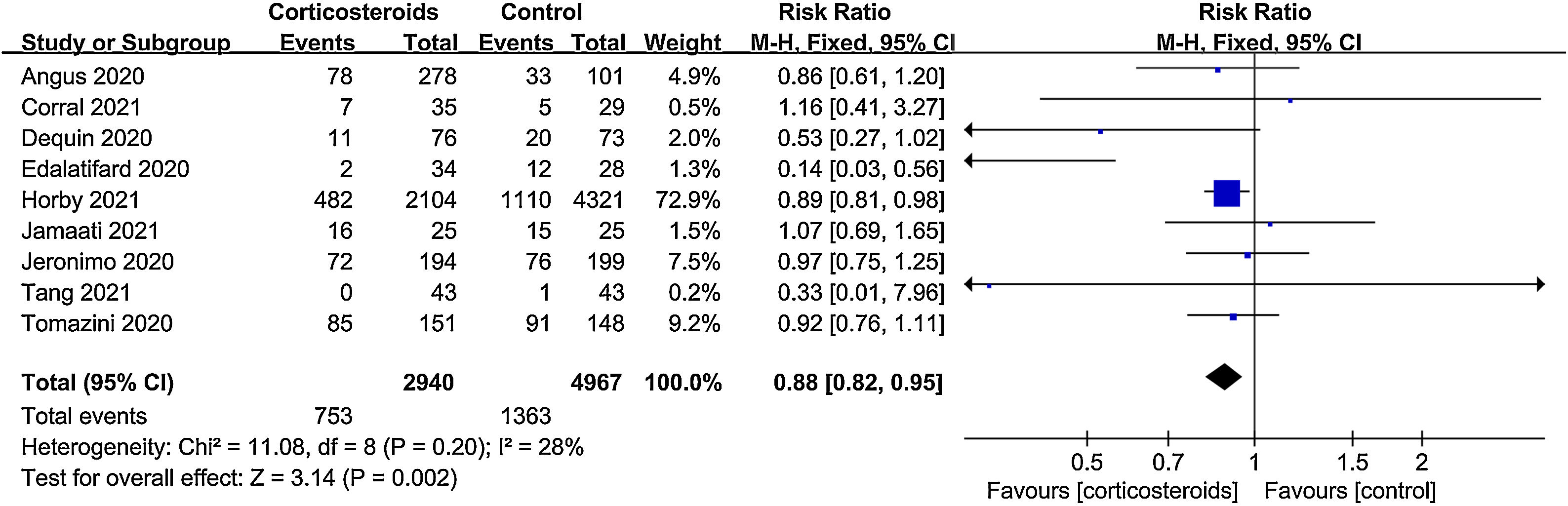
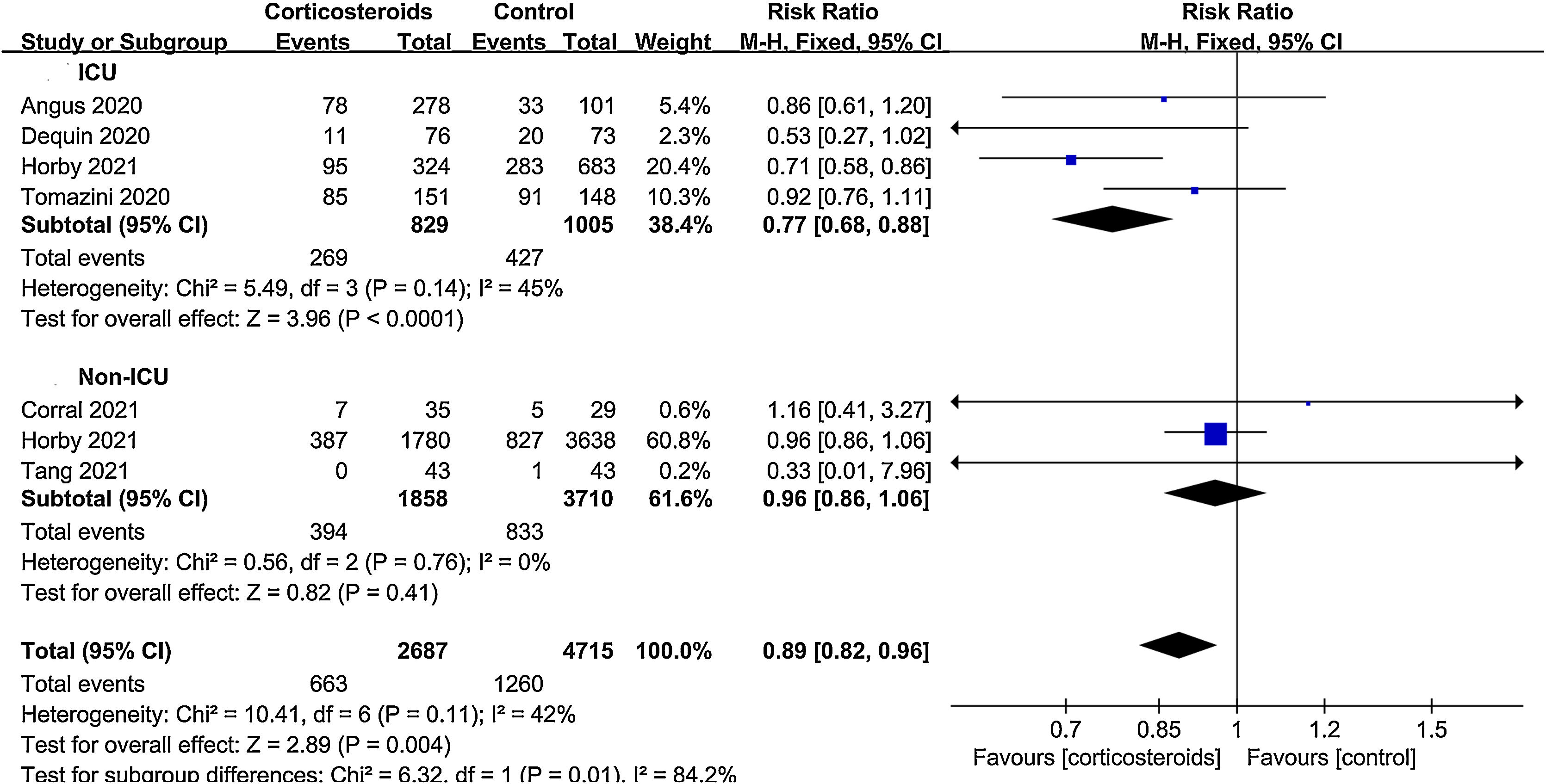
All-cause mortalityAll 9 trials reported data on all-cause mortality. There were 753 deaths among the 2940 patients in the corticosteroid group (25.6%) and 1363 deaths among the 4967 patients in the control group (27.4%). The pooled result indicated that corticosteroids treatment could significantly reduce all-cause mortality in patients with COVID-19 (RR=0.88, 95% CI [0.82, 0.95], P=0.002, I2=28%) (Fig. 3). When subgroup analyses were performed, we found that corticosteroid use was associated with decreased all-cause mortality in severe COVID-19 (RR=0.77, 95% CI [0.68, 0.88], P<0.0001, I2=45%) (Fig. 4). However, no obvious difference was observed in all-cause mortality of non-severe COVID-19 between the corticosteroid and control group (RR=0.96, 95% CI [0.86, 1.06], P=0.41, I2=0%) (Fig. 4). Interestingly, a low dose (RR=0.89, 95% CI [0.82, 0.97], P=0.007, I2=0%) of dexamethasone (RR=0.9, 95% CI [0.83, 0.98], P=0.01, I2=0%) with a long treatment course (RR=0.89, 95% CI [0.82, 0.98], P=0.02, I2=5%) could reduce all-cause mortality in COVID-19 patients (Figs. 5–7).
Duration of hospitalizationTwo studies evaluated the length of hospital stay of non-severe COVID-19 patients; Data from the studies were pooled, and meta-analysis showed that corticosteroid use was significantly associated with longer length of hospital stay (MD=3.83, 95% CI [1.11, 6.56], P=0.006, I2=0%) (Fig. 8).
