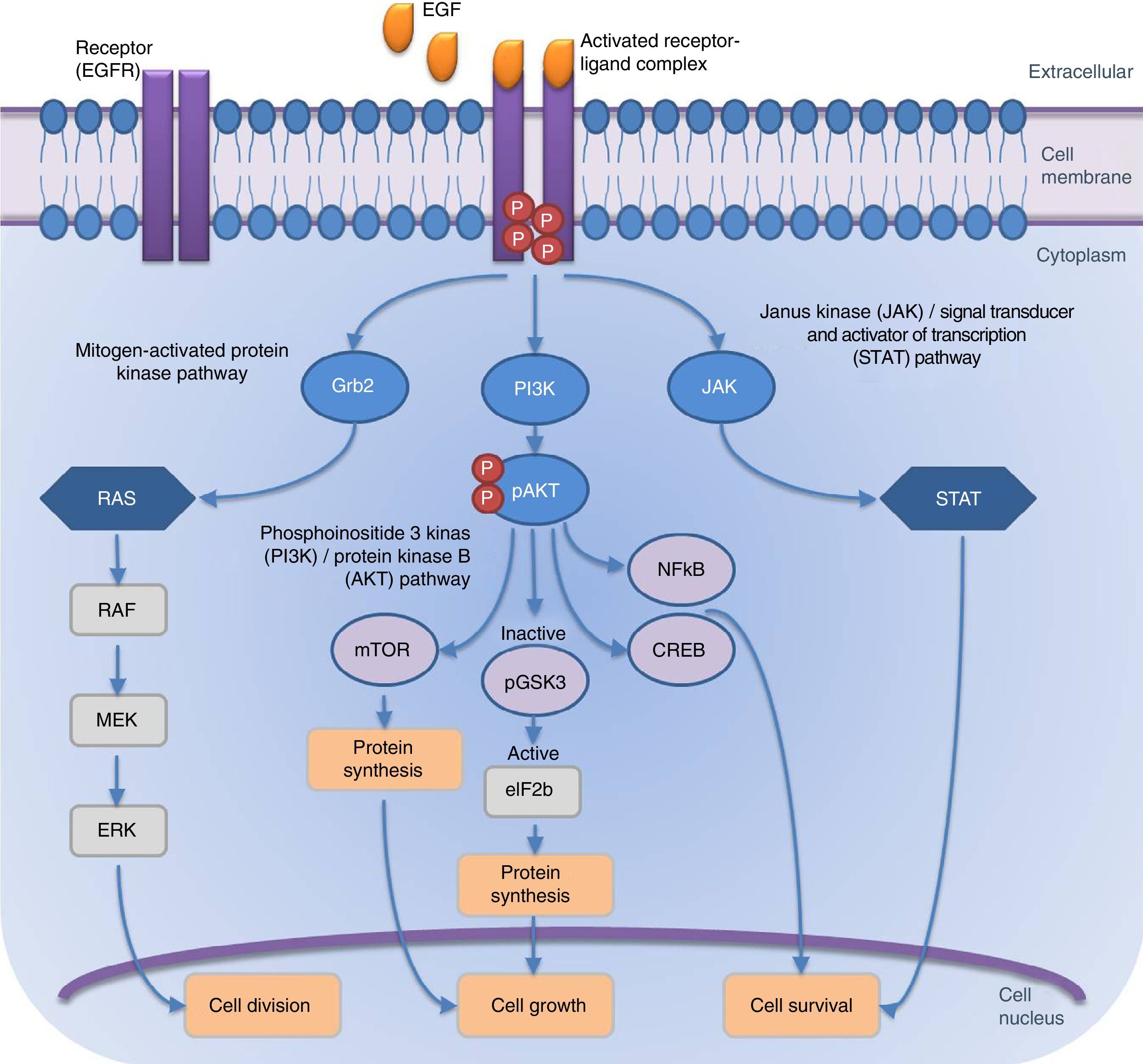
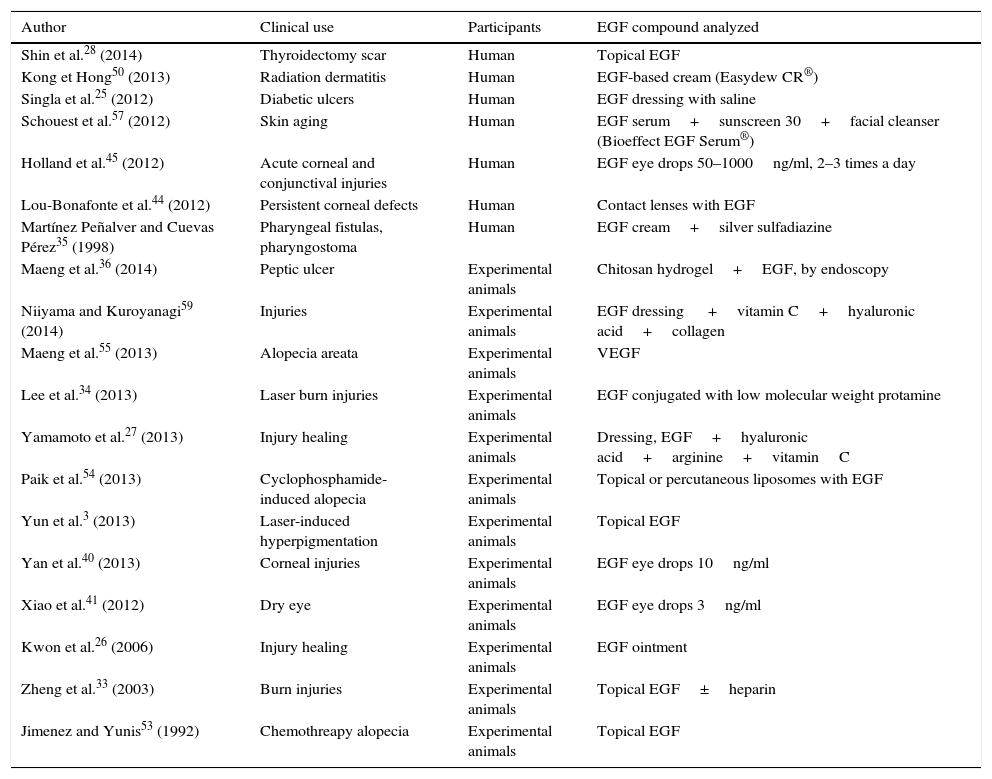
Bioidentical recombinant human epidermal growth factor (rhEGF) is available in concentrations and purity suitable for therapeutic use in long time stable formulations. Beneficial effects in several skin pathologies and lesions have been reported (traumatic and surgical wound healing, laser induced wounds, abnormal scars, keloids, radiation or chemotherapy induced dermatitis, post inflammatory hyperpigmentation or for skin aging damage repairing) and also may be considered for the treatment of several oropharingeal and high gastroesophageal tract mucosa diseases (mouth sores, pharyngeal fistulas, ulcers), and several corneal or conjunctive mucosa lesions.
rhEGF has not shown any important side or collateral effects in humans or in laboratory experimentation animals, showing optimal tolerability and safety with continuous use for months.
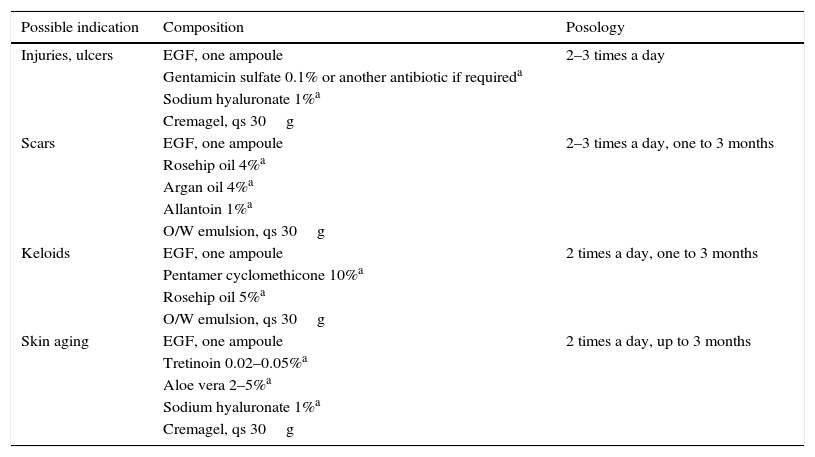
Compounding gives advantages of versatility, individualization, personalization, molecular stability, safety and effectiveness in ideal conditions, showing good tissue penetration, both on intact skin and skin lesions that expose the lower planes to the surface.
rhEGF compounds can be considered for prevention or as a treatment of diverse skin and mucosa diseases and conditions through compounding preparations.
