To analyze the effect of sodium glucose cotransporter 2 (SGLT2) inhibitors in a group of insulin-dependent type 2 diabetes mellitus (T2D) patients.
Patients and methodsOne hundred and five insulin treated T2D patients were enrolled. Primary endpoints were: fasting plasma glucose, HbA1c, weight, total insulin doses (TDI), total basal insulin (TDB) and total rapid insulin (TDR). Secondary variables were: total cholesterol, LDL cholesterol (cLDL), HDL cholesterol (cHDL), triglycerides and systolic (SBP) and diastolic (DBP) blood pressure. Safety and tolerance were evaluated through the appearance of severe hypoglycemia, ketoacidosis and infections.
ResultsAfter 4 months follow-up, a 0.7 (1.0)% HbA1c reduction was found, accompanied by a −2.8 (11.5) UI/day TDI decrease. Weight dropped for 73.7% of patients, with an average −2.0 (2.7) kg reduction. A global cHDL increase was noted after treatment, while no differences were observed for total cholesterol, triglycerides or cLDL. SBP dropped significantly, but no change in DBP was observed.
ConclusionThe use of SGLT2 inhibitors in insulin treated T2D patients resulted in reduction of HbA1c, which was associated to weight loss, cHDL increase and SBP decrease.
Analizar el efecto de los inhibidores del cotransportador sodio glucosa tipo 2 (iSGLT2) en u grupo de pacientes con diabetes mellitus tipo 2 (DM2) tratados con insulina.
Pacientes y métodosSeleccionamos ciento cinco pacientes con diabetes tipo 2 tratados con insulina. Los objetivos primarios: glucosa plasmática ayunas, HbA1c, peso, dosis total de insulina (DTI), dosis de insulina basal (DTB), dosis de insulina rápida (DTR). Como secundarios: Colesterol total, LDL colesterol (cLDL), HDL colesterol (cHDL), triglicéridos and tensión sistólica (TAS) and diastólica (TAD). La seguridad y tolerancia se valoró como hipoglucemia, cetoacidosis o infecciones.
ResultadosTras 4 meses, la HbA1c se redujo en 0,7 (1,0)% acompañado de una disminución en DTI de −2,8 (11,5) UI/día. El 73,7% de los pacientes perdieron peso, con un descenso medio de −2,0 (2,7) kg. El cHDL aumentó, mientras que no observamos diferencias en el colesterol total, triglicéridos o cLDL. TAS disminuyó significativamente sin observar cambios en TAD.
ConclusiónEl uso de iSGLT2 en pacientes con DM2 tratados con insulina reduce la HbA1c asociando pérdida de peso, aumento enl cHDL y descenso en TAS.
T2D affects 13% of the Spanish population. It is associated to obesity, dyslipidemia and hypertension (HT) and constitutes one of the main causes of cardiovascular disease in Spain.
T2D is usually treated with insulin to maintain glycemic control the major limitations of this treatment being hypoglycaemia and weight gain.1
SGLT2 inhibitors prevent the reabsorption of glucose at the proximal renal tubule, producing osmotic diuresis and glycosuria and thus improving blood sugar control in an insulin-independent manner, with low risk of hypoglycemia. They are also associated with weight loss and blood pressure reduction due to their natriuretic effect. Therefore, the latest diabetes clinical guidelines recommend their use for insulin dependent patients whose diabetes is not well controlled, especially hypertensive obese patients.2
In this communication, data on the outcomes of a group of insulin-dependent patients with poorly controlled T2D to whom SGLT2 inhibitors were prescribed in addition to their medication are presented.
Patients and methodsThis is a multicentre, retrospective observational study performed in the Central Hospital of Asturias (Oviedo), the Álvarez Buylla Hospital (Mieres) and the San Agustín Hospital (Avilés) between 2014 and 2016.