DiscussionSARS-CoV-2 is a highly transmissible virus that caused the greatest pandemic of the century. At present, we are looking for effective treatments to control this deadly and evolving disease. In this meta-analysis, we have assessed the effect of corticosteroids in COVID-19 patients. Corticosteroids therapy could significantly reduce all-cause mortality in patients with COVID-19. When subgroup analysis was performed according to disease severity, we found that corticosteroid use was associated with a decreased all-cause mortality in severe COVID-19, but not in patients with non-severe COVID-19. Moreover, we performed other subgroup analyses according to the dosage, type and treatment duration of corticosteroids. The pooled results suggested that a low dosage and long-term use of dexamethasone could reduce all-cause mortality in COVID-19 patients. Finally, we also concluded that corticosteroid use might be associated with a longer length of hospital stay in non-severe COVID-19 patients.
COVID-19-related respiratory or multi-organ failure might be due to an excessive immune response that damages pulmonary alveoli, leading to severe cytokine and chemokine storms with systemic effects.14 To dampen the inflammatory dysfunction, the administration of corticosteroids has attracted significant attention. In the past, corticosteroids have been used extensively in acute respiratory distress syndrome (ARDS), caused by SARS-CoV or Middle East respiratory syndrome (MERS)-CoV.15,16 Nevertheless, there is no consensus that corticosteroid administration is helpful for COVID-19 patients, considering the possibility of delayed viral clearance, increased secondary infections or severe adverse events.