El recombinant human epidermal growth factor (rhEGF, «factor de crecimiento epidérmico recombinante humano bioidéntico»), disponible en concentraciones y pureza aptas para uso terapéutico, dispone de formulaciones estables en el tiempo. Hay evidencias de efectos terapéuticos beneficiosos en diversas enfermedades y lesiones de la piel (heridas, cicatrices, queloides, dermatitis o alopecia por radiación o por quimioterápicos, hiperpigmentaciones posinflamación o daño causado por la edad), e igualmente puede ser considerado para el tratamiento de algunas afecciones de la mucosa orofaríngea y digestiva alta (aftosis, fístulas faríngeas, mucosas esofágica y gástrica) o para lesiones corneales y conjuntivales.
El rhEGF no muestra efectos secundarios o colaterales en humanos ni en animales de experimentación, evidenciando una óptima tolerabilidad y seguridad.
La formulación magistral otorga versatilidad, individualización, personalización, estabilidad molecular, seguridad y efectividad en condiciones idóneas, evidenciando una buena capacidad de penetración en el tejido, tanto en piel intacta como sobre lesiones cutáneas que exponen los planos inferiores a la superficie.
Se pueden considerar los compuestos con rhEGF para la prevención o tratamiento de diversas afecciones y enfermedades cutáneas o mucosas a través de preparados de formulación magistral.
Artículo
Comprando el artículo el PDF del mismo podrá ser descargado
Precio 19,34 €
Comprar ahora