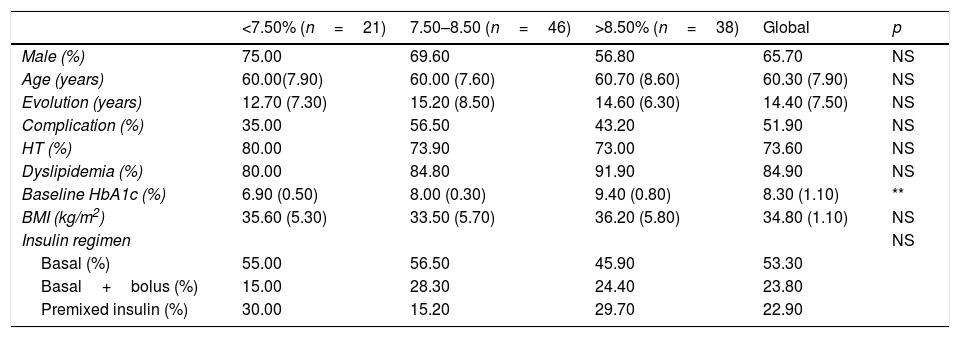
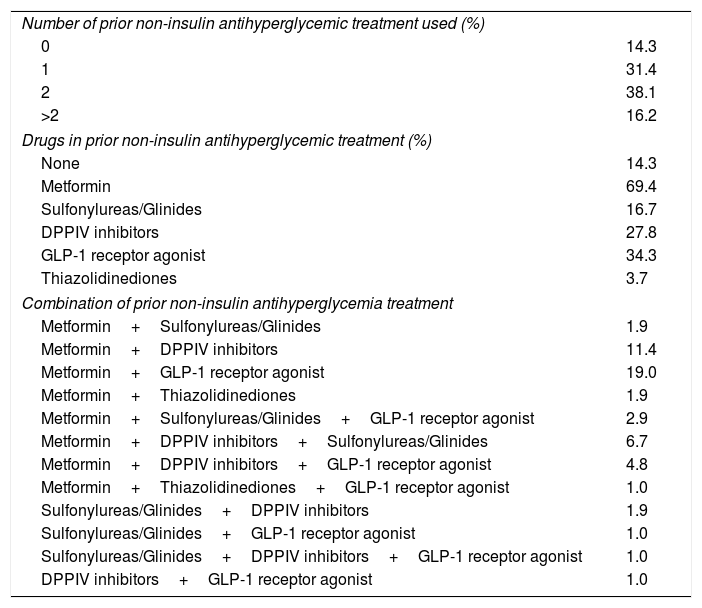
The patients (105) were classified into three groups based on glycaemic control as measured by basal HbA1c: <7.5%, 7.5–8.5% and >8.5%, which presented similar characteristics at baseline (Table 1). All were over 18 years old and received insulin, either basal once a day, premixed or basal-bolus therapy and 85.6% were on additional non-insulin antihyperglycemic treatment (Table 2). The combination of GLP-1 receptor agonist and SGLT2 inhibitors was justified to reduce weight.
Characteristics of patients as a function of their baseline HbA1c blood concentration.
| <7.50% (n=21) | 7.50–8.50 (n=46) | >8.50% (n=38) | Global | p | |
|---|---|---|---|---|---|
| Male (%) | 75.00 | 69.60 | 56.80 | 65.70 | NS |
| Age (years) | 60.00(7.90) | 60.00 (7.60) | 60.70 (8.60) | 60.30 (7.90) | NS |
| Evolution (years) | 12.70 (7.30) | 15.20 (8.50) | 14.60 (6.30) | 14.40 (7.50) | NS |
| Complication (%) | 35.00 | 56.50 | 43.20 | 51.90 | NS |
| HT (%) | 80.00 | 73.90 | 73.00 | 73.60 | NS |
| Dyslipidemia (%) | 80.00 | 84.80 | 91.90 | 84.90 | NS |
| Baseline HbA1c (%) | 6.90 (0.50) | 8.00 (0.30) | 9.40 (0.80) | 8.30 (1.10) | ** |
| BMI (kg/m2) | 35.60 (5.30) | 33.50 (5.70) | 36.20 (5.80) | 34.80 (1.10) | NS |
| Insulin regimen | NS | ||||
| Basal (%) | 55.00 | 56.50 | 45.90 | 53.30 | |
| Basal+bolus (%) | 15.00 | 28.30 | 24.40 | 23.80 | |
| Premixed insulin (%) | 30.00 | 15.20 | 29.70 | 22.90 |
** Statistically significant differences.
Prior non-insulin antihyperglycemic treatments.