Previous meta-analytical studies have evaluated the role of corticosteroids in COVID-19 patients and reached mixed conclusions. A meta-analysis written by Lu et al.17 addressing the impact of corticosteroids in adults and children with coronavirus diseases (MERS, SARS, and COVID-19) included five studies available for COVID-19 indicating that corticosteroid use did not reduce mortality and might instead prolong the duration of hospital stay in adults with COVID-19. Similarly, another meta-analysis18 reviewed 5249 patients from 1 randomized clinical trial and 10 cohort involving coronavirus-related diseases caused by SARS-CoV-2, SARS-CoV, and MERS-CoV. Of the 5249 patients, 1426 were COVID-19 patients. Taking everything into account, corticosteroids use in patients affected by coronavirus diseases delayed virus clearing, failed to improve survival. Notably, SARS, MERS and COVID-19 are phenotypically heterogeneous despite their close virus phylogeny19 and may pose significant selection bias, collectively reducing the quality of the conclusion. Contrary to previous results, a prospective meta-analysis20 published in JAMA concluded that corticosteroids were associated with lower 28-day all-cause mortality in critically ill patients with COVID-19. Subsequently, Ma et al.,21 conducted a meta-analysis of 7 RCTs that showed decreased all-cause mortality in severe COVID-19 patients following corticosteroid treatment. These two meta-analyses mainly focused on the effect of corticosteroid treatment on severe COVID-19 patients only and did not include non-severe COVID-19 patients.
The results of our meta-analysis show that corticosteroids could reduce all-cause mortality in COVID-19 patients. Subsequently, subgroup analyses for mortality stratified by severity of disease, corticosteroids dosage, type and treatment duration were performed in this meta-analysis. Corticosteroid use was associated with decreased all-cause mortality in severe COVID-19 (RR=0.77, 95% CI [0.68, 0.88], P<0.0001, I2=45%), but not in non-severe COVID-19 patients (RR=0.96, 95% CI [0.86, 1.06], P=0.41, I2=0%). Survival benefit was observed with a low dosage (RR=0.89, 95% CI [0.82, 0.97], P=0.007, I2=0%) and long treatment course (RR=0.89, 95% CI [0.82, 0.98], P=0.02, I2=5%) of dexamethasone (RR=0.9, 95% CI [0.83, 0.98], P=0.01, I2=0%) in COVID-19 patients. Furthermore, our findings showed that corticosteroid use in non-severe COVID-19 patients might be related to a lengthier hospital stay (MD=3.83, 95% CI [1.11, 6.56], P=0.006, I2=0%). In non-severe COVID-19 people, an effective immune response with neutralizing antibodies promotes viral clearance and a short-lived inflammatory response.22 However, the immune response in patients with severe SARS-CoV-2 infection is quite strong, often resulting in ARDS or multi-organ dysfunction.23 Corticosteroids can reduce capillary dilation, inflammatory cell exudation, leukocyte infiltration, and phagocytosis in the early phase of inflammation, and also inhibit the excessive proliferation of capillaries and fibroblasts in the late stage.24 In conjunction with our results, treatment with corticosteroids appeared to be more beneficial in severe COVID-19. Moreover, the most effective type of corticosteroid treatment is another concern that needs to be addressed. In our study, dexamethasone could reduce all-cause mortality in COVID-19 patients, while a recent RCT demonstrated that methylprednisolone treatment was more beneficial than dexamethasone in COVID-19 treatment.25 Therefore, there is an urgent need for more RCTs to confirm and validate previous findings.
LimitationsNevertheless, this meta-analysis has several limitations. Firstly, the study contained some high-quality RCTs, but the sample size of patients was dominated by the RECOVERY trial. Secondly, both severe and non-severe COVID-19 patients were included in our research, which might increase the overall heterogeneity. Thirdly, there was no unified standard for the type, time and dosage of corticosteroids used in the various studies. Fourthly, due to the limited data, we could not provide a meta-analysis on other outcomes such as organ support-free days, length of ICU stay, or duration of virus shedding. Lastly, some studies presented the data for continuous variables as the median and interquartile range (IQR), and we had to convert to mean and standard deviation.
ConclusionsThe present meta-analysis revealed that corticosteroid therapy was related to reduced all-cause mortality in severe COVID-19 patients. However, in patients with non-severe COVID-19, the use of corticosteroids did not decrease the all-cause mortality and might instead prolong the duration of hospital stay. More importantly, we uncovered that extended use of low-dose dexamethasone could reduce all-cause mortality in COVID-19 patients. Nevertheless, more RCTs are needed to substantiate our conclusions.
Author contributionsYQZ, WZZ, and BHY searched databases and performed analysis. YQZ and WZZ wrote the manuscript. ZL and BHY designed the study and revised the manuscript. All authors read and approved the final manuscript.
FundingThis research received no external funding.
Conflicts of interestThe authors declare no conflict of interest.