| Number of prior non-insulin antihyperglycemic treatment used (%) | |
| 0 | 14.3 |
| 1 | 31.4 |
| 2 | 38.1 |
| >2 | 16.2 |
| Drugs in prior non-insulin antihyperglycemic treatment (%) | |
| None | 14.3 |
| Metformin | 69.4 |
| Sulfonylureas/Glinides | 16.7 |
| DPPIV inhibitors | 27.8 |
| GLP-1 receptor agonist | 34.3 |
| Thiazolidinediones | 3.7 |
| Combination of prior non-insulin antihyperglycemia treatment | |
| Metformin+Sulfonylureas/Glinides | 1.9 |
| Metformin+DPPIV inhibitors | 11.4 |
| Metformin+GLP-1 receptor agonist | 19.0 |
| Metformin+Thiazolidinediones | 1.9 |
| Metformin+Sulfonylureas/Glinides+GLP-1 receptor agonist | 2.9 |
| Metformin+DPPIV inhibitors+Sulfonylureas/Glinides | 6.7 |
| Metformin+DPPIV inhibitors+GLP-1 receptor agonist | 4.8 |
| Metformin+Thiazolidinediones+GLP-1 receptor agonist | 1.0 |
| Sulfonylureas/Glinides+DPPIV inhibitors | 1.9 |
| Sulfonylureas/Glinides+GLP-1 receptor agonist | 1.0 |
| Sulfonylureas/Glinides+DPPIV inhibitors+GLP-1 receptor agonist | 1.0 |
| DPPIV inhibitors+GLP-1 receptor agonist | 1.0 |
Exclusion criteria were: T1D or diabetes with other aetiologies, pregnancy, a history of repeated urinary infections or a glomerular filtration rate below 60ml/min/1.73m2.
Patients also received antihypertensive and hypolipidemic treatments according to the American Diabetes Association (ADA) recommendations.
The evolution of the following parameters was evaluated: fasting plasma glucose, HbA1c, weight and BMI (kg/m2), TDI/weight, TDB and TDR. In addition, total cholesterol, cLDL, cHDL, triglycerides and SBP and DBP were determined.
Safety and tolerance were evaluated through the occurrence of severe hypoglycemia, ketoacidosis or genitourinary infections.
Statistical analysisThe data are expressed as mean (±standard error) for quantitative variables and as percentages for categorical variables. Means were compared for groups by repeated measures ANOVA with a Greenhouse–Geisser correction and Bonferroni post hoc test and contingency tables to calculate χ2 when comparing categorical variables. The correlation analyses were assessed with the Pearson correlation coefficient and with multivariate linear and logistic regression studies. All the analyses were carried out with the statistical package SPSS v.20.0 (SPSS, Inc., Chicago, IL).
ResultsOf the 112 patients selected, 8 were eliminated due to side effects (pruritus, drug allergic reaction and genitourinary infections) or endocrine pancreatic insufficiency. No severe hypoglycemia or ketoacidosis episodes were recorded.
Of the 105 patients that completed the trial, 65.7% were male, average age was 60.3 (7.9) years (range: 38–79) and average time since diagnosis was 14.4 (7.5) years (range: 1–40). The average HbA1c blood concentration was 8.3% (range: 5.9–11.5), 75.9% were obese and average BMI was 34.8 (5.8)kg/m2. In addition, 73.4% suffered from HT, 84.9% from dyslipidaemia and 51.9% from angiopathy, the most common being diabetic retinopathy.
In addition to their habitual treatment, all patients were given a SGLT2 inhibitor: 10mg dapagliflozin (78.1%), 10mg empagliflozin (13.3%), 100mg canagliflozin (8.6%). Revisions were set after between 2 and 9 months (average, 4 months) of treatment.
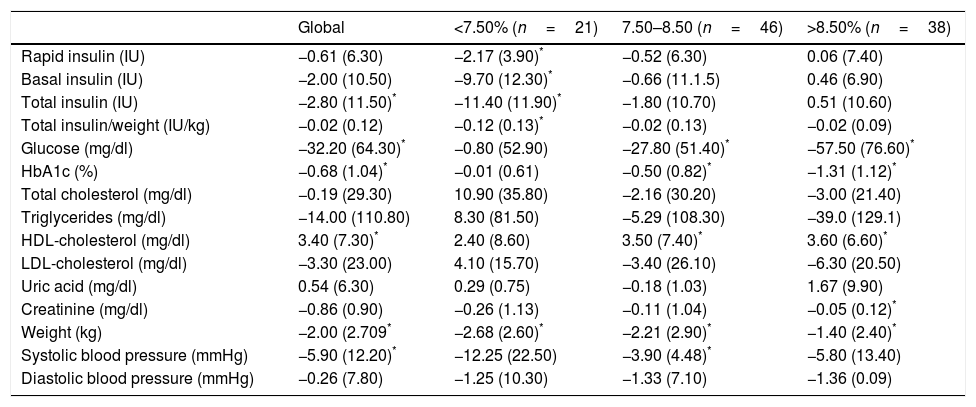
Glycaemic control and insulin dose reductionAfter 4 months follow-up average, significant HbA1c reductions were observed [8.3 (1.1)% to 7.6 (1.1)% (p<0.05)] which was accompanied by TDI reductions from 60.3 (42.7) to 57.5 (47.3)UI/day (p<0.05) (Table 3). However, patients with good glycaemic control did not change their HbA1c levels during treatment with SGLT2 inhibitors [6.9 (0.5)% vs 7.0 (0.8)%; p=NS], the reduction being attributed to those with a poorer control (Table 3). The logistic regression shows that changes in HbA1c posttreatment correlate with initial HbA1c [β=−0.64 (p<0.05)] after adjusting for changes in body weight.
Changes in insulin dose, HbA1c, metabolic and anthropometric parameters and blood pressure after administration of SGLT2 inhibitors for four months to patients subjected to standard T2D treatments.
| Global | <7.50% (n=21) | 7.50–8.50 (n=46) | >8.50% (n=38) | |
|---|---|---|---|---|
| Rapid insulin (IU) | −0.61 (6.30) | −2.17 (3.90)* | −0.52 (6.30) | 0.06 (7.40) |
| Basal insulin (IU) | −2.00 (10.50) | −9.70 (12.30)* | −0.66 (11.1.5) | 0.46 (6.90) |
| Total insulin (IU) | −2.80 (11.50)* | −11.40 (11.90)* | −1.80 (10.70) | 0.51 (10.60) |
| Total insulin/weight (IU/kg) | −0.02 (0.12) | −0.12 (0.13)* | −0.02 (0.13) | −0.02 (0.09) |
| Glucose (mg/dl) | −32.20 (64.30)* | −0.80 (52.90) | −27.80 (51.40)* | −57.50 (76.60)* |
| HbA1c (%) | −0.68 (1.04)* | −0.01 (0.61) | −0.50 (0.82)* | −1.31 (1.12)* |
| Total cholesterol (mg/dl) | −0.19 (29.30) | 10.90 (35.80) | −2.16 (30.20) | −3.00 (21.40) |
| Triglycerides (mg/dl) | −14.00 (110.80) | 8.30 (81.50) | −5.29 (108.30) | −39.0 (129.1) |
| HDL-cholesterol (mg/dl) | 3.40 (7.30)* | 2.40 (8.60) | 3.50 (7.40)* | 3.60 (6.60)* |
| LDL-cholesterol (mg/dl) | −3.30 (23.00) | 4.10 (15.70) | −3.40 (26.10) | −6.30 (20.50) |
| Uric acid (mg/dl) | 0.54 (6.30) | 0.29 (0.75) | −0.18 (1.03) | 1.67 (9.90) |
| Creatinine (mg/dl) | −0.86 (0.90) | −0.26 (1.13) | −0.11 (1.04) | −0.05 (0.12)* |
| Weight (kg) | −2.00 (2.709* | −2.68 (2.60)* | −2.21 (2.90)* | −1.40 (2.40)* |
| Systolic blood pressure (mmHg) | −5.90 (12.20)* | −12.25 (22.50) | −3.90 (4.48)* | −5.80 (13.40) |
| Diastolic blood pressure (mmHg) | −0.26 (7.80) | −1.25 (10.30) | −1.33 (7.10) | −1.36 (0.09) |
Conversely, the highest TDI improvement was observed on patients with baselines for HbA1c<7.5%, which dropped by 23%, while four patients stopped taking insulin (Table 3). However, no TDI reduction was observed for HbA1c >7.5% patients (Table 3).
Body weightA 73.7% of patients reduced their weight, with an average global reduction of −2.0 (2.7) kg (range −1.4 to −2.68kg). This effect was independent of the glucose control (Table 3). In addition, 54% of patients experienced concomitant weight loss and HbA1c reduction. There were no differences between the patients treated with dapagliflozin, empagliflozin and canagliflozin.
Lipid profile and blood pressureThe treatment with SGLT2 inhibitors did not alter the lipid profile and the total cholesterol, triglycerides and cLDL. However, a cHDL increase was noted [41.2 (16.6)mg/dl to 44.6 (16.4)mg/dl (p<0.01)] (Table 3). There were no differences between the groups of patients treated with the three SGLT2 inhibitors.
The SBP dropped significantly [141.1 (21.0)mmHg to 135.2 (18.4)mmHg (p<0.01)] following treatment with SGLT2 inhibitors; however, no DBP changes were observed.
DiscussionThe use of SGLT2 inhibitors on poorly controlled, insulin treated T2D patients resulted in significant improvement of their HbA1c blood concentrations, which was associated to weight loss, cHDL increase and SBP lowering.
These data agree with previous reports indicating that the addition of dapagliflozin,3 empagliflozin4 or canagliflozin5 to patients that received basal insulin and OADs led to improvements in HbA1c and reductions in the quantity of insulin needed (7).
However, this is the first study, to our knowledge, that included patients with good glycaemic control (HbA1c<7.5%). In this group no significant changes in HbA1c levels were evidenced but the treatment allowed reduction of the insulin doses and resulted in weight loss and cHDL increase.
Weight loss was not limited to the glycaemic controlled patients and may have been due, in part, to glycosuria since patients treated with SGLT2 inhibitors exhibited calorific losses between 240 and 400kcal/day in their urine. Consequently, SGLT2 inhibitors are recommended for T2D overweight patients.
Our results on the lipid profile demonstrated an increase in the cHDL concentration but no changes in the total cholesterol, triglycerides or cLDL levels. A recently published study of T2D patients treated with dapagliflzin who experienced a significant increase of 18% in cHDL concentration with no changes in cLDL.6
The decrease in SBP shown by our patients might have been due to the natriuretic effect of SGLT2 inhibitors, which may result in improvement of hemodynamic parameters such as blood pressure.
With regard to safety, no cases of severe hypoglycemia or normoglycaemic ketoacidosis were recorded among our patients. Perhaps the exclusion of T1D patients along with those exhibiting low values of peptide C and the fact that poorly controlled patients maintained their total insulin dose levels contributed to this finding. Another common side effect of SGLT2 inhibitors, linked to the glycosuria they induce, is genitourinary infection, especially for women. Correspondingly, in our patient sample candidiasis was the most common side effect observed, although only 2 patients had to stop the treatment as a result of this.
Limitations of this study are its retrospectivity, the small number of patients enrolled and that it only comprised 4 months of follow-up.
In conclusion, in insulin treated T2D patients, the addition of SGLT2 inhibitors brought about a reduction in HbA1c blood concentration that was associated to weight loss, cHDL increase and SBP decrease, even for patients with good glycaemic control, which should be taken into account when selecting the best hypoglycemia treatment.
Conflicts of interestThe authors have no conflicts of interest to declare.